Abstract
Myelodysplasia is a diagnostic feature of myelodysplastic syndromes (MDSs) but is also found in other myeloid neoplasms. Its molecular basis has been recently elucidated by means of massive parallel sequencing studies. About 90% of MDS patients carry ≥1 oncogenic mutations, and two thirds of them are found in individuals with a normal karyotype. Driver mutant genes include those of RNA splicing (SF3B1, SRSF2, U2AF1, and ZRSR2), DNA methylation (TET2, DNMT3A, and IDH1/2), chromatin modification (ASXL1 and EZH2), transcription regulation (RUNX1), DNA repair (TP53), signal transduction (CBL, NRAS, and KRAS), and cohesin complex (STAG2). Only 4 to 6 genes are consistently mutated in ≥10% MDS patients, whereas a long tail of ∼50 genes are mutated less frequently. At presentation, most patients typically have 2 or 3 driver oncogenic mutations and hundreds of background mutations. MDS driver genes are also frequently mutated in other myeloid neoplasms. Reliable genotype/phenotype relationships include the association of the SF3B1 mutation with refractory anemia with ring sideroblasts, TET2/SRSF2 comutation with chronic myelomonocytic leukemia, and activating CSF3R mutation with chronic neutrophilic leukemia. Although both founding and subclonal driver mutations have been shown to have prognostic significance, prospective clinical trials that include the molecular characterization of the patient’s genome are now needed.
Introduction
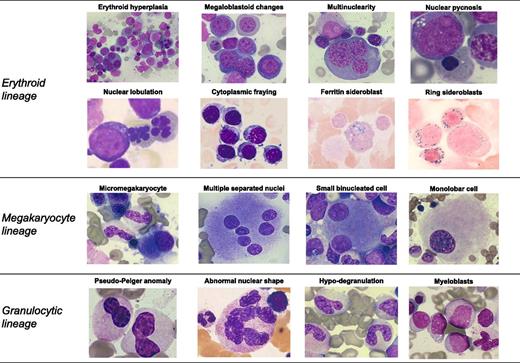
Myelodysplasia is a term used in pathology for describing morphologic abnormalities, or dysplasia, in ≥1 of the major myeloid cell lines of hematopoiesis and is a typical feature of myelodysplastic syndromes (MDSs).1 In the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues, MDSs are defined as clonal hematopoietic stem cell disorders characterized by cytopenia, myelodysplasia, ineffective hematopoiesis, and increased risk of progression to acute myeloid leukemia (AML).1 Representative examples of morphologic abnormalities of myelodysplasia are reported in Figure 1.
Representative examples of morphologic abnormalities of myelodysplasia. May Grünwald Giemsa staining in all cases with the only exception of ring sideroblasts (Perls staining). Magnification from 200× to 1000×, courtesy of Erica Travaglino.
Representative examples of morphologic abnormalities of myelodysplasia. May Grünwald Giemsa staining in all cases with the only exception of ring sideroblasts (Perls staining). Magnification from 200× to 1000×, courtesy of Erica Travaglino.
Myelodysplasia is not restricted to MDS but may be found also in other myeloid neoplasms of the WHO classification (Table 1). Although the different subtypes of myeloid neoplasms have distinctive characteristics, they may share morphologic abnormalities. The paradigmatic example is refractory anemia with ring sideroblasts associated with marked thrombocytosis (RARS-T), which has both the myelodysplastic features of RARS and the myeloproliferative characteristics of essential thrombocythemia. This suggests that the myelodysplastic features of various myeloid neoplasms may reflect common underlying genetic lesions and that these latter contribute to determining clinical phenotypes.
WHO classification of myeloid neoplasms
| Major subgroups . | Conditions . |
|---|---|
| MDS | Refractory cytopenia with unilineage dysplasia, RARS, refractory cytopenia with multilineage dysplasia (RCMD), refractory cytopenia with excess blasts (RAEB, type 1 and 2), MDS with isolated del(5q) |
| Myeloproliferative neoplasms (MPN) | Chronic myeloid leukemia (CML, BCR-ABL1 positive), chronic neutrophilic leukemia (CNL), polycythemia vera, essential thrombocythemia, primary myelofibrosis (PMF), chronic eosinophilic leukemia, mastocytosis |
| MDS/MPN | CMML, atypical chronic myeloid leukemia (aCML, BCR-ABL1 negative), juvenile myelomonocytic leukemia (JMML), RARS-T |
| AML | Various subtypes based on blast morphology and/or underlying genetic lesion(s) |
| Major subgroups . | Conditions . |
|---|---|
| MDS | Refractory cytopenia with unilineage dysplasia, RARS, refractory cytopenia with multilineage dysplasia (RCMD), refractory cytopenia with excess blasts (RAEB, type 1 and 2), MDS with isolated del(5q) |
| Myeloproliferative neoplasms (MPN) | Chronic myeloid leukemia (CML, BCR-ABL1 positive), chronic neutrophilic leukemia (CNL), polycythemia vera, essential thrombocythemia, primary myelofibrosis (PMF), chronic eosinophilic leukemia, mastocytosis |
| MDS/MPN | CMML, atypical chronic myeloid leukemia (aCML, BCR-ABL1 negative), juvenile myelomonocytic leukemia (JMML), RARS-T |
| AML | Various subtypes based on blast morphology and/or underlying genetic lesion(s) |
Information is from Swerdlow et al.1 The WHO classification of myeloid neoplasms also includes myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1.
In this article, we will review the most recent advances in our understanding of the genetic basis of myelodysplasia and will discuss its clinical relevance. The Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium has just completed a study of targeted gene sequencing in a large cohort of patients with MDS and closely related neoplasms.2 For additional information on the genomic characterization of myeloid neoplasms, the reader is referred to recent landmark studies of genomic and epigenomic landscapes of AML,3,4 and a review article in Blood.5 A very detailed analysis of what has been learned about cancer genomes in the last few years has been published recently by Vogelstein et al.6 Definitions for basic terms used in studies of the genetic basis of myeloid neoplasms are reported in the Appendix.
Clonal expansion of myelodysplastic stem cells, ineffective hematopoiesis, and leukemic transformation: a working model of the pathophysiology of myelodysplasia
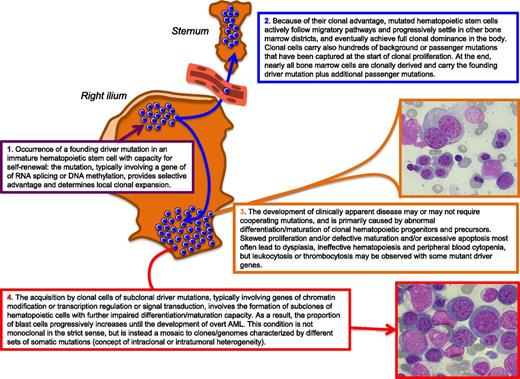
For a better understanding of the pathophysiology of myelodysplasia, we summarized the current concepts in the model reported in Figure 2. Some steps of this model are still working hypotheses, which will hopefully be verified in the near future.
Schematic representation of our current understanding of the pathophysiology of myelodysplasia. In this example, the founding driver mutation is assumed to occur in a hematopoietic cell located in the bone marrow of the right ilium, and the sternum is shown as an anatomically separated bone marrow district to illustrate the concept of mutated stem cell migration through peripheral blood. Bone marrow microphotographs: magnification from 600×, courtesy of Erica Travaglino.
Schematic representation of our current understanding of the pathophysiology of myelodysplasia. In this example, the founding driver mutation is assumed to occur in a hematopoietic cell located in the bone marrow of the right ilium, and the sternum is shown as an anatomically separated bone marrow district to illustrate the concept of mutated stem cell migration through peripheral blood. Bone marrow microphotographs: magnification from 600×, courtesy of Erica Travaglino.
An essential component of the WHO definition of MDS is the clonal nature of myelodysplastic hematopoiesis.1 Although various approaches can be used to prove the existence of a clonal population of hematopoietic cells, the more straightforward one is the use of chromosomal abnormalities or discrete gene rearrangements to show the uniform presence of these markers in purified hematopoietic cell populations.7 Using this approach, chromosomal aberrations were found to be restricted to committed myeloid progenitor cells in MDS patients, suggesting that the genetic lesion occurred in a hematopoietic cell with the capacity to differentiate into mature myeloid cells.8,9
In a landmark study, Walter et al10 used whole genome sequencing to identify somatic mutations in bone marrow samples from patients with AML developing from MDS and then genotyped in each patient a bone marrow sample obtained during the antecedent MDS phase. About 85% to 90% of unfractionated bone marrow cells were found to be clonal in these patients, both in the MDS and in AML phase, irrespective of the number of blasts. This study formally proved that almost all cells of the bone marrow myeloid cell lines (ie, immature red cells, granulocytic/monocytic precursors, and megakaryocytes) are clonally derived in MDS patients at any stage of the disease and not only after AML transformation.10
The clonal architecture of MDS has been elegantly studied by Delhommeau et al11 following the identification of somatic TET2 mutations. CD34+ cells from MDS patients were fractionated into immature CD34+CD38− and mature CD34+CD38+ progenitors. Although TET2 mutations were detected in only a small fraction of CD34+CD38− cells, they were present in a high proportion of more mature progenitors. This suggests that the initial somatic TET2 mutation occurred in a CD34+CD38− cell and was then transmitted to its CD34+CD38+ progeny. A similar clonal architecture has been more recently observed also in patients with chronic myelomonocytic leukemia (CMML).12
The occurrence in an immature hematopoietic stem cell of a somatic mutation that provides survival and growth advantage (for instance, lower propensity to apoptosis) leads to formation of a local clone (Figure 2, step 1). For this clone to become fully dominant in the whole body, the mutated stem cells must have additional advantages. In adulthood, migration and trafficking of hematopoietic stem cells are of crucial importance in maintaining homeostasis of the hematopoietic system.13,14 Despite several investigations, the mechanisms by which neoplastic hematopoietic cells leave the primary site and migrate to other bone marrow districts remain largely unclear.13 Ultimately, however, mutated hematopoietic stem cells achieve full clonal dominance in the bone marrow, and the vast majority of circulating mature cells derive from the dominant clone (Figure 2, step 2).
Once the myelodysplastic clone has become fully dominant in the bone marrow, the disease may or may not become clinically apparent. For instance, a somatic SF3B1 mutation appears to be able to cause a clinical phenotype per se,15,16 whereas a driver TET2 mutation can determine clonal hematopoiesis without hematologic manifestations,17 suggesting that cooperating mutant genes might be required for phenotypic expression. Myelodysplastic hematopoiesis is characterized by excessive apoptosis of hematopoietic precursors, at least in patients with low-risk disease.18 Ineffective hematopoiesis, ie, the premature intramedullary death of erythroblasts, immature granulocytes/monocytes, and megakaryocytes, is primarily responsible for the defective production of mature blood cells and peripheral blood cytopenia. We must therefore assume that the somatic mutation responsible for gain of function at the stem cell level involves loss-of-function at the hematopoietic precursor level (Figure 2, step 3). RARS associated with SF3B1 mutation represents an illustrative example of gain of function at the hematopoietic stem cell level combined with loss of function (excessive apoptosis of immature red cells) at the hematopoietic precursor level.16 In CMML, the early clonal dominance of TET2 mutations has been shown to lead to granulo-monocytic differentiation skewing at the expense of erythroid and megakaryocytic differentiation.12
During the natural course of the disease, patients with MDS are at high risk of progressing to AML.1 The most likely interpretation is that the acquisition of additional driver mutations leads to formation of subclones of hematopoietic cells with further impaired differentiation and/or maturation capacity. The proportion of blast cells progressively increases over time, and overt AML eventually develops (Figure 2, step 4). This has been demonstrated by Walter et al10 in their study of the clonal architecture of secondary AML. In each of the 7 patients studied, in fact, progression to AML was characterized by the persistence of the antecedent founding myelodysplastic clone and the emergence of ≥1 subclone harboring new somatic mutations. Thus, a secondary AML developing from MDS is not monoclonal in the strict sense but is instead a mosaic of several clones/genomes with different sets of somatic mutations, illustrating the concept of intraclonal or intratumoral heterogeneity.10
Recurrent chromosomal abnormalities are mostly secondary genetic events with established clinical relevance in MDS
Recurrent chromosomal abnormalities have been very important thus far for diagnosis and prognostication of MDS. Regarding diagnosis, the detection of a cytogenetic aberration in a patient with peripheral cytopenia and bone marrow dysplasia provides an important marker of clonal proliferation. By contrast, the diagnosis of MDS may be difficult in patients with a normal karyotype or noninformative cytogenetics.19
Recurrent chromosomal abnormalities are detected in about half of patients with MDS,20 and the most common single cytogenetic aberrations include del(5q), trisomy 8, del(20q), and monosomy 7 or del(7q).20-22 These are likely secondary genetic events, deriving from the genome instability caused by the founding genetic mutation.5 The only exception to the rule known thus far is isolated del(5q), which characterizes the 5q- syndrome: in fact, haploinsufficiency for RPS14 and miR-145, mapping to the common deleted region, represents the pathophysiological basis of this MDS subtype.23-25
With respect to the prognostic relevance of recurrent chromosomal abnormalities, in a recent collaborative study aimed to develop the revised International Prognostic Scoring System (IPSS-R) for MDS, patient databases from international institutions were coalesced to assemble a combined database including 7012 patients.26 Cytogenetic abnormalities were categorized into 5 prognostic subgroups that were shown to have significant prognostic relevance with different median survival and risk of evolution into AML (Table 2).
MDS cytogenetic scoring system: prognostic relevance of cytogenetic abnormalities in 7012 patients included in the International Working Group for Prognosis in MDS (IWG-PM) database
| Prognostic cytogenetic subgroup . | Cytogenetic abnormalities . | Proportion of MDS patients, % . | Median overall survival, years . | Median time to 25% AML evolution, years . |
|---|---|---|---|---|
| Very good | −Y, del(11q) | 4 | 5.4 | Not reached |
| Good | Normal karyotype, del(5q), del(12p), del(20q), double abnormality including del(5q) | 72 | 4.8 | 9.4 |
| Intermediate | del(7q), +8, +19, i(17q), any other single or double independent clones | 13 | 2.7 | 2.5 |
| High | −7, inv(3)/t(3q)/del(3q), double including −7/del(7q), complex: 3 abnormalities | 4 | 1.5 | 1.7 |
| Very high | Complex karyotype: >3 abnormalities | 7 | 0.7 | 0.7 |
| Prognostic cytogenetic subgroup . | Cytogenetic abnormalities . | Proportion of MDS patients, % . | Median overall survival, years . | Median time to 25% AML evolution, years . |
|---|---|---|---|---|
| Very good | −Y, del(11q) | 4 | 5.4 | Not reached |
| Good | Normal karyotype, del(5q), del(12p), del(20q), double abnormality including del(5q) | 72 | 4.8 | 9.4 |
| Intermediate | del(7q), +8, +19, i(17q), any other single or double independent clones | 13 | 2.7 | 2.5 |
| High | −7, inv(3)/t(3q)/del(3q), double including −7/del(7q), complex: 3 abnormalities | 4 | 1.5 | 1.7 |
| Very high | Complex karyotype: >3 abnormalities | 7 | 0.7 | 0.7 |
Reproduced from Greenberg et al.26
The 5-group cytogenetic risk classification reported in Table 2 was recently found to predict the outcome of allogeneic hematopoietic stem cell transplantation in MDS patients.27 In particular, patients with a complex karyotype (very poor cytogenetic subgroup) had a very poor outcome after transplantation. This was also true for monosomal karyotype, defined as the karyotype of patients who had ≥2 autosomal monosomies or 1 monosomy in combination with other structural abnormalities.27
Somatic gene mutations in myelodysplasia: from studies of candidate genes to whole genome sequencing
Our understanding of the molecular basis of MDS has improved dramatically in the last 4 years. The first major breakthrough was the identification of somatic mutations of TET2 in patients with rearrangements of chromosome 4q24, where the gene maps.11,29 Subsequently, Bejar et al30 used next-generation sequencing and mass spectrometry–based genotyping to screen mutational hotspots in 111 genes in 439 patients with MDS. In the last 2 years, studies of whole exome or whole genome sequencing led to the discovery of mutations in the RNA splicing machinery,15,31,32 of SETBP1 mutations in aCML,33 and of CSF3R mutations in CNL.34
Recent studies have performed a systematic analysis of panels of known or putative genes relevant in myelodysplasia by coupling high-throughput sample handling with massive parallel sequencing2,35 or by combining deep sequencing with array-based genomic hybridization (Seishi Ogawa, Kyoto University, personal communication, August 9, 2013). The panels used included from 94 to 111 candidate genes, and in the 2 largest studies enrolling >700 patients, oncogenic mutations were identified with confidence in about half of the genes sequenced. Accounting for both gene mutations and chromosomal aberrations, the overall frequency of genetic lesions range from 78% to 90%. A list of the most common recurrently mutated genes in patients with MDS or MDS/MPNs, based on studies published thus far, is reported in Table 3.
Most common driver genes in patients with MDS and MDS/MPN
| Biological pathways and genes . | Frequency, %* . | Timing of mutation acquisition† . | Relationship between mutant gene and clinical phenotype . | Prognostic or predictive relevance of mutant gene . |
|---|---|---|---|---|
| RNA splicing | ||||
| SF3B1 | 15-30% | More often a founding mutation | Strictly associated with ring sideroblasts phenotype (RARS, RARS-T) | Associated with good overall survival and low risk of leukemic evolution |
| SRSF2 | 10-20% | More often a founding mutation | Associated with RCMD or RAEB, co-mutated with TET2 in CMML | Associated with poor overall survival and high risk of leukemic evolution |
| U2AF1 | <10% | More often a founding mutation | Mainly associated with RCMD or RAEB | Associated with high risk of leukemic evolution |
| ZRSR2 | <10% | More often a founding mutation | Not defined | Not defined |
| DNA methylation | ||||
| TET2 | 20-30% | More often a founding mutation | Found in all MDS subtypes, high mutation frequency (50-60%) in CMML | No impact on overall survival, may predict response to hypomethylating agents |
| DNMT3A | ∼10% | More often a founding mutation | Found in all MDS subtypes, co-mutated with SF3B1 in RARS | Associated with unfavorable clinical outcome (negative prognostic relevance mitigated by SF3B1 co-mutation in RARS) |
| IDH1/IDH2 | ∼5% | More often a founding mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome |
| Chromatin modification | ||||
| ASXL1 | 15-20% | More often a subclonal mutation | Associated with RCMD or RAEB, high mutation frequency (40%) in CMML | Associated with unfavorable clinical outcome in all myeloid neoplasms (MDS, MDS/MPN, MPN) |
| EZH2 | ∼5% | More often a subclonal mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome in all myeloid neoplasms |
| Transcription | ||||
| RUNX1 | ∼10% | Typical subclonal mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome |
| BCOR | <5% | Typical subclonal mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome |
| DNA repair control | ||||
| TP53 | ∼5% | Typical subclonal mutation | Associated with advanced disease and complex karyotype, mutated in 20% of patients with MDS with del(5q) | Associated with poor overall survival and high risk of leukemic evolution, predicts poor response to lenalidomide in MDS with del(5q) |
| Cohesin | ||||
| STAG2 | <10% | More often a subclonal mutation | Associated with RCMD or RAEB. Mutated in about 10% of patients with AML | Associated with unfavorable clinical outcome |
| RAS pathway | ||||
| CBL | <5% | More often a subclonal mutation | Found in different MDS subtypes, associated with JMML in children | Not defined in MDS |
| NRAS/KRAS | <5% | More often a subclonal mutation | Found in different MDS subtypes, associated with JMML in children | Not defined in MDS |
| NF1 | <5% | More often a subclonal mutation | Found in different MDS subtypes, associated with JMML in children | Not defined in MDS |
| DNA replication | ||||
| SETBP1 | <5% | More often a subclonal mutation | Found in 25% of patients with aCML and in subsets of patients with advanced MDS or CMML | Associated with poor overall survival and high risk of leukemic evolution |
| Receptors | ||||
| CSF3R | <1% | Founding driver mutation in CNL | Strictly associated with CNL, found in a subset of patients with aCML | Mutation type may predict response to specific inhibitors |
| Biological pathways and genes . | Frequency, %* . | Timing of mutation acquisition† . | Relationship between mutant gene and clinical phenotype . | Prognostic or predictive relevance of mutant gene . |
|---|---|---|---|---|
| RNA splicing | ||||
| SF3B1 | 15-30% | More often a founding mutation | Strictly associated with ring sideroblasts phenotype (RARS, RARS-T) | Associated with good overall survival and low risk of leukemic evolution |
| SRSF2 | 10-20% | More often a founding mutation | Associated with RCMD or RAEB, co-mutated with TET2 in CMML | Associated with poor overall survival and high risk of leukemic evolution |
| U2AF1 | <10% | More often a founding mutation | Mainly associated with RCMD or RAEB | Associated with high risk of leukemic evolution |
| ZRSR2 | <10% | More often a founding mutation | Not defined | Not defined |
| DNA methylation | ||||
| TET2 | 20-30% | More often a founding mutation | Found in all MDS subtypes, high mutation frequency (50-60%) in CMML | No impact on overall survival, may predict response to hypomethylating agents |
| DNMT3A | ∼10% | More often a founding mutation | Found in all MDS subtypes, co-mutated with SF3B1 in RARS | Associated with unfavorable clinical outcome (negative prognostic relevance mitigated by SF3B1 co-mutation in RARS) |
| IDH1/IDH2 | ∼5% | More often a founding mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome |
| Chromatin modification | ||||
| ASXL1 | 15-20% | More often a subclonal mutation | Associated with RCMD or RAEB, high mutation frequency (40%) in CMML | Associated with unfavorable clinical outcome in all myeloid neoplasms (MDS, MDS/MPN, MPN) |
| EZH2 | ∼5% | More often a subclonal mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome in all myeloid neoplasms |
| Transcription | ||||
| RUNX1 | ∼10% | Typical subclonal mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome |
| BCOR | <5% | Typical subclonal mutation | Associated with RCMD or RAEB | Associated with unfavorable clinical outcome |
| DNA repair control | ||||
| TP53 | ∼5% | Typical subclonal mutation | Associated with advanced disease and complex karyotype, mutated in 20% of patients with MDS with del(5q) | Associated with poor overall survival and high risk of leukemic evolution, predicts poor response to lenalidomide in MDS with del(5q) |
| Cohesin | ||||
| STAG2 | <10% | More often a subclonal mutation | Associated with RCMD or RAEB. Mutated in about 10% of patients with AML | Associated with unfavorable clinical outcome |
| RAS pathway | ||||
| CBL | <5% | More often a subclonal mutation | Found in different MDS subtypes, associated with JMML in children | Not defined in MDS |
| NRAS/KRAS | <5% | More often a subclonal mutation | Found in different MDS subtypes, associated with JMML in children | Not defined in MDS |
| NF1 | <5% | More often a subclonal mutation | Found in different MDS subtypes, associated with JMML in children | Not defined in MDS |
| DNA replication | ||||
| SETBP1 | <5% | More often a subclonal mutation | Found in 25% of patients with aCML and in subsets of patients with advanced MDS or CMML | Associated with poor overall survival and high risk of leukemic evolution |
| Receptors | ||||
| CSF3R | <1% | Founding driver mutation in CNL | Strictly associated with CNL, found in a subset of patients with aCML | Mutation type may predict response to specific inhibitors |
Approximate proportion of patients with MDS carrying the mutant gene reported in studies published so far.
Based on values for mutant allele burden or variant allele frequency.
Spliceosome mutations: their unexpected promotion of clonal proliferation and the concept of genetic predestination
Pre-mRNA splicing is catalyzed by the spliceosome, a macromolecule composed of 5 small nuclear RNAs associated with proteins to form particles termed small nuclear ribonucleoproteins (snRNP).16 More than 50% of patients with myelodysplasia carry somatic mutations in spliceosome genes encoding proteins involved in the 3′ splice site recognition and U2 snRNP function.2 Spliceosome mutations are rarely found in childhood myeloid neoplasms, suggesting that they are typically acquired in the elderly.36
Mutations of the RNA splicing machinery are largely mutually exclusive and are most often founding events. In fact, the mutant allele burden is typically between 40% and 50%, indicating a dominant bone marrow clone that is heterozygous for the mutation.37,38 Mutation hotspots have been shown in the 3 most frequently mutated genes, ie, SF3B1, SRSF2, and U2AF1, and almost all described mutations are missense, with no evidence of nonsense or frameshift changes.15,31,32 Different patterns of missplicing associated with the above mutant genes have been described.32,39,40 Altogether, the available evidence suggests that spliceosome mutations affecting the 3′ splice site recognition and U2 snRNP function are likely to create novel protein isoforms that can drive clonal dominance of mutated hematopoietic stem cells.5 This conclusion was totally unpredictable since, as emphasized by Vogelstein et al,6 a spliceosome mutation was expected to lead to a plethora of nonspecific cellular abnormalities rather than to promote clonal proliferation.
The different spliceosome mutations are associated with different phenotypes and different clinical outcomes (Table 3).2,38 Somatic SF3B1 mutations are found almost exclusively in patients with refractory anemia with ring sideroblasts without or with thrombocytosis (RARS and RARS-T, respectively), and this clearly suggests a causal relationship between mutation and ring sideroblast formation.16 In addition, the vast majority of patients with SF3B1 mutation have a good clinical outcome with a low propensity to AML transformation.37 SRSF2 mutations are found mainly in patients with multilineage dysplasia and/or excess blasts and, at variance with SF3B1 mutations, predict for high risk of leukemic evolution and poor survival.41-43 SRSF2 mutations have been detected in about one fifth of cases of AML transformed from MPN44 and, in particular, have been reported in ∼40% to 50% of patients with CMML, where they are frequently associated with TET2 mutations.12,31 Somatic mutations of U2AF1 have been reported in various MDS subtypes and found to be predictive of a high risk of leukemic evolution and shorter survival.32,41
The observation that spliceosome mutations are mainly founding mutations associated with different clinical outcomes has led Papaemmanuil et al2 to hypothesize that they give rise to initiating clones with different genetic predestination. More specifically, the initial driver mutation would shape the future trajectory of clonal evolution through constraints on the repertoire of cooperating genetic lesions. The molecular mechanisms underlying genetic predestination remain to be defined.
Somatic mutations in genes encoding epigenetic regulators
In a recent review article in Blood, Issa45 described cellular differentiation as an epigenetic process that requires specific and highly ordered DNA methylation and chromatin modification programs. The disordered differentiation of MDS is often associated with somatic mutations in genes that control DNA methylation (TET2, DNMT3A, and IDH1/IDH2) or regulate chromatin modification (ASXL1 and EZH2).5,45
Somatic TET2 mutations were first described in patients with myeloid neoplasms in 2009.11,29 TET2 is mutated in 20% to 25% of patients with MDS2,30 and in 50% to 60% of patients with CMML.46 In an elegant study, Busque et al47 found recurrent somatic TET2 mutations in elderly females who had clonal hematopoiesis demonstrated by X-chromosome inactivation skewing but no hematologic phenotype. This and other observations support the notion that TET2 mutation can lead per se to increased hematopoietic stem cell self-renewal and clonal myeloid proliferation.48,49 TET2 mutations are frequently found in patients with normal karyotype, and therefore represent a useful marker of clonality in these subjects.30 In addition, co-occurrence of TET2 and SRSF2 mutation is typically found in CMML.2 Thus far, no prognostic relevance in terms of overall survival has been clearly defined,30,50 but recent studies suggest that TET2 mutation may predict response to hypomethylating agents.51,52 Interestingly, TET2 mutations were found to be associated with reduced overall survival among patients with intermediate-risk AML.53
In a study based on massively parallel DNA sequencing, Ley et al54 found that DNMT3A mutations are highly recurrent in patients with de novo AML and are associated with a poor outcome. Somatic DNMT3A mutations have later been detected in ∼10% to 15% of patients with different subtypes of MDSs.35,55,56 They are associated with unfavorable clinical outcome and more rapid progression to AML in patients with RCMD or RAEB55 but not in those with RARS, likely because the co-occurrence of the SF3B1 mutation mitigates the negative effect of DNMT3A mutation.56 As suggested by Papaemmanuil et al,2 this observation may indicate that some genes may only be transforming in specific genomic contexts.
Recurrent mutations in the isocitrate dehydrogenase genes IDH1 and IDH2 are found in AML and MDS.5,57 In AML, co-occurrence of NPM1 and IDH1 or IDH2 mutations is associated with a good clinical outcome.53 By contrast, IDH1 mutation has been found to be associated with a short leukemia-free survival in MDS.58
Two genes involved in chromatin modification and regulation are recurrently mutated in MDS: ASXL1, which interacts with the polycomb-group repressive complex 1 and 2 (PRC1, PRC2),59,60 and EZH2, which belongs to PRC2.2,30,61-65 In cellular and animal models, ASXL1 mutations have been shown to promote myeloid transformation through loss of PRC2-mediated gene repression.60 ASXL1 mutations are common not only in MDS, but also in AML, CMML, and PMF, and are generally associated with poor clinical outcome in all myeloid neoplasms.46,61,62,66 Of note, ASXL1 mutation has been recently incorporated into a prognostic scoring system for CMML as a negative prognostic factor.46 Similarly, EZH2 mutations were found to be significantly associated with a shorter overall in lower risk MDS, and their incorporation in a prognostic model improved risk stratification in these patients.56
Several studies, recently reviewed in Blood by Issa,45 have shown that MDS patients not only carry epigenetic effector mutations but also an abnormal epigenome. Some of these patients respond to epigenetic therapy, including hypomethylating drugs (azacitidine and decitabine) and drugs that inhibit multiple histone deacetylases.45 However, although the efficacy of some of these treatments has been demonstrated in prospective clinical trials,67 we still lack reliable predictors of response to epigenetic therapy.
Somatic mutations in other cellular pathways
Acquired mutations of transcription factors have been described not only in AML but also in MDS.4,30 Somatic mutations of RUNX1 are found in ∼7% to 8% of all patients with MDS and are generally associated with advanced disease, severe thrombocytopenia, and poor clinical outcome.2,30,56
The gene TP53, located on chromosome 17p13.1, encodes p53, which coordinates transcription programs contributing to tumor suppression, and mutant p53 proteins have been identified in various cancers.6,68 TP53 mutations are found in about 5% of patients with MDS, mainly in subjects with advanced disease, complex karyotype, abnormalities of chromosome 17, or deletions of chromosome 5 and 7.30,55 MDS patients carrying TP53 mutation have an unfavorable clinical outcome and a high risk of leukemic evolution,2,30 and the same is true for patients with MPN.69 In particular, TP53 mutated subclones may occur at an early disease stage in MDS with del(5q), where they are associated with a lower response to lenalidomide and an increased risk of progression to AML.70
The Ras superfamily includes small GTP-binding proteins involved in intracellular signal transduction. Several genes of this superfamily have been found to be mutated in patients with myelodysplasia, including NRAS, KRAS, NF1, PTPN11, and CBL.2 Somatic or germ-line mutations of RAS pathway gene are present in ∼90% of patients with juvenile CMML,71 an MDS/MPN in which secondary mutations of SETBP1 and JAK3 may cause disease progression.72
SETBP1 encodes a protein that binds the SET nuclear oncogene involved in DNA replication. Although heterozygous de novo germ-line mutations in SETBP1 have been shown to be associated with the Schinzel-Giedion midface retraction syndrome,73 somatic mutations have been recently detected in patients with myeloid malignancies.33,74 In particular, SETBP1 mutations are found in ∼25% to 30% of patients with aCML.33,75 SETBP1 has a direct role in the transcriptional regulation of other genes,76 and SETBP1 mutations are more often genetic events associated with disease progression in MDS.74,75,77
CSF3R encodes the receptor for colony stimulating factor 3. The acquisition of nonsense mutations in this gene, resulting in the expression of truncated CSF3R protein, has been previously found to be associated with progression to MDS/AML in patients with severe congenital neutropenia.78 Activating somatic mutations in CSF3R have been recently detected in ∼90% of patients with CNL and in ∼40% of those with aCML.34 This study also showed that distinguishing between these 2 disorders may be difficult using the WHO criteria, whereas follow-up investigations demonstrated that CSF3R and SETBP1 mutations are not mutually exclusive.34 As pointed out by Gotlib et al,79 CNL and aCML are likely overlapping neoplasms: although the pathogenesis of the former is mainly characterized by CSF3R mutation, that of aCML is likely more multifactorial.
Cohesin is a highly conserved 4-subunit ring structure that encircles sister chromatids during metaphase, allowing their cohesion, and also plays critical roles in transcriptional regulation and post-replicative DNA repair.80 Somatic mutations in STAG2, a gene of the cohesin complex, have been found in ∼6% of MDS patients.10 In a recent work, Kon et al81 detected mutations and deletions involving various genes of the cohesin complex (STAG2, RAD21, SMC1A, and SMC3) in 8% of patients with MDS, 10% of those with CMML, and 12% of those with AML. A similar frequency was previously reported in AML patients,3 suggesting that altered cohesin function plays a role in myeloid leukemogenesis.
The gene BCOR, located on chromosome Xp11.4, encodes a corepressor of BCL6, a POZ/zinc finger transcription repressor that is required for germinal center formation and may influence apoptosis. Germ-line mutations of this gene are associated with oculofaciocardiodental and Lenz microphthalmia syndromes.82 Inactivating somatic mutations of BCOR have been described in AML with normal karyotype83 and more recently in a subset of patients with MDS.2,84 These are typical subclonal driver mutations, associated with a poor clinical outcome.84
Familial MDSs and genetic predisposition to acquisition of somatic mutations associated with myelodysplasia: germ-line GATA2 mutations
For a deeper analysis of this subject, the reader is referred to a comprehensive review article by Liew and Owen.85 Familial syndromes predisposing to MDS or AML include bone marrow failure inherited disorders (Diamond-Blackfan, dyskeratosis congenita, severe congenital neutropenia), DNA repair deficiency syndromes, Noonan syndrome and neurofibromatosis I, Down syndrome, and familial platelet disorder with propensity to myeloid malignancy (associated with germ-line mutations of RUNX1 or CEBPA).
More recently, germ-line mutations of GATA2 have been described in familial syndromes characterized by predisposition to MDS and AML.86 In 3 families, subjects carrying the GATA2 (C1061T) mutation had macrocytic anemia and developed MDS/AML between the second and fifth decade of life. Various germ-line mutations of GATA2 have been detected in patients with the Emberger syndrome, characterized by primary lymphedema associated with a predisposition to AML.87 Finally, several germ-line mutations in GATA2 have been reported to be associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome, which predisposes to myeloid malignancy.88-90 Typically these patients have a combination of severe monocytopenia with mild neutropenia and marginally reduced hemoglobin level, and progression to MDS/AML typically occurs in the second or third decade of life.90 It should be noted that, although germ-line mutations of both RUNX1 or GATA2 may predispose to MDS,91 somatic mutations of the same genes may represent mechanisms of disease progression in myeloid neoplasms.2
Several driver genes may cause myelodysplasia, and genotype/phenotype relationships have been defined
In their recent article, Vogelstein et al6 concluded that there are ∼140 genes whose intragenic mutations contribute cancer. Based on currently available data, the number of potential myelodysplasia driver genes is likely somewhat lower (50-60). However, reliable estimates can be provided only by whole genome sequencing studies.10,35 Whole exome sequencing and candidate gene sequencing are clearly less informative and may not allow the identification of more rarely mutated genes.
The patient cohorts studied thus far are heterogeneous, and all studies are basically retrospective. Taking into account these limitations, only 4 to 6 genes (SF3B1, TET2, SRSF2, ASXL1, DNMT3A, and RUNX1) have been found to be consistently mutated in ≥10% of MDS patients, whereas a long tail of about 40 to 50 additional genes are mutated in smaller subsets.2 In the study by Walter et al,35 the 2 most frequently mutated genes were TP53 and U2AF1: this likely reflects the fact that their patient cohort included a low proportion of low risk and a high proportion of high-risk MDS subtypes.
At presentation, most MDS patients have 2 or 3 oncogenic driver mutations and hundreds of background or passenger mutations. Considering the variant allele frequency, some mutant genes, typically those of RNA splicing and DNA methylation, appear to be mainly associated with the initial clonal proliferation, whereas others are mainly involved in subclonal evolution (Table 3). However, the temporal order of acquisition of driver mutations is not fixed and varies from subject to subject. Thus, the same mutant gene, eg, TET2, may be an early driver in some patients and a subclonal driver in others. Walter et al35 observed that mutations in driver genes belonging to the same biologic pathway tended not to co-occur, suggesting that a second mutation in the same pathway provides no additional growth advantage or is not even tolerated.
According to Vogelstein et al,6 most human cancers are caused by 2 to 8 sequential genetic lesions that develop over the course of 20 to 30 years. In their studies of whole genome sequencing, Welch et al3 estimated that as few as 2 key somatic mutations are needed to cause the malignant founding clone and clinically manifest AML. The available evidence suggests that this may apply also to MDS: at variance with AML, however, the genetic lesions responsible for MDS likely occur sequentially over years, rather than over months or weeks, at least in low-risk subtypes with long natural history of disease, as is typically RARS.92
Unfortunately only few functional studies linking the various mutant genes to a cellular or disease phenotype have been performed. In an animal model of conditional Tet2 loss, Tet2 haploinsufficiency was shown to lead to a disorder resembling human CMML.48 Another study reported findings suggesting that SF3B1 or Sf3b1 haploinsufficiency leads to ring sideroblast formation in human cells or heterozygous knockout mice, respectively.93 The CSF3R (T618I) mutation has been shown to drive a lethal myeloproliferative disorder in a murine model.94
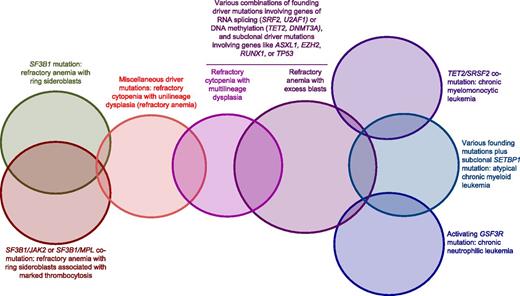
Genotype/phenotype relationships have been defined in MDS, MDS/MPN, and related myeloid neoplasms. A tentative schematic representation of our current knowledge of these relationships is reported in Figure 3. Although reliable conclusions will be made possible only by prospective studies, this scheme provides a proof of concept of the potential feasibility of a molecular classification of MDS and related myeloid neoplasms. More specifically, SF3B1 mutation appears to be strictly associated with refractory anemia with ring sideroblasts with or without marked thrombocytosis,15,37 the combination of SRSF2 and TET2 mutation with CMML,2 and an activating CSF3R mutation with CNL.34 Refractory anemia has no peculiar genotype thus far, raising the question as to whether it should be considered as a separate entity. RCMD and refractory anemia with blast excess can be associated with different combinations of founding mutations, primarily involving genes of RNA splicing (with the only exclusion of SF3B1) or epigenetic regulation and subclonal driver mutations.
Schematic representation of our current knowledge of genotype/phenotype relationships in MDS, MDS/MPN, and a related MPN like CNL. The SF3B1 mutation is strictly associated with RARS, whereas the combination of the SF3B1 mutation with subclonal driver mutations in JAK2 or MPL is associated with RARS-T. Thus far, no conclusive genotype/phenotype relationship has been defined within refractory cytopenia with unilineage dysplasia. Various combinations of founding and subclonal driver mutations can be found in RCMD and RAEB. CMML has a relatively well-defined molecular basis, involving primarily somatic mutations of TET2 and SRSF2: comutation of these genes is almost invariably associated with CMML, whereas the ASXL1 mutation involves poor outcome. Within MDS/MPN, aCML is characterized by various founding mutations plus a subclonal mutation of SETBP1. Activating mutations of CSF3R are strictly associated with CNL.
Schematic representation of our current knowledge of genotype/phenotype relationships in MDS, MDS/MPN, and a related MPN like CNL. The SF3B1 mutation is strictly associated with RARS, whereas the combination of the SF3B1 mutation with subclonal driver mutations in JAK2 or MPL is associated with RARS-T. Thus far, no conclusive genotype/phenotype relationship has been defined within refractory cytopenia with unilineage dysplasia. Various combinations of founding and subclonal driver mutations can be found in RCMD and RAEB. CMML has a relatively well-defined molecular basis, involving primarily somatic mutations of TET2 and SRSF2: comutation of these genes is almost invariably associated with CMML, whereas the ASXL1 mutation involves poor outcome. Within MDS/MPN, aCML is characterized by various founding mutations plus a subclonal mutation of SETBP1. Activating mutations of CSF3R are strictly associated with CNL.
Interestingly, as shown in Table 4, a molecular classification appears to be already feasible in MDS/MPN, a group of myeloid neoplasms thus far defined on the basis of cumbersome clinical, hematologic, and morphologic criteria.1 It is also apparent that specific driver genes are responsible for the myeloproliferative component of the different MDS/MPN, like JAK2 or MPL in RARS-T, SETBP1 in aCML, and CSF3R in CNL. We have previously shown that RARS-T develops from RARS through the occurrence of a subclonal driver mutation in JAK2 or MPL in the initial SF3B1 mutated clone.37,95 A similar evolutionary process likely operates in CMML and aCML, which may develop from a preexisting MDS through the acquisition of subclonal driver mutations that cause monocytosis and granulocytic leukocytosis, respectively.
Somatic mutations that characterize the different types of MDS/MPN
| Myeloid neoplasm according to the 2008 WHO classification . | Main diagnostic/clinical feature(s) . | Most common mutant gene(s) . |
|---|---|---|
| CMML (classified as MDS/MPN) | Persistent peripheral blood monocytosis (>1 × 109/L). | Co-occurrence of TET2 and SRSF2 mutations, or combinations of ASXL1 mutations with other mutant driver genes. ASXL1 mutation is associated with poor overall survival and high risk of progression to AML |
| aCML (classified as MDS/MPN) | Peripheral leukocytosis (≥13 × 109/L) with dysgranulopoiesis and 10% or more circulating immature granulocytes. | Combinations of founding mutations in various genes and subclonal mutations of SETBP1 or ASXL1. |
| CNL (classified as MPN) | Neutrophilic leukocytosis (≥25 × 109/L) with less than 10% circulating immature granulocytes. | Activating somatic mutations of CSF3R (encoding the receptor for G-CSF) in the vast majority of patients. Oncogenic lesions can be classified as truncation or membrane proximal mutations, involving different preferential downstream kinase signaling. |
| JMML (classified as MDS/MPN) | Persistent peripheral blood monocytosis (>1 × 109/L) in children. | Somatic mutations of the RAS pathway (NRAS, KRAS, NF1, PTPN11, and CBL). Heterozygous germ-line CBL mutations may predispose to JMML. Subclonal driver mutations of SETBP1 and JAK3 may cause disease progression. |
| RARS-T (classified as a provisional entity within MDS/MPN) | Macrocytic anemia, ring sideroblasts, and thrombocytosis. | Combinations of a founding somatic mutation of SF3B1 and subclonal driver mutations of JAK2 or MPL (and likely of other as-yet-unknown genes). |
| Myeloid neoplasm according to the 2008 WHO classification . | Main diagnostic/clinical feature(s) . | Most common mutant gene(s) . |
|---|---|---|
| CMML (classified as MDS/MPN) | Persistent peripheral blood monocytosis (>1 × 109/L). | Co-occurrence of TET2 and SRSF2 mutations, or combinations of ASXL1 mutations with other mutant driver genes. ASXL1 mutation is associated with poor overall survival and high risk of progression to AML |
| aCML (classified as MDS/MPN) | Peripheral leukocytosis (≥13 × 109/L) with dysgranulopoiesis and 10% or more circulating immature granulocytes. | Combinations of founding mutations in various genes and subclonal mutations of SETBP1 or ASXL1. |
| CNL (classified as MPN) | Neutrophilic leukocytosis (≥25 × 109/L) with less than 10% circulating immature granulocytes. | Activating somatic mutations of CSF3R (encoding the receptor for G-CSF) in the vast majority of patients. Oncogenic lesions can be classified as truncation or membrane proximal mutations, involving different preferential downstream kinase signaling. |
| JMML (classified as MDS/MPN) | Persistent peripheral blood monocytosis (>1 × 109/L) in children. | Somatic mutations of the RAS pathway (NRAS, KRAS, NF1, PTPN11, and CBL). Heterozygous germ-line CBL mutations may predispose to JMML. Subclonal driver mutations of SETBP1 and JAK3 may cause disease progression. |
| RARS-T (classified as a provisional entity within MDS/MPN) | Macrocytic anemia, ring sideroblasts, and thrombocytosis. | Combinations of a founding somatic mutation of SF3B1 and subclonal driver mutations of JAK2 or MPL (and likely of other as-yet-unknown genes). |
CNL has been included because of its overlapping features with aCML.
Myelodysplasia driver genes are recurrently mutated also in other myeloid neoplasms
The driver genes whose mutations are responsible for MDS (Table 3) are frequently mutated also in other myeloid neoplasms listed in Table 1. This is primarily true for AML,3,4,53 although, as underlined by Walter et al,35 some genes are overrepresented in MDS compared with AML and vice versa. Indeed, in the recent study by the Cancer Genome Atlas Research Network, the 20 most recurrently mutated genes in AML were FLT3, NPM1, DNMT3A, IDH1/2, TET2, RUNX1, TP53, N/KRAS, CEPBPA, WT1, PTPN11, KIT, U2AF1, SMC1A, SMC3, PHF6, STAG2, and RAD21.4 Only half of these genes are within the 20 most recurrently mutated ones in MDS (Table 3). Although the molecular pathophysiology of MDS is different from that of AML, some AML driver genes might behave as subclonal drivers in MDS and thereby drive leukemic transformation.
Somatic mutations of CBL, TET2, ASXL1, and IDH/IDH2 have been detected in the advanced phase of chronic myeloid leukemia.96 Several of the genes reported in Table 3 can also be mutated in PMF, in combination with JAK2 (V617F) or MPL exon 10 mutations, and comutation has been shown to have a negative impact on the clinical course of this MPN.66 Recently, somatic mutations of TET2, SRSF2, ASXL1, CBL, and RUNX1 have been detected in ∼90% of patients with advanced mastocytosis, and overall survival was found to be significantly shorter in patients with additional mutations than in those carrying KIT (D816V) only.97
Myelodysplasia driver genes may also interact with somatic mutations involving lymphoid cell lines, thus giving rise to peculiar phenotypes. T-cell large granular lymphocytic leukemia is characterized by clonal expansion of CD3+ cytotoxic T lymphocytes and may be associated with autoimmune disorders and immune-mediated cytopenias.98 The expansion of clonal T cells has been shown to be caused by somatic mutations of STAT3 or STAT5b.99,100 Autoimmune processes have been shown to contribute to cytopenia in a subset of MDS patients, and these patients may benefit from immunosuppressive treatment.101 Interestingly, a recent study describes the occurrence of STAT3 mutated T-cell clones in a subset of patients with MDS, suggesting that this may represent a molecular mechanism for the occurrence of autoimmune phenomena.102
Relevance of gene mutations in the diagnostic approach to myelodysplasia: toward a molecular classification of myeloid neoplasms
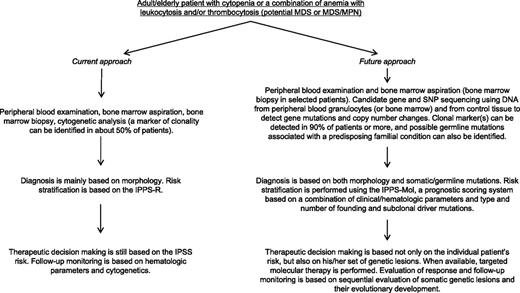
The current diagnostic approach to MDS includes peripheral blood and bone marrow morphology to evaluate abnormalities of peripheral blood cells and hematopoietic precursors (Figure 1), bone marrow biopsy to assess marrow cellularity, fibrosis, and topography,103 and cytogenetics to identify nonrandom chromosomal abnormalities (Table 2).104
Massive parallel sequencing has the potential of dramatically improving our approach to diagnosis of MDS as illustrated in Figure 4. The price of whole genome sequencing is expected to drop below $1000 in the next years, whereas that of targeted gene sequencing2 should be definitely lower. Deep sequencing may allow the simultaneous detection of both somatic gene mutations and copy number changes, the cytogenetic abnormalities typical of MDS, in a single assay.2 Although whole genome sequencing is clearly more informative, massive parallel sequencing of a panel of myeloid genes is more feasible in a clinical laboratory. The genes to be sequenced may include ∼50 to 60 myelodysplasia driver genes,2,35 genes associated with inherited disorders that predispose to MDS, and a reasonable number of germ-line single nucleotide polymorphisms that allow reliable detection of copy number changes from sequencing data.2
Current approach to diagnosis and prognostication of MDS and MDS/MPN and a hypothetical future approach based on massive parallel sequencing.
Current approach to diagnosis and prognostication of MDS and MDS/MPN and a hypothetical future approach based on massive parallel sequencing.
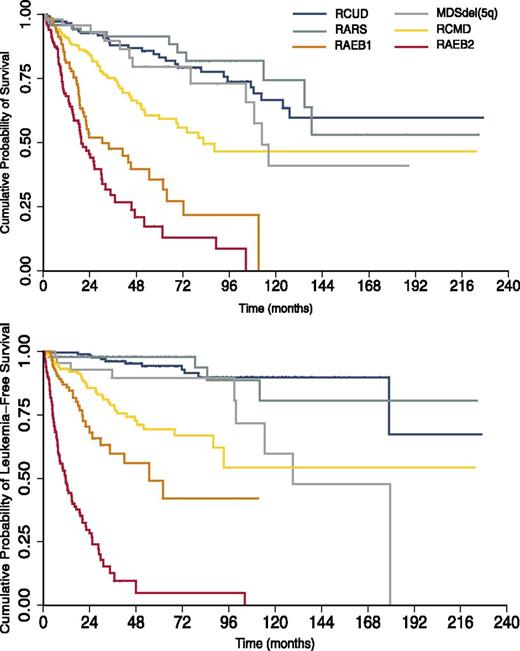
Kaplan-Meier analysis of overall survival and leukemia-free survival of 1110 patients diagnosed with MDS at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, between 1990 and 2012. MDS patients are stratified according to the 2008 WHO classification categories. Multilineage dysplasia and excess of blasts have a considerable impact on outcomes.
Kaplan-Meier analysis of overall survival and leukemia-free survival of 1110 patients diagnosed with MDS at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, between 1990 and 2012. MDS patients are stratified according to the 2008 WHO classification categories. Multilineage dysplasia and excess of blasts have a considerable impact on outcomes.
Prognostic and predictive significance of driver mutations and development of molecular models for clinical decision making
As shown in Figure 5, the current WHO classification of MDS has valuable prognostic relevance, with blast percentage and multilineage dysplasia representing the most relevant parameters from this point of view. However, the reproducibility of these latter parameters is far from optimal,105 and there is a need for more robust prognostic factors. The IPSS-R26 clearly represents a step forward but does take into account only cytogenetic abnormalities, which are secondary genetic events and not the driver lesions.
The definition of founding and subclonal driver mutations might considerably improve prognostication of MDS and more generally clinical decision-making in this field. First, the identification of the mutant gene responsible for the initial clone is relevant to clinical outcome. For instance, ring sideroblasts may be found not only in patients with a founding mutation in SF3B1, but also in those with an initiating oncogenic lesion in SRSF2.31 However, the median leukemia-free survival is >10 years in the former vs <2 years in the latter.2,37 Second, the detection of subclonal driver mutations associated with small clones may allow early diagnosis of disease progression, including evolution into AML. In chronic lymphocytic leukemia, the presence of subclonal driver mutations adversely impacts clinical outcome.106 Third, the number of driver mutations per patient represents an important prognostic factor per se. In the recent study by Papaemmanuil et al,2 the median leukemia-free survival was >3 years in patients with 1 or 2 driver mutations vs <2 years in patients with ≥3 driver mutations.
A few studies have already suggested that incorporation of somatic mutations into prognostic scoring systems can improve prognostication of MDS.30,56 Solary and coworkers46 have proposed a new prognostic score for CMML that includes not only age and hematologic parameters, but also ASXL1 mutations status. Under the aegis of the MDS Foundation, the International Working Group for Prognosis in MDS has started a research project aimed to develop a prognostic scoring system (IPSS-Mol) that includes clinical, hematologic, and molecular parameters. Our preliminary evidence suggests that parameters such as hemoglobin level, blast count, and high-risk cytogenetic abnormalities will continue to retain strong independent prognostic value (M.C., L.M., and M.G.D.P., unpublished data, August 8, 2013). Additional parameters that may significantly contribute to a refined risk assessment of MDS include gene expression profiling-based signatures.107
Finally, characterization of the patient’s genome may guide therapeutic programs, and its inclusion in prospective clinical trials is therefore of crucial importance. TET2 mutations might be associated with response to hypomethylating agents,52 whereas U2AF1 mutations would independently predict for poor outcome after allogeneic stem cell transplantation.108 There is considerable therapeutic potential for epigenetic-targeted therapies in AML,109 and this may be also true in MDS. Several drugs that target the spliceosome are being investigated for potential use in various malignancies,110 whereas drugs targeting oncogenic Ras signaling111 might be useful in many myeloid neoplasms. Case reports suggest that ruxolitinib may be effective in CNL associated with CSF3R mutation34 and in chronic eosinophilic leukemia associated with a PCM1-JAK2 fusion gene.112 More generally, the identification of the biologic pathways that are activated by mutation might allow personalized treatment in the individual patient with myelodysplasia. It should also be considered that characterization of the patient’s genome before and after treatment may allow a correct assessment of response, in particular, the impact of treatment on clonal pattern of hematopoiesis.
For the above expectations to be realized, functional studies of the mutant genes and prospective clinical trials that include the molecular characterization of the patient’s genome are now needed.
Acknowledgments
The authors thank the many colleagues of the Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium and those of the Associazione Italiana per la Ricerca sul Cancro (AIRC) Gruppo Italiano Malattie Mieloproliferative (AGIMM).
The studies on the genetic basis of myeloid neoplasms conducted at the Department of Hematology Oncology, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, and the Department of Molecular Medicine, University of Pavia, were supported by grants from AIRC, Fondazione Cariplo, Fondo per gli Investimenti della Ricerca di Base (FIRB, project RBAP11CZLK), and Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 2010-2011) (to M.C.) and Fondazione Berlucchi (to M.G.D.P.). In particular, M.C. acknowledges funding from the AIRC Special Program “Molecular Clinical Oncology 5 per mille” (project 1005).
Authorship
Contribution: M.C. conceived this review article, analyzed the literature, wrote the manuscript, and prepared the illustrations; and M.G.D.P. and L.M. analyzed the literature and contributed to the manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.
Appendix: Definitions for basic terms used in studies of the genetic basis of myeloid neoplasms
| Clone: A group of cells that derive from a common parent cell and share its genome. |
| • Malignant clone: A clone generated by the occurrence in the parent cell of a somatic mutation that alters the cell biology and function. |
| • Founding or initiating clone: A malignant clone generated by a founding somatic mutation. |
| • Subclone: A clone generated by the occurrence of an additional driver mutation in a cell of an already established clone. |
| • Clonal architecture: Clonal composition of a neoplasm based on its clonal origin and subclonal evolution. |
| • Clonal dominance: A condition in which most cells of a tissue belong to a clone. In myelodysplastic syndromes, about 85-90% of bone marrow cells are clonally derived. |
| • Subclonal evolution or expansion. The process by which the founding malignant clone generates subclones through the acquisition of additional driver mutations. In some patients, the order of genetic changes, i.e., the temporal order of acquisition of driver mutations, can be inferred by means of massive parallel sequencing though calculation of the mutant allele burden or variant allele frequency (VAF). |
| • Intraclonal (intratumoral) heterogeneity. A founding malignant clone that has undergone subclonal evolution is no longer monoclonal in the strict sense, but is instead a mosaic of several clones/genomes with different sets of somatic mutations. |
| Mutation: A change of the nucleotide sequence of the genome. |
| • Germline mutation: A mutation that is inherited though a germ cell (oocyte or spermatozoon) at the time of conception, and is therefore present in all cells of a developed body. |
| • Somatic mutation: A mutation that occurs in a non-germ cell of a body after conception (the ancient Greek somatikos means “of the body”). |
| • Nonsynonymous mutation: A nucleotide mutation that alters the amino acid sequence of a protein. |
| • Driver mutation: A mutation that causes a selective advantage in a cell with capacity for self-renewal, leading to formation of a clone of mutated cells. |
| − Founding or initiating driver mutation: A driver mutation that gives rise to the initial clone of a malignancy. |
| − Subclonal or cooperating driver mutation: A driver mutation that occurs in a cell of an already established clone and generates a subclone carrying both the founding and the newly acquired mutation. |
| • Background or passenger mutation: A mutation that occurs in a tissue before neoplastic transformation and has no pathophysiological significance. In most tissues, including hematopoietic stem cells, the number of passenger mutations is a function of age. Whole genome sequencing studies have shown that in hematopoietic stem cells, their number may range from about 100 to 1000, depending on age. When an initiating driver mutation gives rise to a malignant clone (neoplastic transformation), all background or passenger mutations present at that time are captured and carried forward. Additional passengers mutations can be captured during subclonal evolution. |
| Mutant allele burdenorVAF: The relative proportion of a mutant or variant allele (i.e., the allele carrying a somatic mutation) in a tissue or tumor sample. The mutant allele burden can be estimated using various approaches: i) by means of allele-specific quantitative PCR [e.g., the procedure employed for estimating the proportion of JAK2 (V617F)-mutant alleles in granulocytes from a patient with polycythemia vera]; or ii) more directly by assessing variant and wild-type reads using next-generation sequencing. A mutant allele burden or VAF of about 50% in regions of diploid DNA content in a homogeneous cell population (e.g., circulating granulocytes in myeloid neoplasms) suggest a fully clonal population of cells that are heterozygous for the mutation. In myeloid neoplasms, the mutant allele burden in a bone marrow sample is most commonly between 40 and 50% due to the presence of non-myeloid cells. |
| Variant-allele clusters: Group of mutation with similar VAF, potentially belonging to the same clone or subclone. |
| Whole exome sequencing: A procedure that allows to sequence all the coding regions (exomes) of a genome. |
| Whole genome sequencing: A procedure that allows to sequence the coding and non coding regions of a genome. |
| Clone: A group of cells that derive from a common parent cell and share its genome. |
| • Malignant clone: A clone generated by the occurrence in the parent cell of a somatic mutation that alters the cell biology and function. |
| • Founding or initiating clone: A malignant clone generated by a founding somatic mutation. |
| • Subclone: A clone generated by the occurrence of an additional driver mutation in a cell of an already established clone. |
| • Clonal architecture: Clonal composition of a neoplasm based on its clonal origin and subclonal evolution. |
| • Clonal dominance: A condition in which most cells of a tissue belong to a clone. In myelodysplastic syndromes, about 85-90% of bone marrow cells are clonally derived. |
| • Subclonal evolution or expansion. The process by which the founding malignant clone generates subclones through the acquisition of additional driver mutations. In some patients, the order of genetic changes, i.e., the temporal order of acquisition of driver mutations, can be inferred by means of massive parallel sequencing though calculation of the mutant allele burden or variant allele frequency (VAF). |
| • Intraclonal (intratumoral) heterogeneity. A founding malignant clone that has undergone subclonal evolution is no longer monoclonal in the strict sense, but is instead a mosaic of several clones/genomes with different sets of somatic mutations. |
| Mutation: A change of the nucleotide sequence of the genome. |
| • Germline mutation: A mutation that is inherited though a germ cell (oocyte or spermatozoon) at the time of conception, and is therefore present in all cells of a developed body. |
| • Somatic mutation: A mutation that occurs in a non-germ cell of a body after conception (the ancient Greek somatikos means “of the body”). |
| • Nonsynonymous mutation: A nucleotide mutation that alters the amino acid sequence of a protein. |
| • Driver mutation: A mutation that causes a selective advantage in a cell with capacity for self-renewal, leading to formation of a clone of mutated cells. |
| − Founding or initiating driver mutation: A driver mutation that gives rise to the initial clone of a malignancy. |
| − Subclonal or cooperating driver mutation: A driver mutation that occurs in a cell of an already established clone and generates a subclone carrying both the founding and the newly acquired mutation. |
| • Background or passenger mutation: A mutation that occurs in a tissue before neoplastic transformation and has no pathophysiological significance. In most tissues, including hematopoietic stem cells, the number of passenger mutations is a function of age. Whole genome sequencing studies have shown that in hematopoietic stem cells, their number may range from about 100 to 1000, depending on age. When an initiating driver mutation gives rise to a malignant clone (neoplastic transformation), all background or passenger mutations present at that time are captured and carried forward. Additional passengers mutations can be captured during subclonal evolution. |
| Mutant allele burdenorVAF: The relative proportion of a mutant or variant allele (i.e., the allele carrying a somatic mutation) in a tissue or tumor sample. The mutant allele burden can be estimated using various approaches: i) by means of allele-specific quantitative PCR [e.g., the procedure employed for estimating the proportion of JAK2 (V617F)-mutant alleles in granulocytes from a patient with polycythemia vera]; or ii) more directly by assessing variant and wild-type reads using next-generation sequencing. A mutant allele burden or VAF of about 50% in regions of diploid DNA content in a homogeneous cell population (e.g., circulating granulocytes in myeloid neoplasms) suggest a fully clonal population of cells that are heterozygous for the mutation. In myeloid neoplasms, the mutant allele burden in a bone marrow sample is most commonly between 40 and 50% due to the presence of non-myeloid cells. |
| Variant-allele clusters: Group of mutation with similar VAF, potentially belonging to the same clone or subclone. |
| Whole exome sequencing: A procedure that allows to sequence all the coding regions (exomes) of a genome. |
| Whole genome sequencing: A procedure that allows to sequence the coding and non coding regions of a genome. |