Abstract
The C-terminus of CBFβ-SMMHC, the fusion protein produced by a chromosome 16 inversion in acute myeloid leukemia subtype M4Eo, contains domains for self-multimerization and transcriptional repression, both of which have been proposed to be important for leukemogenesis by CBFβ-SMMHC. To test the role of the fusion protein's C-terminus in vivo, we generated knock-in mice expressing a C-terminally truncated CBFβ-SMMHC (CBFβ-SMMHCΔC95). Embryos with a single copy of CBFβ-SMMHCΔC95 were viable and showed no defects in hematopoiesis, whereas embryos homozygous for the CBFβ-SMMHCΔC95 allele had hematopoietic defects and died in mid-gestation, similar to embryos with a single-copy of the full-length CBFβ-SMMHC. Importantly, unlike mice expressing full-length CBFβ-SMMHC, none of the mice expressing CBFβ-SMMHCΔC95 developed leukemia, even after treatment with a mutagen, although some of the older mice developed a nontransplantable myeloproliferative disease. Our data indicate that the CBFβ-SMMHC's C-terminus is essential to induce embryonic hematopoietic defects and leukemogenesis.
Key Points
Heterozygous CBFβ-SMMHCΔC95 knock-in mice have normal hematopoiesis.
Heterozygous CBFβ-SMMHCΔC95 knock-in mice do not develop leukemia.
Introduction
Inversion 16 (Inv16) results in the fusion of the transcription factor gene, CBFB, and MYH11, which encodes smooth muscle myosin heavy chain (SMMHC).1 Expression of CBFβ-SMMHC is probably the initiating event in Inv16 AML2 ; however, its mechanism is not well understood.
Previously, we generated knock-in mice expressing Cbfb-MYH113 to address the role of the fusion gene in vivo. Mice expressing a single allele of the fusion gene (Cbfb+/MYH11) showed defective primitive hematopoiesis, blocked definitive hematopoiesis, and embryonic lethality.3,4 Interestingly, except for the primitive hematopoietic defect, this phenotype is indistinguishable from mice lacking functional Cbfb5-7 or its dimerization partner Runx1,8,9 implying that Cbfb-MYH11 has dominant negative activities. Importantly, nearly 100% of the Cbfb-MYH11 mice develop AML within 6 months after treatment with a mutagen.2,10 This demonstrates that expression of Cbfb-MYH11 is necessary, but not sufficient for AML.
The C-terminus of CBFβ-SMMHC consists of repeated α-helical rod domains that facilitate self-dimerization and multimerization.11-13 Work in myeloid cells indicates CBFβ-SMMHC's ability to form multimers correlates with its ability to repress proliferation and transcription.12,13 In addition, CBFβ-SMMHC C-terminus can interact with transcriptional corepressors,14,15 providing a possible mechanism for its dominant negative effects on transcriptional regulation by CBFβ/RUNX1. CBFβ-SMMHC multimerization and transcriptional repression are potential mechanisms for leukemogenesis, implying that the C-terminal region is critically important. To test the requirement of this region in vivo, we generated knock-in mice expressing a truncated Cbfb-MYH11 that encodes a CBFβ-SMMHC fusion protein missing the C-terminal 95 amino acids.
Methods
The construction of the targeting vector, the generation of the targeted mouse ES cells, and the production of chimeric mice with the ES cells were performed as previously described.3,10,16 The mouse studies were approved by the National Human Genome Research Institute Animal Care and Use Committee, and followed relevant National Institutes of Health and national guidelines and regulations. Western blot analysis using antibodies against CBFβ and tubulin were performed as previously described.4,16,17 Flow cytometry analysis and histologic analysis were conducted as previously described.4,16 Colony forming assays were performed as previously described.4,16 The MCSFR reporter assay was performed as described before except that the cells were transfected in 96-well plates.16,18 The pCMV-CBFB-MYH11ΔC95 plasmid was generated using the EcoRI-NotI fragment from pCBFB-MYH11ΔC9511. For microarray analysis (GSE42238), RNA was prepared from peripheral blood cells of E12.5 embryos, hybridized in triplicate, and analyzed as previously described.4 All comparisons were performed to wild-type samples from the same litter as the indicated knock-in samples. Functional analysis was performed using Ingenuity Pathway Analysis (Ingenuity Systems, http://www.ingenuity.com) with Benjamini-Hochberg corrected P values. Excluding microarray analysis, statistical significance was assessed using the student t test by Excel (Microsoft). Transplantation experiments were performed as described,16 with injection of 106 or 107 cells from the spleens or bone marrows of aged Cbfb+/ΔC95 mice into isogenic, wild-type C57BL/6 × 129 F1 hybrid mice. The recipient mice were monitored by monthly blood draws for blood smear, hematocrits, and flow cytometry analysis. At the end of 1 year, the mice were killed and grossly analyzed for organ infiltrations.
Results and discussion
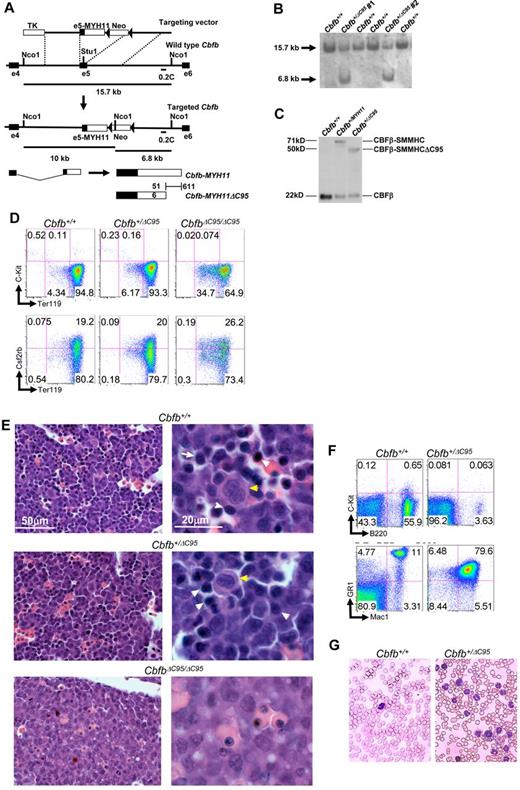
We generated knock-in mice with the Cbfb-MYH11ΔC95 (CbfbΔC95) allele using a strategy similar to that used previously for the full-length Cbfb-MYH11 (Cbfb+/MYH11) allele (Figure 1A).3,16 After screening for proper targeting and confirming expression of the fusion protein (Figure 1B-C), 2 ES cell clones were used to establish independent lines of mice (no. 1 and no. 2), which showed nearly identical phenotypes. Surprisingly, Cbfb+/ΔC95 mice were viable and fertile, unlike Cbfb+/MYH11mice.3
The C-terminal 95 amino acids of CBFβ-SMMHC are required for defects in hematopoiesis and leukemogenesis. (A) Targeting strategy used to replace exon 5 of Cbfb with the targeting construct. Locations of probe 0.2C and the sizes of NcoI fragments detected by 0.2C are indicated. Filled triangles represent lox-P sites. (B) Southern blot hybridization of NcoI-digested DNA from transfected ES cell clones with probe 0.2C. The 15.7 kb band corresponds to the wildtype Cbfb allele, and the 6.8 kb band corresponds to the knockin allele. (C) Western blot analysis of parental (Cbfb+/+), full-length CBFβ-SMMHC (Cbfb+/MYH11) expressing, and CBFβ-SMMHCΔC95 (Cbfb+/ΔC95) expressing ES cell clones. The blot was probed with an antibody specific to CBFβ. The calculated molecular weights for each protein are indicated. (D) Representative FACS plots for Ter119, C-Kit, and Csf2rb staining of primitive blood from E10.5 embryos from line No. 1 of the indicated genotypes. Percentage of cells in each gate is given. N ≥ 3 for each genotype. (E) Histologic sections of fetal livers from E12.5 embryos from line No. 2 of the indicated genotypes. Yellow arrows indicate megakaryocytes. White arrows indicate hematopoietic progenitors. Similar results were found in embryos from line no. 1 (data not shown). (F) Representative FACS plots of B220, C-Kit, Gr1, and Mac1 of peripheral blood from older than 10-month-old mice from line no. 1 of the indicated genotypes. Percentage of cells in each gate is given. N > 3 for each genotype. (G) Wright-Giemsa stained peripheral blood smears from older than 10-month-old mice of the indicated genotypes from line no. 1. Similar results were observed in mice from line no. 2 (data not shown).
The C-terminal 95 amino acids of CBFβ-SMMHC are required for defects in hematopoiesis and leukemogenesis. (A) Targeting strategy used to replace exon 5 of Cbfb with the targeting construct. Locations of probe 0.2C and the sizes of NcoI fragments detected by 0.2C are indicated. Filled triangles represent lox-P sites. (B) Southern blot hybridization of NcoI-digested DNA from transfected ES cell clones with probe 0.2C. The 15.7 kb band corresponds to the wildtype Cbfb allele, and the 6.8 kb band corresponds to the knockin allele. (C) Western blot analysis of parental (Cbfb+/+), full-length CBFβ-SMMHC (Cbfb+/MYH11) expressing, and CBFβ-SMMHCΔC95 (Cbfb+/ΔC95) expressing ES cell clones. The blot was probed with an antibody specific to CBFβ. The calculated molecular weights for each protein are indicated. (D) Representative FACS plots for Ter119, C-Kit, and Csf2rb staining of primitive blood from E10.5 embryos from line No. 1 of the indicated genotypes. Percentage of cells in each gate is given. N ≥ 3 for each genotype. (E) Histologic sections of fetal livers from E12.5 embryos from line No. 2 of the indicated genotypes. Yellow arrows indicate megakaryocytes. White arrows indicate hematopoietic progenitors. Similar results were found in embryos from line no. 1 (data not shown). (F) Representative FACS plots of B220, C-Kit, Gr1, and Mac1 of peripheral blood from older than 10-month-old mice from line no. 1 of the indicated genotypes. Percentage of cells in each gate is given. N > 3 for each genotype. (G) Wright-Giemsa stained peripheral blood smears from older than 10-month-old mice of the indicated genotypes from line no. 1. Similar results were observed in mice from line no. 2 (data not shown).
We inter-crossed the Cbfb+/ΔC95 mice and examined their progeny for defects in hematopoiesis. Using differentiation markers Ter119, C-Kit, and Csf2rb, we found that the Cbfb+/ΔC95 embryos did not show the primitive blood defects seen in Cbfb+/MYH11 embryos (Figure 1D, supplemental Figure 1).4 Homozygous CbfbΔC95/ΔC95 embryos showed a trend toward increased immature Ter119low, C-Kit− cells and decreased mature Ter119high, C-Kit− cells, but these changes were statistically significant only in line no. 1 (supplemental Figure 1A). In addition, there was no change in the number of Csf2rb+ cells in CbfbΔC95/ΔC95 embryos (supplemental Figure 1B). These data indicate that the C-terminal 95 amino acids are required for CBFβ-SMMHC's primitive hematopoiesis defects and may mediate the Runx1-repression independent activity previously described.4
At embryonic day 12.5 (E12.5), Cbfb+/ΔC95 mice showed normal definitive hematopoiesis as indicated by the presence of hematopoietic cells in the fetal liver (Figure 1E), and normal colony forming abilities in culture (supplemental Figure 1C). However, the CbfbΔC95/ΔC95 mice showed a block in definitive hematopoiesis (Figure 1E) and central nervous system hemorrhaging (data not shown), both characteristics of Cbfb+/MYH11 and Cbfb−/− mice.3,5-7 In addition, from inter-crosses of Cbfb+/ΔC95 mice, we never observed live CbfbΔC95/ΔC95 embryos after E13.5, and at birth we observed 56 Cbfb+/+, 66 Cbfb+/ΔC95, but no CbfbΔC95/ΔC95 progenies, indicating embryonic lethality; another characteristic of Cbfb+/MYH11 and Cbfb−/− mice. These findings indicate that CBFβ-SMMHC's C-terminal 95 amino acids are required for it is dominant negative activity during definitive hematopoiesis.
To test whether CBFβ-SMMHC's C-terminal 95 amino acids were required for leukemogenesis, we monitored Cbfb+/ΔC95 mice for leukemia development. To promote leukemogenesis, Cbfb+/ΔC95 mice were treated with N-ethyl-N-nitrosourea (ENU). Of the 20 ENU-treated Cbfb+/ΔC95 mice observed, none developed leukemia. These mice were followed for more than a year, which is significantly longer than required for leukemia development in Cbfb+/MYH11 mice.2,10,16 With advanced age (> 10 months), some Cbfb+/ΔC95 mice developed a myeloproliferative disease with increased Mac1/Gr1 double-positive cells (Figure 1G, supplemental Figure 2) in the peripheral blood, and increased myelopoiesis in the bone marrow and spleen (supplemental Figure 3). Colony forming assays of bone marrow cells from 6-month-old Cbfb+/ΔC95 mice showed increased total colony number and increased granulocyte and macrophage (CFU-GM) colonies (supplemental Figure 4). This myeloproliferative disease was clearly distinct from Cbfb-MYH11+ leukemia as these cells did not express C-Kit (Figure 1F) and did not have a blast like appearance (Figure 1G), both hallmarks of leukemia in Cbfb+/MYH11 mice.2,10,16 It was also different from myeloid dysplasia because the mice did not suffer from cytopenia in the peripheral blood. Unlike leukemia, even though these abnormal cells were increased in the spleen and disrupted splenic structure, they showed only mild infiltration into other organs (supplemental Figure 5). In addition, when these abnormal myeloid cells (> 5 Cbfb+/ΔC95 donors) were transplanted into isogenic mice, none of the recipient mice showed abnormal myelopoiesis up to 1 year after transplantation. This is in contrast to leukemic cells from Cbfb+/MYH11 mice, which show severe infiltration in other organs and are readily transplantable.2 Collectively, these results demonstrate that, although CBFβ-SMMHCΔC95 retains some ability to disrupt myelopoiesis, it lost its leukemogenic capability.
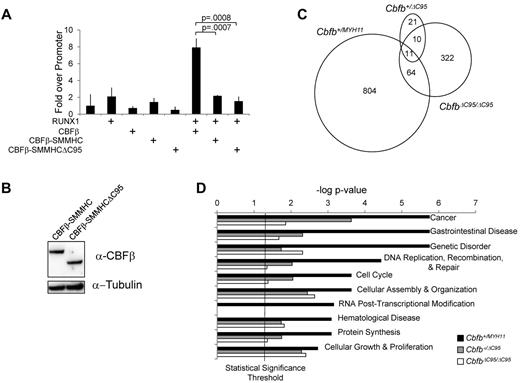
The C-terminus of CBFβ-SMMHC contains a RUNX1–repression domain that is partially deleted in CBFβ-SMMHCΔC95.14,15 Therefore, it is possible that the loss of CBFβ-SMMHCΔC95's leukemogenic activity is related to impaired repression of RUNX1. Therefore, we tested CBFβ-SMMHCΔC95's ability to repress RUNX1 activation of the MCSFR promoter.18 Cells transfected with RUNX1 and CBFβ-SMMHCΔC95 failed to activate the MCSFR reporter, similar to full-length CBFβ-SMMHC in this assay (Figure 2A-B). These data suggest that CBFβ-SMMHCΔC95 is still capable of repressing RUNX1 transactivation, which does not correlate with its leukemogenic activity.
The C-terminal 95 amino acids of CBFβ-SMMHC are not required for RUNX1 repression, but are important for the deregulation of gene expression. (A) Bar graph from a representative experiment showing fold change in luciferase activity in 293-0 cells transfected with expression plasmids for the indicated proteins. To control for differences in transfection efficiencies, luciferase activity was standardized to renilla activity, which was expressed from a constitutively active promoter. Each transfection was performed in triplicate, and the assay was performed more than 3 times, each time with the same relative luciferase activity. (B) Western blot of 293-0 cells transfected with plasmids expressing the indicated proteins. The blot was probed with an antibody specific to CBFβ, stripped, and reprobed with an antibody specific to tubulin. (C) Venn diagrams representing the numbers of genes that showed > 2-fold change in expression and a P < .05 in the peripheral blood from E12.5 embryos from line no. 2 of the indicated genotypes compared with their wild-type littermate controls. (D) Bar graph of the 10 biologic functions most significantly associated with the differentially expressed genes in Cbfb+/MYH11 embryos, and the P value of these functions for the differentially expressed genes in Cbfb+/ΔC95 and CbfbΔC95/ΔC95 embryos. The vertical line is the statistical significance threshold at the −log of P = .05, therefore functions with P values above this line (to the right) are considered statistically significant.
The C-terminal 95 amino acids of CBFβ-SMMHC are not required for RUNX1 repression, but are important for the deregulation of gene expression. (A) Bar graph from a representative experiment showing fold change in luciferase activity in 293-0 cells transfected with expression plasmids for the indicated proteins. To control for differences in transfection efficiencies, luciferase activity was standardized to renilla activity, which was expressed from a constitutively active promoter. Each transfection was performed in triplicate, and the assay was performed more than 3 times, each time with the same relative luciferase activity. (B) Western blot of 293-0 cells transfected with plasmids expressing the indicated proteins. The blot was probed with an antibody specific to CBFβ, stripped, and reprobed with an antibody specific to tubulin. (C) Venn diagrams representing the numbers of genes that showed > 2-fold change in expression and a P < .05 in the peripheral blood from E12.5 embryos from line no. 2 of the indicated genotypes compared with their wild-type littermate controls. (D) Bar graph of the 10 biologic functions most significantly associated with the differentially expressed genes in Cbfb+/MYH11 embryos, and the P value of these functions for the differentially expressed genes in Cbfb+/ΔC95 and CbfbΔC95/ΔC95 embryos. The vertical line is the statistical significance threshold at the −log of P = .05, therefore functions with P values above this line (to the right) are considered statistically significant.
Previously, we showed that CBFβ-SMMHC can induce gene expression changes independent of Runx1 repression.4 To address whether such activity requires the C-terminal 95 amino acids, we performed microarray analysis. We first identified the differentially expressed genes (DEGs) in Cbfb+/ΔC95 and CbfbΔ95/ΔC95 E12.5 embryos by comparing the gene expression profiles in these embryos with that in the littermate wild-type embryos (Cbfb+/+). We then compared the Cbfb+/ΔC95 and CbfbΔ95/ΔC95 DEGs with DEGs in the Cbfb+/MYH11 embryos, as we previously described.4 We found that the DEGs in the Cbfb+/ΔC95 and CbfbΔ95/ΔC95 embryos were very different from those in the Cbfb+/MYH11 embryos (Figure 2C). We also analyzed the biologic functions associated with these 3 sets of DEGs. Consistent with the failure of Cbfb+/ΔC95 mice to develop leukemia, the DEGs in Cbfb+/ΔC95 and CbfbΔ95/Δ95 embryos showed decreased association with functions probably important for leukemogenesis, such as cancer, cell cycle, and hematologic disease (Figure 2D). These results imply that the C-terminal 95 amino acids of CBFβ-SMMHC are critical for gene expression changes that are central to its leukemogenic activity.
To understand whether any of these gene expression changes are because of altered RUNX1 activity, we compared the list of DEGs from the CbfbΔ95/Δ95 embryos to genes shown to be bound by RUNX1.19 We found that 134 DEGs (32.9%) are bound by RUNX1 (supplemental Table 1). 4 of these genes (CD53,20 Cpa3,20 ItgaL,21,22 Mpo23,24 ) are targets of RUNX1 and many of these genes are deregulated in other models of impaired RUNX1 activity.4,25,26 These findings imply that at least some of the gene expression changes induced by CBFβ-SMMHCΔC95 are because of altered RUNX1 activity.
The data presented here demonstrate that the C-terminal 95 amino acids are critical for CBFβ-SMMHC's activities, particularly leukemogenesis. Blocking the function of this region in the fusion protein may be a potential therapeutic strategy in the future for the treatment of Inv16 AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Abdel Elkahloun and the National Human Genome Research Institute (NHGRI) microarray core for their assistance with microarray hybridization and reading; Bhavesh Borate and Niraj Trivedi of the NHGRI bioinformatics core for their assistance with the microarray analysis; Stephen Wincovitch and the NHGRI cytogenetics and fluorescent microscopy core for their assistance in karyotyping ES cell clones and imaging histology slides; Stacie Anderson and Martha Kirby of the NHGRI flow cytometry core for their assistance with FACS analysis; and Lauren R. Brinster for analysis of the pathologic slides.
This work was support by the Intramural Research Program of the National Human Genome Research Institute.
National Institutes of Health
Authorship
Contribution: Y.K. designed and performed experiments and analyzed data; R.K.H. designed and performed experiments, analyzed data, and wrote the paper; L.Z. designed and performed experiments and analyzed data; L.A. and C.R. performed experiments; L.G. performed experiments and analyzed data; and P.P.L. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Y.K. is Division of Gene Therapy Science, Graduate School of Medicine, Osaka University, Osaka, Japan.
Correspondence: Yasuhiko Kamikubo, Oncogenesis and Development Section, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD 20892; e-mail: pliu@mail.nih.gov.
References
Author notes
Y.K., R.K.H., and L.Z. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal