Abstract
Chronic graft-versus-host disease (GVHD) is a major transplantation toxicity that impacts quality-of-life and long-term survival. Corticosteroids are standard-of-care for initial therapy of chronic GVHD despite limited efficacy and significant long-term toxicity. Proteasome-inhibition has immunomodulatory effects potentially beneficial in GVHD. We previously reported on peri-transplantation bortezomib (day +1, +3, +7) for GVHD prophylaxis. We now report on bortezomib plus corticosteroids for initial therapy of chronic GVHD.
We undertook a single-arm prospective phase II trial for participants 18 years or older and 100 days or more after allogeneic transplantation from HLA-matched related, unrelated or mismatched donors (MRD, MUD, MMUD) with no active malignant relapse, and limited or extensive chronic GVHD requiring initiation of systemic therapy. IV bortezomib was dosed at 1.3 mg/m2 on days 1, 8, 15 and 22 of each 35 day cycle for 3 cycles (15 weeks). Concurrent prednisone dose was initially 0.5 mg/kg/d, but the study was later amended to allow up to 1 mg/kg/d. The suggested steroid taper after cycle 1 was 10-25% every 1-2 weeks. No addition or subtraction of other immunosuppressive medications was permitted. Topical GVHD therapies (e.g. eye, mouth) were allowed. The primary endpoint was week 15 overall response rate (ORR) per NIH chronic GVHD criteria, excluding ocular/oral sites where topical treatment was permitted. Secondary endpoints included the proportion of participants tolerating 50% or greater prednisone dose reduction at 15 weeks, prednisone dose requirement at 1 year, and long-term overall and progression-free survival (OS, PFS).
Between March 2009 and November 2011, 22 participants with a median age of 51 years (range, 25-69) comprising 5, 13, and 4 recipients of 8/8 MRD, MUD, and 7/8 MMUD PBSC grafts, respectively, were enrolled. 45% were male. 18% had prior acute GVHD (1 gr. 1; 2 gr. 2; 1 gr. 3). The median time from transplantation to chronic GVHD onset was 215 days (range, 133-666), and to initiation of chronic GVHD therapy was 257 days (range, 186-2070). Participants had a median of 3 (range, 1-6) sites of chronic GVHD, with liver (73%), oral (68%), skin (64%), ocular (46%) and joint/muscle/fascia (J/M/F) (27%) the most common. The median follow up duration in survivors was 25 months (range, 19.3-48.5).
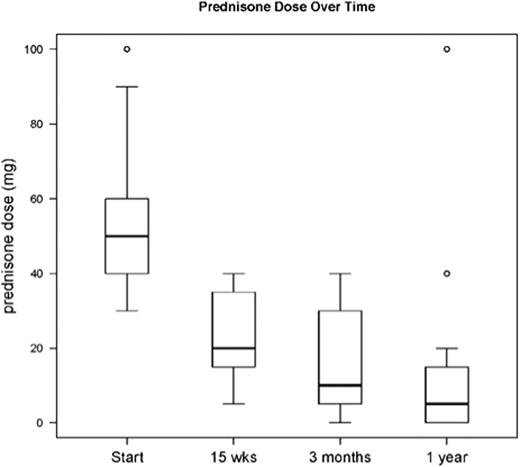
Bortezomib plus prednisone as initial chronic GVHD therapy was well tolerated, with 1 grade 3 CTCAE (sensory neuropathy) possibly related to bortezomib. One participant died of relapsed AML after 2 cycles of treatment and was not evaluable for toxicity or response. Another died of fungal infection during cycle 1 and was not evaluable for response. 18 of 22 participants (82%) completed the 3 cycles of therapy. For the 20 participants evaluable for response, the week 15 ORR (per NIH chronic GVHD global score) was 80% (CR: 2 [10%], PR: 14 [70%]). Additionally, 1 participant (5%) had a mixed response, with CR at sentinel sites (liver, skin) but interval progression of pulmonary GVHD; 1 participant (5%) had stable disease; and 2 participants (10%) with prednisone starting dose of 0.5 mg/kg/d had progressive GVHD. The change of median NIH chronic GVHD global score from baseline to week 15 was 5.5 (range, 1-7) → 3 (range, 0-6), respectively. Sites of response included skin (n = 11; CR = 8 [73%], PR = 1, SD = 2); liver (n = 15; CR = 8 [53%], PR = 5, PD = 2); and J/M/F (n = 6; CR = 2 [33%], PR = 1, SD = 3). The change of median NIH chronic GVHD organ score from baseline to week 15 was: liver 3 (range, 1-3) → 0 (range, 0-2); skin 2 (range, 1-3) → 0 (range, 0-2); J/M/F 1 (range, 1-2) → 1 (range, 0-2). 14 participants (70%) tolerated a 50% or greater steroid dose reduction by week 15. The median steroid dose taper from baseline to week 15 was 50 mg (range, 30-100) → 20 mg (range, 5-40) (p<0.001). The ability to taper steroids persisted beyond the 3 treatment cycles (Figure). The cohort 2-year OS and PFS was 73% each, with non-relapse mortality and relapse incidence of 14% each.
Off Label Use: Bortezomib is a proteasome inhibitor with a number of immunomodulatory effects that are potentially beneficial in GVHD. We studied bortezomib plus prednisone as initial therapy for chronic GVHD.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal