Abstract
Engineering of T cells with chimeric antigen receptors (CARs) can impart novel T cell specificity for an antigen of choice, and anti-CD19 CAR T cells have been shown to effectively eradicate CD19+ malignancies. Most patients with acute myeloid leukemia (AML) are incurable with standard therapies and may benefit from a CAR-based approach, but the optimal antigen to target remains unknown. CD123, the IL3Rα chain, is expressed on the majority of primary AML specimens, but is also expressed on normal bone marrow (BM) myeloid progenitors at lower levels. We describe here in vitro and in vivostudies to evaluate the feasibility and safety of CAR-based targeting of CD123 using engineered T cells (CART123 cells) as a therapeutic approach for AML. Our CAR consisted of a ScFv derived from hybridoma clone 32716 and signaling domains from 4-1-BB (CD137) and TCR-ζ.
Among 47 primary AML specimens we found high expression of CD123 (median 85%, range 6-100%). Quantitative PCR analysis of FACS-sorted CD123dim populations showed measurable IL3RA transcripts in this population, demonstrating that blasts that are apparently CD123dim/neg by flow cytometry may in fact express CD123. Furthermore, FACS-sorted CD123dimblasts cultured in methylcellulose up-regulated CD123, suggesting that anti-CD123 immunotherapy may be a relevant strategy for all AML regardless of baseline myeloblast CD123 expression.
CART123 cells incubated in vitro with primary AML cells showed specific proliferation, killing, and robust production of inflammatory cytokines (IFN-α, IFN-γ, RANTES, GM-CSF, MIP-1β, and IL-2 (all p<0.05).
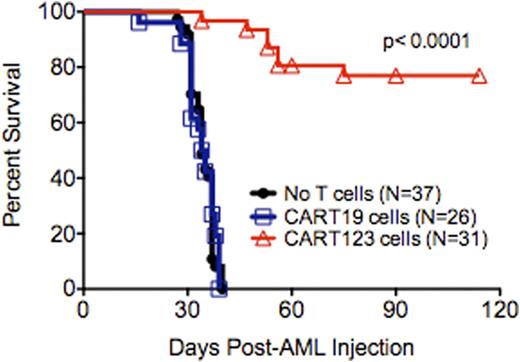
In NOD-SCID-IL2Rγc-/- (NSG) mice engrafted with the human AML cell line MOLM14, CART123 treatment eradicated leukemia and resulted in prolonged survival in comparison to negative controls of saline or CART19-treated mice (see figure). Upon MOLM14 re-challenge of CART123-treated animals, we further demonstrated robust expansion of previously infused CART123 cells, consistent with establishment of a memory response in animals.
A crucial deficiency of tumor cell line models is their inability to represent the true clonal heterogeneity of primary disease. We therefore engrafted NSG mice that are transgenic for human stem cell factor, IL3, and GM-CSF (NSGS mice) with primary AML blasts and treated them with CART123 or control T cells. Circulating myeloblasts were significantly reduced in CART123 animals, resulting in improved survival (p = 0.02, n=34 CART123 and n=18 control animals). This observation was made regardless of the initial level of CD123 expression in the primary AML sample, again confirming that apparently CD123dimAML may be successfully targeted with CART123 cells.
Given the potential for hematologic toxicity of CART123 immunotherapy, we treated mice that had been reconstituted with human CD34+ cells with CART123 cells over a 28 day period. We observed near-complete eradication of human bone marrow cells. This finding confirmed our finding of a significant reduction in methylcellulose colonies derived from normal cord blood CD34+ cells after only a 4 hour in vitro incubation with CART123 cells (p = 0.01), and was explained by: (i) low level but definite expression of CD123 in hematopoietic stem and progenitor cells, and (ii) up-regulation of CD123 upon myeloid differentiation.
In summary, we show for the first time that human CD123-redirected T cells eradicate both primary human AML and normal bone marrow in xenograft models. As human AML is likely preceded by clonal evolution in normal or “pre-leukemic” hematopoietic stem cells (Hong et al. Science 2008, Welch et al. Cell 2012), we postulate that the likelihood of successful eradication of AML will be enhanced by myeloablation. Hence, our observations support CART-123 as a viable therapeutic strategy for AML and as a novel cellular conditioning regimen prior to hematopoietic cell transplantation.
Gill:Novartis: Research Funding; American Society of Hematology: Research Funding. Carroll:Leukemia and Lymphoma Society: Research Funding. Grupp:Novartis: Research Funding. June:Novartis: Research Funding; Leukemia and Lymphoma Society: Research Funding. Kalos:Novartis: Research Funding; Leukemia and Lymphoma Society: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal