Key Points
JAK2-V617F cells show a competitive advantage over wild-type cells in BM transplantation assays.
A preclinical mouse model allows the examination of the effects of therapeutic agents on blood parameters and JAK2-V617F mutant allele burden.
Abstract
To establish a preclinical animal model for testing drugs with potential effects on myeloproliferative neoplasms (MPNs), we first performed a detailed phenotypic characterization of Cre-inducible transgenic JAK2-V617F mice. Deleting the conditional mouse Jak2-knockout alleles increased erythropoiesis and accentuated the polycythemia vera phenotype, but did not alter platelet or granulocyte levels. In a transplantation assay, JAK2-V617F+ BM cells had an advantage over wild-type competitor cells. Using this competitive repopulation assay, we compared the effects of INC424 (ruxolitinib), a dual Jak1/Jak2 inhibitor, and hydroxyurea (HU). HU led to weight loss, but did not reduce spleen weight. The hematologic parameters were lowered and a slight decrease of the mutant allele burden was noted. INC424 had little effect on body weight, but strongly decreased spleen size and rapidly normalized RBC and neutrophil parameters. No significant decrease in the mutant allele burden was observed. INC424 reduced the phospho-Stat5 levels, whereas HU strongly increased phospho-Stat5, most likely because of the elevated erythropoietin levels in response to the HU-induced anemia. This compensatory increase in JAK/STAT signaling may counteract the beneficial effects of cytoreduction at higher doses of HU and represents an adverse effect that should be avoided.
Introduction
Myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell diseases characterized by increased proliferation of the erythroid, megakaryocytic, or myeloid lineages. An acquired somatic mutation in the JAK2 gene resulting in a valine to phenylalanine substitution at position 617 (JAK2-V617F) is present in the majority of patients with MPNs.1-4 Several mouse models expressing JAK2-V617F have been established that mimic the MPN phenotypes observed in patients (for review, see Van Etten et al5 and Li et al6 ). We described previously a Cre-loxP–inducible transgenic mouse model that expresses human JAK2-V617F under the endogenous JAK2 promoter and displays a phenotype resembling polycythemia vera (PV) or essential thrombocythemia (ET).7 The phenotype was correlated with the ratio between the BM mRNA levels for the mutant human JAK2-V617F and the wild-type mouse Jak2 with a PV phenotype present at ratios of 1:1 (V617F: wild-type), whereas ET was observed at ratios of approximately 1:2. In the present study, we corroborated this correlation by studying the effects of decreased expression of the wild-type mouse Jak2 by crossing JAK2-V617F–transgenic mice with an inducible Jak2-knockout strain. Using a competitive transplantation assay, we established a preclinical mouse model that allows monitoring drug effects on the mutant JAK2-V617F allele burden. We used this mouse model to compare the efficacy of hydroxyurea (HU), which is considered the standard therapy for MPN, with the Jak1/Jak2 inhibitor INC424 (ruxolitinib).8,9
Methods
Transgenic mice
Mice carrying a Cre-recombinase–inducible human JAK2-V617F transgene (FF1) and a conditional knockout of Jak2 have been described previously.7,10 To activate the FF1 transgene or to induce the JAK2 knockout, the FF1 mice were crossed with MxCre,11 SclCre,12 or VavCre13 mice. Cre expression in MxCre mice was induced by a single IP injection of 300 μg of polyinosine-polycytosine (pIpC) or in SclCre mice by 2 mg of tamoxifen for 5 consecutive days. For competitive repopulation, BM cells from 8-week-old female C57BL/6 mice (Harlan Laboratories) or UBC-GFP mice expressing enhanced green fluorescent protein (GFP) were used.14 Mice were kept under specified pathogen-free conditions with free access to food and water. All experiments were done in strict adherence to Swiss laws for animal welfare and were approved by the Swiss Cantonal Veterinary Office of Basel-Stadt.
Compounds and formulations
INCB018424/ruxolitinib phosphate salt (herein referred to as INC424) was supplied by Incyte and Novartis Pharma. HU (H8627) was obtained from Sigma-Aldrich. Every 4 days, INC424 was freshly formulated in 0.5% hydroxypropyl methylcellulose. HU solution was prepared freshly every day by dissolving in 0.9% NaCl.
Competitive repopulation and inhibitor studies
Total BM cells from JAK2-V617F or C57BL/6 donor mice were mixed 1:1 with UBC-GFP BM cells and transplanted into C57BL/6 female recipient mice lethally irradiated with 12 Gy. Alternatively, JAK2-V617F/UBC-GFP+ BM cells mixed 1:1 with C57BL/6 were used. Blood counts were performed 5 weeks after transplantation. Mice that displayed a PV phenotype were randomized into 5 groups and dosed with INC424, HU, or vehicle twice daily (BID) for 21 consecutive days. INC424 was administered by oral gavage (30 or 90 mg/kg BID) and HU was injected IP (10, 50, 100, or 200 mg/kg BID). Control mice received 0.5% hydroxypropyl methylcellulose (vehicle). On day 21 of treatment, the recipients were euthanized by CO2 asphyxiation and tissue and blood samples were taken for further analysis.
Blood analyses
Blood was collected into EDTA-coated Microtainers (BD Biosciences) by tail vein sampling prior to the start of dosing and by cardiac puncture during the take-down procedure. Complete blood counts were determined on the ADVIA120 Hematology Analyzer using Multispecies Version 5.9.0-MS software (Bayer).
Flow cytometry analysis
Whole blood or single-cell suspensions from BM and spleen were stained with PE-conjugated, anti–mouse monoclonal antibodies against Ter119, CD61, Mac1, and Gr1 and biotin-conjugated anti–mouse antibody against CD71 (BioLegend). Heart, liver, and aorta cells were stained with PE-conjugated CD45 and Pe-Cy7–conjugated CD31 anti–mouse antibodies (BioLegend). The samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Hematopoietic progenitor assays
Erythroid progenitors were assayed with or without 3 units/mL of erythropoietin (EPO) in methylcellulose-based medium (Stem-Alpha) supplemented with 100 ng/mL of SCF (PeproTech), 310 μg/mL of human transferrin, and 200μM hemin (Sigma-Aldrich). BM cells (2 × 105) were plated in 30-mm dishes in triplicate. CFU-erythrocyte (CFU-E) colonies were scored after 4-5 days and BFU-E colonies after 9-10 days in culture. Myeloid progenitor assays were performed with 1 × 104 BM cells in triplicate in M3434 medium (STEMCELL Technologies) and CFU-granulocyte (CFU-G), CFU-macrophage (CFU-M), and CFU-granulocyte/macrophage (CFU-GM) colonies were counted after 7 days of culture. CFU-megakaryocyte (CFU-MK) colonies were grown in chamber slides with or without 50 ng/mL of recombinant human thrombopoietin in collagen-based medium (MegaCult-C; STEMCELL Technologies) containing 50 ng/mL of IL-11, 10 ng/mL of recombinant mouse IL-3 and 20 ng/mL of recombinant human IL-6 (PeproTech). BM cells (1 × 105) were cultured for 8 days on slide chambers in duplicates. The slides were then fixed and stained and CFU-MK colonies were counted.
Sample preparation and bioanalytical method
Concentrations of INC424 in blood were determined using quantitative ultra-performance liquid chromatography tandem mass spectrometry. For details, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
EPO ELISA assay
Mouse EPO levels were quantified in spleen homogenates by immunoassay (R&D Systems) according to the manufacturer's instructions. Spleen homogenates (50 μL) in RIPA buffer were assayed in triplicate.
Histology and immunohistochemistry
BM (from the sternum and femur), spleens, and livers were fixed in 4% phosphate-buffered formalin, embedded in paraffin, and sectioned. Tissue sections were stained with H&E for morphology or with Gömöri for the analysis of reticulin fibers. Images were taken using an Olympus BX43 microscope (numeric aperture of the objective lenses, 0.75/20× and 0.95/40×) and an Olympus DP73 camera (Olympus Schweiz AG). The software for image acquisition was cellSense Version 1.6. Immunohistochemistry with rabbit anti–phospho-STAT5 antibodies (#9359; Cell Signaling Technology) was performed as described previously.15 Evaluation and quantification of immunostained nuclei were performed using the Zeiss Mirax slide scanner (Scan Software Version 1.12; Zeiss). Automated quantitative assessment of phospho-STAT5+ and phospho-STAT5− cell nuclei was performed using eCognition Version XD 1.5 software (Definiens). The results were expressed as a percentage of phospho-STAT5+ nuclei of total nuclei. For details, see supplemental Methods.
Statistical analysis
Results are presented as means ± SEM. To assess the statistical significance among individual cohorts, 1-way ANOVA with subsequent Bonferroni posttest (Prism Version 4.00 software; GraphPad) or the Mann-Whitney rank sum test was used. P < .05 was considered significant.
Results
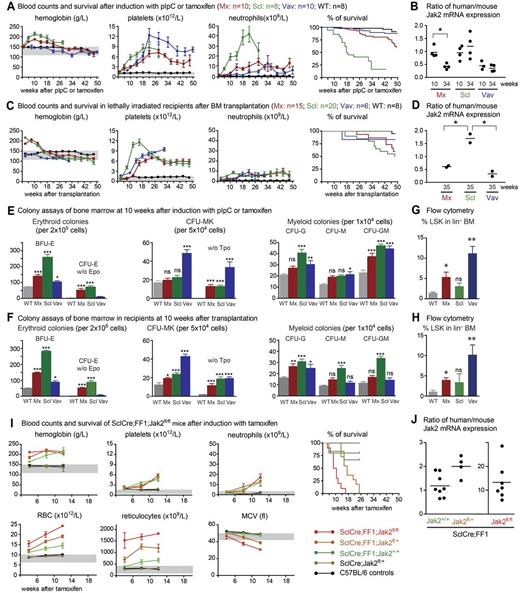
To characterize the phenotypic variations of our previously described Cre-loxP–inducible JAK2-V617F–transgenic mice (FF1), we crossed them with 3 different Cre-recombinase–expressing strains: the IFN-inducible MxCre, the tamoxifen-inducible SclCre, and the constitutively expressed VavCre strain. In all of these mice, the mutant JAK2-V617F expression is driven by the endogenous JAK2 promoter.7 Cre-recombinase is solely required for the flipping of the sequences encoding the kinase domain into an active configuration.7 We confirmed our previous finding that mice double transgenic for MxCre and JAK2-V617F (MxCre;FF1) display a PV phenotype, which was detectable within 4 weeks after induction by pIpC (Figure 1A). At approximately 26 weeks after pIpC administration, a decrease in hemoglobin and other RBC parameters occurred, but the platelets and neutrophils remained elevated. An even more pronounced PV phenotype was observed when SclCre;FF1 double-transgenic mice were induced with tamoxifen. At later stages, a trend toward lower hemoglobin values was also noted in SclCre;FF1 mice. However, longer observation was not possible because of a markedly shortened survival of these mice (Figure 1A). The cause of death in most cases remained unclear, but in some animals, bleeding in the abdominal cavity was found at autopsy (not shown). As described previously, VavCre;FF1 mice displayed normal hemoglobin levels, but very high platelet counts and mild neutrophilia resembling a pure ET phenotype (Figure 1A). Their survival was prolonged in comparison with SclCre;FF1 or MxCre;FF1 mice. Spleen weights were notably elevated in all 3 double-transgenic lines compared with wild-type controls (supplemental Figure 1A).
Comparison of hematopoiesis and survival of JAK2-V617F–transgenic mice activated by 3 different Cre transgenes. (A) Blood counts and survival after induction with pIpC or tamoxifen. (B) Ratio between human JAK2-V617F and mouse Jak2 in the BM of JAK2-V617F–transgenic mice at 10 and 34 weeks. (C) Blood counts and survival after transplantation of 2 × 106 BM cells collected from JAK2-V617F mouse donors into lethally irradiated recipients. (D) Ratio between human JAK2-V617F and mouse Jak2 in the BM of hosts transplanted with JAK2-V617F BM cells. The BM samples were collected 35 weeks after transplantation. Mx indicates MxCre;FF1 mice (red symbols); Scl, SclCre;FF1 mice (green symbols); Vav, VavCre;FF1 mice (blue symbols); and WT, wild-type controls (black symbols). Note that all SclCre;FF1 mice died within 30 weeks after tamoxifen induction and therefore no blood counts were available beyond this time point. Survival is shown as Kaplan-Meier curves. (E-F) Numbers of hematopoietic progenitors assessed by colony assays in methylcellulose or collagen-based medium. BFU-E indicates burst forming unit erythroid; and Tpo, thrombopoietin. BM from 3 mice per group were analyzed on duplicate plates. (G-H) Percentages of LSK cells within the lineage− BM cell population of nontransplanted mice induced by pIpC or tamoxifen (G) and in the lethally irradiated hosts transplanted with the BM cells from JAK2-V617F mice (H). (I) Blood counts and survival of SclCre;FF1 mice with Jak2fl/+ or Jak2fl/fl genotypes after induction with tamoxifen. (J) Ratio between human JAK2-V617F and mouse Jak2 in the BM of SclCre;FF1 mice with the Jak2+/+, Jak2fl/+, or Jak2fl/fl genotypes. Error bars represent SEM. One-way ANOVA is shown for comparisons between wild-type and transgenic mice. ns indicates not significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Comparison of hematopoiesis and survival of JAK2-V617F–transgenic mice activated by 3 different Cre transgenes. (A) Blood counts and survival after induction with pIpC or tamoxifen. (B) Ratio between human JAK2-V617F and mouse Jak2 in the BM of JAK2-V617F–transgenic mice at 10 and 34 weeks. (C) Blood counts and survival after transplantation of 2 × 106 BM cells collected from JAK2-V617F mouse donors into lethally irradiated recipients. (D) Ratio between human JAK2-V617F and mouse Jak2 in the BM of hosts transplanted with JAK2-V617F BM cells. The BM samples were collected 35 weeks after transplantation. Mx indicates MxCre;FF1 mice (red symbols); Scl, SclCre;FF1 mice (green symbols); Vav, VavCre;FF1 mice (blue symbols); and WT, wild-type controls (black symbols). Note that all SclCre;FF1 mice died within 30 weeks after tamoxifen induction and therefore no blood counts were available beyond this time point. Survival is shown as Kaplan-Meier curves. (E-F) Numbers of hematopoietic progenitors assessed by colony assays in methylcellulose or collagen-based medium. BFU-E indicates burst forming unit erythroid; and Tpo, thrombopoietin. BM from 3 mice per group were analyzed on duplicate plates. (G-H) Percentages of LSK cells within the lineage− BM cell population of nontransplanted mice induced by pIpC or tamoxifen (G) and in the lethally irradiated hosts transplanted with the BM cells from JAK2-V617F mice (H). (I) Blood counts and survival of SclCre;FF1 mice with Jak2fl/+ or Jak2fl/fl genotypes after induction with tamoxifen. (J) Ratio between human JAK2-V617F and mouse Jak2 in the BM of SclCre;FF1 mice with the Jak2+/+, Jak2fl/+, or Jak2fl/fl genotypes. Error bars represent SEM. One-way ANOVA is shown for comparisons between wild-type and transgenic mice. ns indicates not significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
The PV and ET phenotypes in our mice were correlated with the ratio between the mutant human JAK2-V617F mRNA and the wild-type mouse Jak2.7 Therefore, we examined whether JAK2-V617F expression levels were correlated with the decrease in hemoglobin values. During the early PV phase (10 weeks), the MxCre:FF1 and SclCre;FF1 mice showed an approximately 1:1 ratio of mutant and wild-type JAK2 mRNA, whereas the ratio in VavCre;FF1 mice with ET was approximately 1:2 (Figure 1B). At later stages (35 weeks), the expression ratio decreased in MxCre;FF1 mice to 1:2 levels that were comparable to VavCre;FF1, but the expression ratio remained at 1:1 in SclCre;FF1 mice (Figure 1B). The transgene copy number in MxCre;FF1 mice decreased from 9 to 6 copies and the number of copies in the active configuration also decreased from 7 to 5 (supplemental Figure 2), most likely reflecting continuous excision induced by endogenous IFN production. In contrast, no reduction in the expression ratio or transgene copy number was observed in SclCre;FF1 mice (Figure 1B and supplemental Figure 2). This strongly suggests that the reduction in Hb is independent of gene dosage.
Primary recipients of BM cells (2 × 106) from MxCre;FF1, VavCre;FF1, and SclCre;FF1 donors taken 6-10 weeks after induction with pIpC or tamoxifen, respectively, showed an MPN phenotype similar to the one observed in nontransplanted mice (Figure 1C), including a significant increase in spleen size (supplemental Figure 1B). The survival of transplanted recipients of SclCre;FF1 BM cells substantially improved compared with nontransplanted SclCre;FF1 mice and was no longer significantly different from mice that received MxCre;FF1 BM (Figure 1C right panel). Therefore, the increased mortality in the nontransplanted SclCre:FF1 mice appears to be related to the expression of JAK2-V617F in nonhematopoietic tissues. Because Cre expression in SclCreER-transgenic mice has also been described in endothelial cells,13 we examined mRNA from purified endothelial cells and found that JAK2-V617F expression was detectable at low levels (supplemental Figure 3A). However, there was no significant difference between SclCre;FF1 and MxCre;FF1 mice. Cre mRNA was also detectable in several nonhematopoietic tissues (supplemental Figure 3B).
We found an increase in erythroid, megakaryocytic, and myeloid colony-forming cells in BM from JAK2-V617F–transgenic mice (Figure 1E). The SclCre;FF1 mice showed the highest numbers of erythroid and myeloid colonies, whereas the VavCre;FF1 mice displayed the highest numbers of megakaryocytic colonies. Thrombopoietin-independent growth was present in all 3 strains, but EPO-independent growth was most prominent in SclCre;FF1 and MxCre;FF1 mice. The percentage of lineage−, Sca-1+, c-kit+ (LSK) cells in the BM was elevated in MxCre;FF1 mice and a trend toward higher levels was also noted in SclCre;FF1 mice, but the highest proportion of LSK cells was present in VavCre;FF1 mice (Figure 1G). Similar results for colony-forming cells and LSK cells were obtained in the BM of transplanted recipients (Figure 1F and H).
To examine the influence of the wild-type Jak2 on the MPN phenotype, we crossed the SclCre;FF1 mice with a conditional knockout of the mouse Jak2 gene.10 After tamoxifen induction, the SclCre;FF1;Jak2fl/fl and SclCre;FF1;Jak2fl/+ mice displayed higher RBC numbers and reticulocyte counts than SclCre;FF1;Jak2+/+ mice (Figure 1I). Initially, the heterozygous and homozygous Jak2 knockouts showed higher hemoglobin values than controls, but at later time points, the hemoglobin values in the 3 genotypes became indistinguishable, although the number of erythrocytes remained higher. Microcytosis with decreases in mean corpuscular volume was noted at 12 weeks after induction. The SclCre;FF1 mice on a heterozygous and homozygous Jak2-knockout background also had drastically reduced survival. The cause of death in most of the mice remains unclear. The mice did not appear to be visibly sick before they died and there were no signs of leukemic transformation. The platelet and neutrophil counts were largely unaffected by the heterozygous or homozygous reduction of the wild-type Jak2 (Figure 1I). The ratio of mutant to wild-type JAK2 increased in mice heterozygous and homozygous for Jak2fl/fl (Figure 1J).
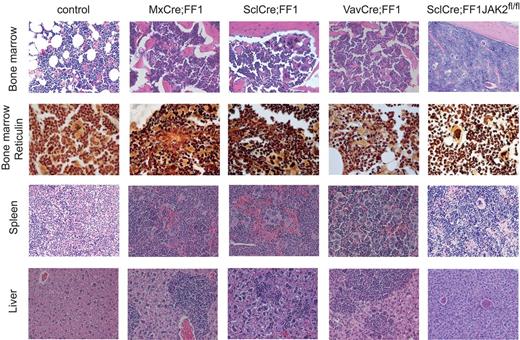
To further characterize the later stages when hemoglobin values decrease, we performed histopathologic analysis of BM trephine sections of the different genotypes (Figure 2 and Table 1). The 3 strains that express JAK2-V617F in the presence of 2 wild-type copies of mouse Jak2 demonstrated hypercellularity with trilineage hyperplasia. Markedly increased numbers of atypical megakaryocytes (hyperchromatic, hyperlobulated nuclei, abnormal nuclear configuration) were present, many of them in clusters. Reticulin stain (Gömöri) revealed a dense network of reticulin fibers sometimes in combination with collagenous fibers. Most of the older JAK2-V617F mice also demonstrated osteosclerosis (Table 1). The spleens showed destruction of normal architecture by infiltrates of highly atypical hematopoiesis. Megakaryocytes were the dominant cell population, displaying the same atypical morphology as in the BM. The livers showed portal tract but also sinusoidal infiltrates of atypical hematopoiesis (Figure 2). SclCre;FF1;Jak2fl/fl mice showed hypercellularity with trilineage hyperplasia including atypical, clustered megakaryocytes. There was no or only a mild increase in reticulin fibers. The spleens showed infiltrates of highly atypical hematopoiesis, but the livers (and kidneys) were mostly unaffected by these changes (Figure 2 and Table 1). Therefore, histopathology suggests that myelofibrosis could be linked to the decrease of hemoglobin at later stages of disease in MxCre;FF1 and SclCre;FF1 mice. When we transplanted BM cells from older SclCre;FF1 mice with myelofibrosis into lethally irradiated recipients, the PV phenotype reappeared in the transplanted mice, further supporting the hypothesis that the decrease in hemoglobin was mainly because of myelofibrosis (data not shown).
Histopathology of H&E-stained tissue samples. Magnification is 20× except for the second row that was prepared with Gömöri staining to visualize reticulin fibers (magnification, 40×). The mice were killed at 28-30 weeks of age (controls and VavCre;FF1) or 28-30 weeks after induction with pIpC (MxCre;FF1) or tamoxifen (SclCre;FF1). Because of the markedly reduced likelihood of survival, SclCre;FF1;Jak2fl/fl mice were killed 14 weeks after induction with tamoxifen.
Histopathology of H&E-stained tissue samples. Magnification is 20× except for the second row that was prepared with Gömöri staining to visualize reticulin fibers (magnification, 40×). The mice were killed at 28-30 weeks of age (controls and VavCre;FF1) or 28-30 weeks after induction with pIpC (MxCre;FF1) or tamoxifen (SclCre;FF1). Because of the markedly reduced likelihood of survival, SclCre;FF1;Jak2fl/fl mice were killed 14 weeks after induction with tamoxifen.
Histologic analysis of myelofibrosis
| . | BM . | Fibrosis . | Osteosclerosis . | Spleen . | Liver . | Comment . |
|---|---|---|---|---|---|---|
| Nontransplanted | ||||||
| WT 1 | Normal | 0 | Normal | Normal | ||
| WT 2 | Normal | 0 | Normal | Normal | ||
| WT 3 | Normal | 0 | Normal | Normal | ||
| MxCre;FF1 1 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis |
| MxCre;FF1 2 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis |
| MxCre;FF1 3 | MPN | 2–3 | Yes | MPN | normal | Osteosclerosis, PGC |
| SclCre;FF1 1 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| SclCre;FF1 2 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| SclCre;FF1 3 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| VavCre;FF1 1 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| VavCre;FF1 2 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| VavCre;FF1 3 | MPN | 2–3 | Yes | MPN | Normal | Osteosclerosis, PGC |
| SclCre;FF1;Jak2fl/fl 1 | MPN | 0 | No | MPN | Normal | |
| SclCre;FF1;Jak2fl/fl 2 | MPN | 0–1 | No | MPN | Normal | |
| SclCre;FF1;Jak2fl/fl 3 | MPN | 0 | No | MPN | Normal | |
| SclCre;FF1;Jak2fl/fl 4 | MPN | 1 | No | MPN | Normal | |
| 1:1 transplantations | ||||||
| WT 1 | Normal | 0 | Normal | Normal | ||
| WT 2 | Normal | 0 | Normal | Normal | ||
| WT 3 | Normal | 0 | Normal | Normal | ||
| MxCre;FF1 1 | MPN | 2 | Yes | MPN | Normal | no iron |
| MxCre;FF1 2 | MPN | 1–2 | Yes | MPN | Normal | iron +, Ec reduced |
| MxCre;FF1 3 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis |
| SclCre;FF1 1 | MPN | 3 | Yes | MPN | EMH | |
| SclCre;FF1 2 | MPN | 3 | Yes | MPN | EMH | |
| SclCre;FF1 3 | MPN | 2 | Yes | MPN | Normal | |
| . | BM . | Fibrosis . | Osteosclerosis . | Spleen . | Liver . | Comment . |
|---|---|---|---|---|---|---|
| Nontransplanted | ||||||
| WT 1 | Normal | 0 | Normal | Normal | ||
| WT 2 | Normal | 0 | Normal | Normal | ||
| WT 3 | Normal | 0 | Normal | Normal | ||
| MxCre;FF1 1 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis |
| MxCre;FF1 2 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis |
| MxCre;FF1 3 | MPN | 2–3 | Yes | MPN | normal | Osteosclerosis, PGC |
| SclCre;FF1 1 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| SclCre;FF1 2 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| SclCre;FF1 3 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| VavCre;FF1 1 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| VavCre;FF1 2 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis, PGC |
| VavCre;FF1 3 | MPN | 2–3 | Yes | MPN | Normal | Osteosclerosis, PGC |
| SclCre;FF1;Jak2fl/fl 1 | MPN | 0 | No | MPN | Normal | |
| SclCre;FF1;Jak2fl/fl 2 | MPN | 0–1 | No | MPN | Normal | |
| SclCre;FF1;Jak2fl/fl 3 | MPN | 0 | No | MPN | Normal | |
| SclCre;FF1;Jak2fl/fl 4 | MPN | 1 | No | MPN | Normal | |
| 1:1 transplantations | ||||||
| WT 1 | Normal | 0 | Normal | Normal | ||
| WT 2 | Normal | 0 | Normal | Normal | ||
| WT 3 | Normal | 0 | Normal | Normal | ||
| MxCre;FF1 1 | MPN | 2 | Yes | MPN | Normal | no iron |
| MxCre;FF1 2 | MPN | 1–2 | Yes | MPN | Normal | iron +, Ec reduced |
| MxCre;FF1 3 | MPN | 2–3 | Yes | MPN | EMH | Osteosclerosis |
| SclCre;FF1 1 | MPN | 3 | Yes | MPN | EMH | |
| SclCre;FF1 2 | MPN | 3 | Yes | MPN | EMH | |
| SclCre;FF1 3 | MPN | 2 | Yes | MPN | Normal | |
Mice older than 26 weeks were analyzed.
PGC indicates pseudo-Gaucher cells; EMH, extramedullary hematopoiesis; and Ec, erythrocytes.
We performed competitive transplantation assays to set up a preclinical model to test Jak2 inhibitors or other drugs used for the treatment of MPN patients and to follow the effects on the blood counts and the JAK2-V617F allele burden (Figure 3). BM cells from the UBC-GFP–transgenic strain expressing the GFP protein in all hematopoietic lineages were used as competitors.14 We mixed UBC-GFP–transgenic BM with cells from the 2 strains that showed a PV phenotype (MxCre;FF1 or SclCre;FF1) at a 1:1 ratio and transplanted a total of 2 × 106 BM cells into lethally irradiated recipients (C57BL/6; Figure 3A). Initially, the MxCre;FF1- and SclCre;FF1-reconstituted recipient mice displayed a PV phenotype, with hemoglobin values returning to the normal range after 18 weeks. The platelet and neutrophil numbers in MxCre;FF1 recipients increased after 16-18 weeks, whereas in recipients of SclCre;FF1 BM cells, the platelets and neutrophils were elevated at earlier time points. The chimerism, measured as the percentage of GFP− cells in the peripheral blood, reached nearly 100% in erythrocytes, with MxCre;FF1 mice showing a slight delay in platelets and neutrophils compared with SclCre;FF1 mice (Figure 3B bottom panel). The apparent rapid initial increase of chimerism in the RBC compartment is in part because of persistence of erythrocytes from the recipient. Controls receiving wild-type BM mixed 1:1 with UBC-GFP BM cells remained at approximately 50% GFP− cells throughout the time of observation. The survival times in the 3 groups of transplanted mice were not significantly different (Figure 3C) and the ratio of mutant to wild-type JAK2 remained approximately 1:1 in MxCre:FF1-transplanted mice at 35 weeks (Figure 3D). At later stages, histologic analysis revealed myelofibrosis in recipients of MxCre;FF1 and SclCre;FF1 BM (data not shown). The chimerism in the BM and spleens of MxCre;FF1-transplanted mice was close to 100% in all lineages tested (Figure 3E) and also in LSK cells (Figure 3F). Because in these experiments chimerism was estimated indirectly as the decrease of GFP+ cells, we also performed transplantation of BM cells from MxCre;FF1 mice that were crossed with the UBC-GFP mice and coexpressed GFP with JAK2-V617F. In the recipients of BM from MxCre;FF1;GFP donors, we confirmed that expressing JAK2-V617F provides a competitive advantage (supplemental Figure 4). We also performed a transplantation experiment into CD45.1 recipients and the contribution of autologous reconstitution with CD45.1 cells was < 1% in all lineages except T cells (data not shown). Therefore, autologous reconstitution was negligible in these experiments.
Competitive repopulation assays. (A) Schematic drawing of the experimental setup. (B) BM cells from a wild-type control mouse (WT, black symbols), from MxCre;FF1 mouse (Mx, red symbols), and a SclCre;FF1 mouse (Scl, green symbols) were harvested at 6 and 8 weeks after induction with pIpC or tamoxifen, respectively, mixed with BM cells from a UBC-GFP transgenic mouse at a 1:1 ratio, and transplanted into 7 lethally irradiated recipients per group (2 × 106 BM cells each). The time course of the blood counts and the percentages of chimerism (bottom panel) as determined by flow cytometry of GFP− cells are shown for the erythroid (Ter119), platelet (CD61), and granulocytic (Gr1) lineages in peripheral blood. Error bars represent SEM. (C) Survival probabilities of the transplantation recipients (Kaplan-Meier plot). Color coding is as in panel B. (D) Ratio between human JAK2-V617F and mouse Jak2 in the BM of mice transplanted with the MxCre;FF1 and UBC-GFP BM cells in a 1:1 ratio. Sampling was performed 35 weeks after transplantation. (E) Percentages of chimerism in the BM and spleens of transplanted mice as determined by the proportion of GFP− cells. (F) Percentages of LSK cells within the lineage− BM cell population and the LSK chimerism determined as the proportion of GFP− cells in the BM of recipient mice. Error bars represent SEM. One-way ANOVA is shown for comparisons between mice transplanted with wild-type (C57BL/6 and UBC-GFP) and mutant (MxCre;FF1 and UBC-GFP) BM cells. ns indicates not significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Competitive repopulation assays. (A) Schematic drawing of the experimental setup. (B) BM cells from a wild-type control mouse (WT, black symbols), from MxCre;FF1 mouse (Mx, red symbols), and a SclCre;FF1 mouse (Scl, green symbols) were harvested at 6 and 8 weeks after induction with pIpC or tamoxifen, respectively, mixed with BM cells from a UBC-GFP transgenic mouse at a 1:1 ratio, and transplanted into 7 lethally irradiated recipients per group (2 × 106 BM cells each). The time course of the blood counts and the percentages of chimerism (bottom panel) as determined by flow cytometry of GFP− cells are shown for the erythroid (Ter119), platelet (CD61), and granulocytic (Gr1) lineages in peripheral blood. Error bars represent SEM. (C) Survival probabilities of the transplantation recipients (Kaplan-Meier plot). Color coding is as in panel B. (D) Ratio between human JAK2-V617F and mouse Jak2 in the BM of mice transplanted with the MxCre;FF1 and UBC-GFP BM cells in a 1:1 ratio. Sampling was performed 35 weeks after transplantation. (E) Percentages of chimerism in the BM and spleens of transplanted mice as determined by the proportion of GFP− cells. (F) Percentages of LSK cells within the lineage− BM cell population and the LSK chimerism determined as the proportion of GFP− cells in the BM of recipient mice. Error bars represent SEM. One-way ANOVA is shown for comparisons between mice transplanted with wild-type (C57BL/6 and UBC-GFP) and mutant (MxCre;FF1 and UBC-GFP) BM cells. ns indicates not significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
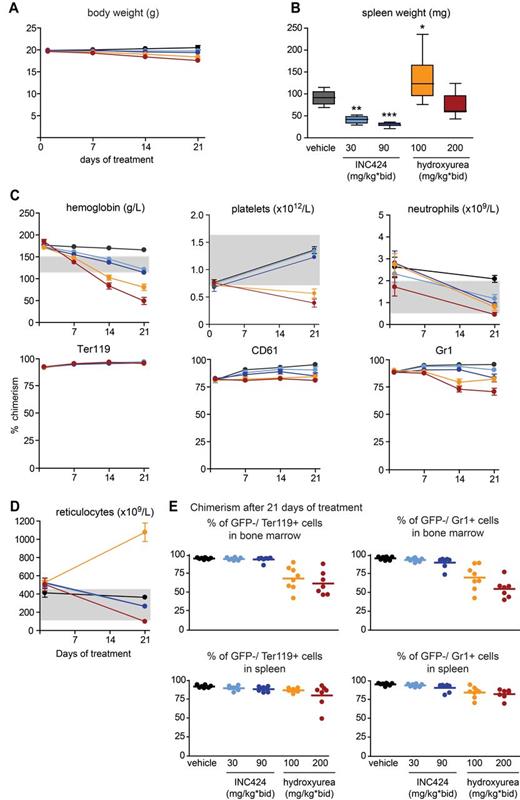
For drug testing, we selected the interval of 4-8 weeks after transplantation, when the recipients of MxCre;FF1 BM already displayed a PV-like phenotype. We examined the effects of INC424, a dual Jak1/Jak2 inhibitor, and of HU, currently a standard drug in the treatment of MPN patients (Figure 4). INC424 treatment did not significantly alter body weight, although a slight trend toward weight loss was observed, particularly at the higher dose (Figure 4A). A more pronounced decrease in body weight was found in mice treated with HU, which reached almost 10% after 21 days at 200 mg/kg BID. Spleen weights were significantly reduced after treatment with INC424, whereas treatment with HU (200 mg/kg) had no effect or even slightly increased spleen weights (100 mg/kg; Figure 4B). INC424 normalized the hemoglobin values at both doses tested, whereas HU treatment resulted in anemia that was particularly pronounced at the higher dose (200 mg/kg; Figure 4C top panel). This reduction in hemoglobin did not alter the mutant allele burden, as indicated by the high percentage of GFP−Ter119+ erythrocytes in the peripheral blood (Figure 4C bottom panel). Interestingly, the reticulocytes were elevated in mice treated with HU at 100 mg/kg, whereas the higher dose (200 mg/kg) resulted in a decrease of the reticulocyte numbers below the normal range (Figure 4D). Platelet levels were not affected by INC424 treatment compared with vehicle control, whereas HU at both doses lowered the platelet counts below the normal range (Figure 4C top panel). A slight decrease in the mutant allele burden in platelets was observed with both drugs, with a maximum decrease of 21% in the HU (200 mg/kg)–treated group (Figure 4C bottom panel). Neutrophil numbers were lowered by both INC424 and HU. The mutant allele burden in neutrophils was reduced in the INC424 (90 mg/kg) cohort by 13%, and in the HU cohort by 14% and 26% at 100 mg/kg and 200 mg/kg, respectively (Figure 4C bottom panel). The chimerism in BM and spleens showed no significant differences between the vehicle and INC424 for the Ter119+ erythroid cells, whereas a slight, nonsignificant decrease was noted in the Gr1+ myeloid compartment at the higher dose of INC424 (Figure 4E). HU-treated mice showed a significant decrease in the mutant allele burden in the BM in both the erythroid and myeloid lineages, whereas in the spleen no significant differences were found (Figure 4E).
Analysis of mice treated with INC424, HU, or vehicle and transplanted with JAK2-V617F mutant (from MxCre;FF1 mice with a PV phenotype) and Jak2 wild-type (from UBC-GFP mice) BM cells in a 1:1 ratio. (A) Body weight during treatment. Black indicates vehicle (0.5% hydroxypropyl methylcellulose); bright blue, INC424 30 mg/kg BID; dark blue, INC424 90 mg/kg BID; orange, HU 100 mg/kg BID; and red, HU 200 mg/kg BID. (B) Spleen weights. Color coding is as in panel A. (C) Blood counts and flow cytometry data showing the chimerism (percentage of GFP− cells) after 21 days of treatment. (D) Reticulocyte counts. Color coding is as in panel A. (E) Chimerism in BM (top panel) and spleen (bottom panel) after 21 days of treatment. One-way ANOVA is shown for comparisons between the vehicle- and INC424- or HU-treated cohorts. ns indicates not significant.*P ≤ .05; **P ≤ .01; ***P ≤ .001.
Analysis of mice treated with INC424, HU, or vehicle and transplanted with JAK2-V617F mutant (from MxCre;FF1 mice with a PV phenotype) and Jak2 wild-type (from UBC-GFP mice) BM cells in a 1:1 ratio. (A) Body weight during treatment. Black indicates vehicle (0.5% hydroxypropyl methylcellulose); bright blue, INC424 30 mg/kg BID; dark blue, INC424 90 mg/kg BID; orange, HU 100 mg/kg BID; and red, HU 200 mg/kg BID. (B) Spleen weights. Color coding is as in panel A. (C) Blood counts and flow cytometry data showing the chimerism (percentage of GFP− cells) after 21 days of treatment. (D) Reticulocyte counts. Color coding is as in panel A. (E) Chimerism in BM (top panel) and spleen (bottom panel) after 21 days of treatment. One-way ANOVA is shown for comparisons between the vehicle- and INC424- or HU-treated cohorts. ns indicates not significant.*P ≤ .05; **P ≤ .01; ***P ≤ .001.
To assess the effects of INC424 and HU on downstream signaling pathways, we analyzed the phosphorylation of STAT5 and STAT3 proteins 2 hours after the last dose. At this time, the levels of INC424 in whole blood were 2.44 ± 0.64 μmol/L for the mice that received 30 mg/kg (n = 8) and 8.91 ± 1.70 μmol/L for the mice that received 90 mg/kg. Unfortunately, we were unable to determine the blood levels of HU. The levels of phosphorylated STAT5 (pSTAT5) protein were clearly decreased in spleen sections of the INC424-treated mice, as assessed by immunohistochemistry, quantitative image analysis, Western blotting, and Meso Scale Discovery (MSD) technology (Figure 5A-D). In contrast, spleen sections of HU-treated mice showed an increase of pSTAT5 compared with the vehicle-treated controls. These findings were confirmed by immunoblot and MSD analysis of spleen lysates (Figure 5C-D). Signals for pSTAT3 in spleen lysates were weak but slightly decreased in the mice that received the higher dose of INC424 (Figure 5C). The striking increase in the levels of splenic STAT5 (but not STAT3) phosphorylation after HU treatment, exceeding the levels observed in the transplanted, vehicle-treated control group, prompted us to investigate whether elevated levels of EPO could be responsible for this effect. EPO concentrations in the spleen lysates of vehicle- and INC424-treated mice were comparable, but the EPO concentrations in HU-treated mice were up to 4-fold elevated (Figure 5E). This finding is likely a consequence of the anemia induced in the HU-treated groups.
Analysis of STAT phosphorylation in spleens after 21 days of treatment. (A) Immunohistochemistry with antibodies against pSTAT5 of spleen sections from mice that were killed 2 hours after the final dose. Scale bars indicate 100 μm. (B) Quantification of the pSTAT5 immunohistochemistry with Definiens image analysis software. The histogram shows the percentage of pSTAT5+ nuclei (mean ± SEM of 4-8 mice per group). Note that the phosphorylation of STAT5 in the HU-treated group was significantly elevated compared with the vehicle-treated group, whereas the pSTAT5 in the INC424-treated groups was lower. (C) Levels of phosphorylated and total STAT3 and STAT5 proteins in spleen homogenates were assessed by immunoblot analysis. (D) Relative levels of pSTAT5 and total STAT5 were measured in spleen homogenates using MSD technology. The values represent the mean ± SEM, n = 8 per group. (E) Measurement of EPO levels in spleen samples after therapy. Spleen samples were homogenized and levels of mouse EPO were determined by ELISA. Values represent means ± SEM (n = 7-8). To fit the results on the graph, the HU-treated samples are shown in a 10-fold expanded scale. *Significantly different from vehicle-treated control group using Mann-Whitney rank sum test (P < .05).
Analysis of STAT phosphorylation in spleens after 21 days of treatment. (A) Immunohistochemistry with antibodies against pSTAT5 of spleen sections from mice that were killed 2 hours after the final dose. Scale bars indicate 100 μm. (B) Quantification of the pSTAT5 immunohistochemistry with Definiens image analysis software. The histogram shows the percentage of pSTAT5+ nuclei (mean ± SEM of 4-8 mice per group). Note that the phosphorylation of STAT5 in the HU-treated group was significantly elevated compared with the vehicle-treated group, whereas the pSTAT5 in the INC424-treated groups was lower. (C) Levels of phosphorylated and total STAT3 and STAT5 proteins in spleen homogenates were assessed by immunoblot analysis. (D) Relative levels of pSTAT5 and total STAT5 were measured in spleen homogenates using MSD technology. The values represent the mean ± SEM, n = 8 per group. (E) Measurement of EPO levels in spleen samples after therapy. Spleen samples were homogenized and levels of mouse EPO were determined by ELISA. Values represent means ± SEM (n = 7-8). To fit the results on the graph, the HU-treated samples are shown in a 10-fold expanded scale. *Significantly different from vehicle-treated control group using Mann-Whitney rank sum test (P < .05).
BM cellularity was increased in vehicle-treated mice, but normal in animals treated with the INC424 30 mg/kg BID dose, whereas hypocellularity was observed with the INC424 90 mg/kg BID dose (supplemental Figure 5). A decrease in the number of megakaryocytes was observed in both INC424-treated groups. BM from HU-treated animals displayed severe hypocellularity (supplemental Figure 5). In the spleens, the regions with white and red pulp were apparent in all treatment groups. However, the in spleens of vehicle-treated animals, enhanced proliferation of erythroid cells was observed (supplemental Figure 6). Spleens from INC424-treated animals displayed reduced extramedullary hematopoiesis and, subsequently, splenic atrophy. The mice treated with HU at 200 mg/kg BID also displayed reduced proliferation of erythroid cells (supplemental Figure 6).
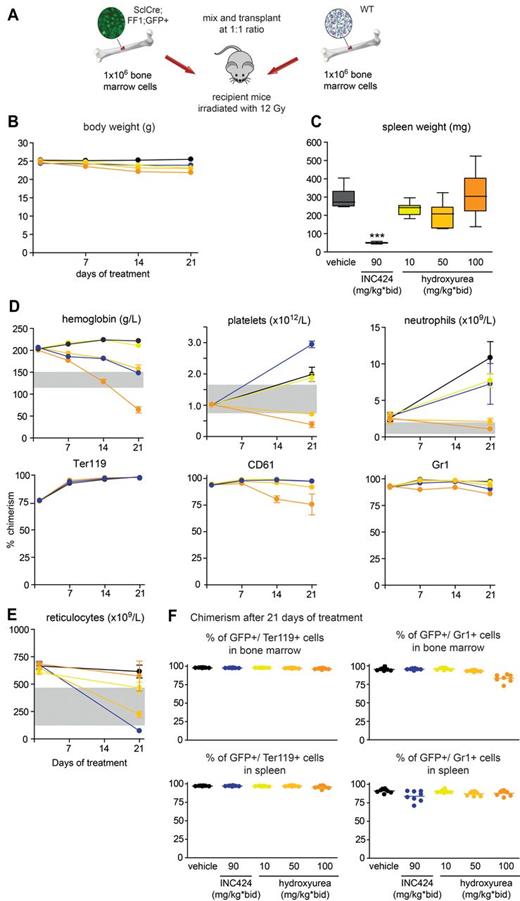
To explore a wider range of HU concentrations and to further improve the experimental setting for drug testing, we repeated the study using recipients that received BM from a SclCre;FF1;GFP donor 6 weeks after tamoxifen induction. In this setting, GFP is coexpressed with JAK2-V617F in the same cells and allows direct monitoring of the mutant allele burden. All recipients developed a PV phenotype with high hemoglobin and neutrophilia and enlarged spleens (Figure 6). The effects of INC424 (90 mg/kg BID) and HU (100 mg/kg BID) on hemoglobin and spleen weight and histology were comparable to the previous experiment (Figure 6 and supplemental Figures 7 and 8). However, INC424 was unable to normalize neutrophils and there was a paradox effect on platelet numbers, which after 21 days of treatment were higher than in the vehicle controls. HU at 50 mg/kg BID still controlled the blood counts, but 10 mg/kg BID was below an effective dose (Figure 6). In this setting, GFP allowed direct monitoring of the percentage of JAK2-V617F+ cells. Again, there was virtually no effect on chimerism detectable with INC424 and only HU at 100 mg/kg BID lowered the mutant allele burden, mainly in the platelet and neutrophil compartment (Figure 6D). Another way of assessing the effect on the total tumor burden is to take into account the reduction of the tumor mass by reducing the spleen size. In the second experiment, INC424 reduced the spleen size compared with vehicle control by approximately 250 mg, whereas HU had no effect. The percentage of GFP+ cells in the spleens was 77% in the INC424-treated mice, 85% in the HU group, and 91% in the vehicle group. By this estimate, INC424 had a greater impact on the tumor load than HU. Similar to the previous experiment, increased levels of pSTAT5 were observed in the spleens and BM samples of mice treated with HU at 100 mg/kg BID, which were correlated with increased concentrations of EPO (supplemental Figure 9).
Analysis of mice treated with INC424, HU, or vehicle and transplanted with JAK2-V617F BM cells expressing GFP (from SclCre;FF1;GFP mice with a PV phenotype) and Jak2 wild-type (from C57BL/6 mice) BM cells in a 1:1 ratio. (A) Schematic drawing of the experimental setup. WT indicates wild-type. (B) Body weight during treatment. Black indicates vehicle (0.5% hydroxypropyl methylcellulose); dark blue, INC424 90 mg/kg BID; yellow, HU 10 mg/kg BID; bright orange, HU 50 mg/kg BID; and dark orange, HU 100 mg/kg BID. (C) Spleen weights. Color coding is as in panel B. (D) Blood counts and flow cytometry data showing the chimerism (percentage of GFP+ cells) after 21 days of treatment. (E) Reticulocyte counts. Color coding is as in panel B. (F) Chimerism (percentage of GFP+ cells) in BM (top panel) and spleen (bottom panel) after 21 days of treatment. One-way ANOVA is shown. ns indicates not significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Analysis of mice treated with INC424, HU, or vehicle and transplanted with JAK2-V617F BM cells expressing GFP (from SclCre;FF1;GFP mice with a PV phenotype) and Jak2 wild-type (from C57BL/6 mice) BM cells in a 1:1 ratio. (A) Schematic drawing of the experimental setup. WT indicates wild-type. (B) Body weight during treatment. Black indicates vehicle (0.5% hydroxypropyl methylcellulose); dark blue, INC424 90 mg/kg BID; yellow, HU 10 mg/kg BID; bright orange, HU 50 mg/kg BID; and dark orange, HU 100 mg/kg BID. (C) Spleen weights. Color coding is as in panel B. (D) Blood counts and flow cytometry data showing the chimerism (percentage of GFP+ cells) after 21 days of treatment. (E) Reticulocyte counts. Color coding is as in panel B. (F) Chimerism (percentage of GFP+ cells) in BM (top panel) and spleen (bottom panel) after 21 days of treatment. One-way ANOVA is shown. ns indicates not significant. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Discussion
To make our inducible transgenic JAK2-V617F mice usable as a preclinical animal model for testing drugs with potential effects on MPNs, we first performed a detailed characterization of the phenotypic variations and examined the capacity of the transgenic BM cells to transplant the phenotype. We previously showed in our transgenic mouse model that a ratio of human JAK2-V617F to wild-type mouse Jak2 of approximately 1:2 resulted in thrombocytosis and neutrophilia with normal hemoglobin closely resembling ET, whereas a ratio of approximately 1:1 between human JAK2-V617F and wild-type mouse Jak2 was associated with a PV phenotype.7 In the present study, we reproduced these findings using the conditional JAK2-V617F transgene in crossings with 2 previously studied Cre strains and with the tamoxifen-inducible SclCreER strain that we have not examined before (Figure 1). The VavCre;FF1 double-transgenic mice showed an ET phenotype and a low ratio of JAK2-V617F to wild-type Jak2, whereas the MxCre;FF1 and the SclCre;FF1 mice showed a PV phenotype and a high ratio of JAK2-V617F to wild-type Jak2 (Figure 1A-B). Surprisingly, SclCre;FF1 mice had a significantly shorter survival time than the other 2 mouse strains. Survival improved when SclCre;FF1 BM was transplanted into irradiated wild-type recipients (Figure 1C), suggesting that nonhematopoietic expression of JAK2-V617F may play a role. In contrast, no improvement in survival was noted in transplanted MxCre;FF1 and VavCre;FF1 mice. Therefore, the beneficial effect in SclCre;FF1 mice does not seem to be related to a general attenuation of disease phenotype in transplantation recipients, as described previously by others.16,17 Using a LacZ reporter gene, expression of Cre recombinase in tamoxifen-treated SclCreER mice was found in hematopoietic cells and endothelial cells of several adult organs.13 Although our analysis of JAK2-V617F expression in endothelial cells did not reveal major differences in expression between MxCre;FF1 and SclCre;FF1 mice (supplemental Figure 3), we cannot exclude that localized expression in some parts of the vascular system could be stronger in SclCre than in MxCre mice.
On longer follow-up, we observed normalization of hemoglobin levels in MxCre;FF1 and SclCre;FF1 mice at approximately 18-26 weeks after induction. Histologic examination revealed myelofibrosis with variable degrees of osteosclerosis as a likely cause for the reduced hemoglobin (Figure 2 and Table 1). Consistent with this being a consequence of myelofibrosis, a full PV phenotype reemerged when BM from older mice with grade 2-3 myelofibrosis was transplanted into wild-type recipients (data not shown). In MxCre;FF1 mice, a decrease of the JAK2-V617F expression ratio (Figure 1B) may, in addition to myelofibrosis, contribute to normalizing the hemoglobin levels. We suspect that this decrease in JAK2-V617F expression is the consequence of endogenous IFN production that could activate the MxCre expression in absence of pIpC and lead to the decrease in transgene copy number (supplemental Figure 2A). SclCre;FF1 mice showed no such decrease in JAK2-V617F expression or copy number.
The effects of JAK2-V617F on erythropoiesis were potentiated by decreasing the wild-type mouse Jak2 alleles (Figure 1I). Although the RBC numbers and reticulocytes were strongly elevated in these mice, the erythrocytes became increasingly microcytic, preventing a further increase of the hemoglobin and hematocrit values. The microcytosis could be a consequence of gastrointestinal bleeding because the stools of these mice were frequently Hemoccult+, but may also reflect imbalance of iron utilization in massively increased erythropoiesis. The survival times of SclCre;FF1 mice with heterozygous or homozygous loss of Jak2 were drastically shorter than those of mice with wild-type Jak2. Overall, these results further strengthen the link between the ratio of JAK2-V617F and wild-type Jak2 for the responsiveness of erythropoiesis to the mutant JAK2. The megakaryocytic and granulocytic lineages seem to be largely unaffected by the loss of the wild-type Jak2.
A correlation between expression levels of the mutant JAK2 and the PV or ET phenotypes has been observed in some, but not all, mouse JAK2-V617F models. In addition, differences in phenotype may also be because of the use of mouse versus human JAK2-V617F (reviewed in Van Etten et al5 and Li et al6 ). The early retroviral models all used the mouse Jak2-V617F and obtained high expression levels of Jak2-V617F with a PV phenotype.1,16,18-20 Retroviral expression of the human JAK2-V617F also produced a PV phenotype with normal platelet levels.7 In contrast, transgenic mice that expressed the mouse Jak2-V617F under the H-2Kb promoter showed an ET phenotype in one line or occasionally a mild PV phenotype with thrombocytosis in another line.21 Transgenic mice expressing the human JAK2-V617F under the Vav promoter resulted in an ET phenotype.22 However, the expression levels of the mutant and wild-type Jak2 have not been studied in detail in these transgenic mice. One knock-in model that used the human JAK2-V617F displayed a mild ET phenotype.17 Other knock-in mouse models that used the mouse Jak2-V617F resulted in a PV phenotype with in most cases a 1:1 ratio of the mutant to the wild-type Jak2.23-25 These knock-in models do not contradict the conclusions from our studies because at a 1:1 expression ratio, we also observed a PV phenotype. The ET phenotype in our model was found when the expression levels of the mutant Jak2 decreased to approximately 1:2. This cannot be modeled in the knock-ins, because the expression is fixed and cannot be modified. Therefore, our MxCre;FF1 mice currently represent the only model that allows modulation of the expression ratio by applying different doses of pIpC induction in otherwise genetically identical mice.7 Recently, additional factors that influence the manifestation of ET and PV phenotypes have been described (eg, the activity of Stat1 signaling or expression of TNFα26,27 ).
Competitive transplantation of BM from MxCre;FF1 or SclCre;FF1 mice mixed with cells from UBC-GFP–transgenic mice at a ratio of 1:1 resulted in a PV phenotype. We first used the MxCre;FF1 repopulation assay. INC424 and HU were chosen because a large amount of data are available from human studies.28 Overall, the mouse model faithfully recapitulated the known effects of these drugs on blood counts and spleen weights (Figure 4). A slight trend to lower mutant allele burden was observed in the HU-treated mice, particularly in the granulocytic lineage in blood and in BM (Figure 4C and E). Because the 200 mg/kg BID dose of HU led to weight loss and anemia, we also repeated the experiment with lower doses of HU. Furthermore, to allow more direct monitoring of the mutant allele burden, we used BM from SclCre;FF1;GFP donors for this experiment. HU at lower doses (50 mg/kg BID) normalized the blood counts and a slight reduction of the mutant allele burden was observed (Figure 6D). Results on the effects of HU on JAK2 mutant allele burden in clinical studies are controversial. A decrease of the JAK2-V617F allele burden was described in some retrospective studies of MPN patients treated with HU.29-32 However, other studies did not confirm this finding.33-35 One study found that the JAK2-V617F allele burden decreased only in patients who received HU as a de novo treatment, whereas patients who had been on HU for a prolonged period of time showed a remarkably stable mutant allele burden.36 Because our competitive repopulation assay shows a major proliferative advantage of the JAK2-V617 clone, the increased sensitivity of the mutant clone to HU could be related to high cell proliferation. Similar to the clinical trials, INC424 showed little effect on the mutant allele burden in our mouse model (Figures 4 and 6). In contrast to the human studies, platelets were not lowered by INC424 in our mouse experiments (Figures 4 and 6). The reason for this difference in responsiveness is currently unknown, but we observed the same result with other Jak2 inhibitors (data not shown). We suspect that the lack of response in the mouse represents an interspecies difference in the regulation of thrombopoiesis.
INC424 treatment significantly reduced the phosphorylation of Stat5 in spleen and BM cells (Figure 5 and supplemental Figure 9). In contrast, the HU-treated mice showed a strong increase in Stat5 phosphorylation that was dose dependent. Stat3 phosphorylation was less affected (Figure 5). Because the HU-treated group also displayed anemia and elevated EPO levels, we suspect that the elevated pStat5 levels are driven by increased cytokine levels. Cytokines other than EPO may also be involved. Hyperactive Jak2/Stat signaling has been shown to have undesired effects on genome stability.37 The HU doses used in our study ranged from lower than effective dose (10 mg/kg BID) to a dose that induced cytopenia (200 mg/kg BID). Using a dose-conversion method that is based on body surface area,38 the 100 mg/kg BID dose in the mouse would correspond to 16.2 mg/kg/d in humans (ie, approximately 1′000 mg/d for a 60-kg person), which is a dose frequently used for maintenance in MPN patients. Therefore, the activation of the Stat pathway by HU is an adverse effect that should be avoided in the treatment of patients with MPNs.
Several studies have tested the effects of Jak inhibitors and other compounds in animal models of JAK2-V617F. Initially, retroviral models expressing JAK2-V617F were used to test the compounds imatinib, dasatinib, AG490, and TG101348.20,39 Other studies used mice transplanted with BaF3 cells that were engineered to express JAK2-V617F to test the effects of TG101209, INCB16562, INCB08424, NVP-BSK805, and SB1518.40-44 More recently, the Jak2-V617F knock-in and transgenic mice were used to assess the effects of TG101348, R723, and G6.21,24 These models allowed the monitoring of blood counts and spleen size, but not changes in the mutant allele burden. Recently, one transgenic model showed that the Jak2 inhibitor G6 was also able to reduce the mutant allele burden in the BM.45 In these mice, all cells carry the human JAK2-V617F transgene under the control of the Vav-promoter,22 but apparently not all cells also express the transgene. How the mRNA expression levels of the transgene can be used to define changes in the allele burden has not been described in detail for this mouse model. Our competitive repopulation assay offers the advantage that JAK2-V617F–expressing cells in all 3 lineages relevant to the MPN disease phenotype, RBCs, platelets, and neutrophils, can be directly monitored in peripheral blood as well as in BM and other organs. This system will allow us to evaluate not only the effects of individual compounds, but also to assess combinations of drugs for the therapy of MPNs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jürg Schwaller and Axel Karow for helpful comments on the manuscript; Dario Sterker for bioanalytics; and Arno Dölemeyer for quantitative assessment of pSTAT5+ nuclei in spleen sections.
This work was supported by grants 310000-108006/1 and 32003BB_135712 from the Swiss National Science Foundation and the Swiss Cancer League (KLS-02398-02-2009) to R.C.S.
Authorship
Contribution: L.K. performed the research, analyzed the data, and wrote the manuscript; P.L., J.G., H.H.-S., V.R., R.A., M.M., K.-U.W., and T.R. performed the research and analyzed the data; S.D. prepared and analyzed the histology samples; and R.C.S. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: V.R., R.A., M.M., and T.R. are full-time employees of Novartis Pharma AG. The inhibitor studies carried out in the laboratory of R.C.S. were supported in part by Novartis. The remaining authors declare no competing financial interests.
Correspondence: Radek C. Skoda, MD, Department of Biomedicine, Experimental Hematology, University Hospital Basel, Hebelstrasse 20, 4031 Basel, Switzerland; e-mail: radek.skoda@unibas.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal