Key Points
Tolerance induced in the absence of CD47 signaling.
C57BL/6 Rag2−/−γc−/−CD47−/− mice humanized without GVHD.
Abstract
The use of C57BL/6 Rag2−/−γc−/− mice as recipients for xenotransplantation with human immune systems (humanization) has been problematic because C57BL/6 SIRPα does not recognize human CD47, and such recognition is required to suppress macrophage-mediated phagocytosis of transplanted human hematopoietic stem cells (HSCs). We show that genetic inactivation of CD47 on the C57BL/6 Rag2−/−γc−/− background negates the requirement for CD47-signal recognition protein α (SIRPα) signaling and induces tolerance to transplanted human HSCs. These triple-knockout, bone marrow, liver, thymus (TKO-BLT) humanized mice develop organized lymphoid tissues including mesenteric lymph nodes, splenic follicles and gut-associated lymphoid tissue that demonstrate high levels of multilineage hematopoiesis. Importantly, these mice have an intact complement system and showed no signs of graft-versus-host disease (GVHD) out to 29 weeks after transplantation. Sustained, high-level HIV-1 infection was observed via either intrarectal or intraperitoneal inoculation. TKO-BLT mice exhibited hallmarks of human HIV infection including CD4+ T-cell depletion, immune activation, and development of HIV-specific B- and T-cell responses. The lack of GVHD makes the TKO-BLT mouse a significantly improved model for long-term studies of pathogenesis, immune responses, therapeutics, and vaccines to human pathogens.
Introduction
The narrow species tropism of HIV prevents direct in vivo studies in animal models. Simian immunodeficiency virus (SIV) or SIV/HIV chimeric virus infection of rhesus macaques has long served as a surrogate model for HIV infection in humans but has limitations, including cost, availability, and outbred genetics. Differences between the immune systems of macaques and humans, as well as substantial variation between the HIV and SIV genomes, also make the extrapolation of findings to human cohorts challenging. Thus, it is desirable to develop a mouse model of HIV infection. The first successful HIV infections in mice used immunodeficient SCID mice reconstituted with human immune cells.1-3 The best current methods to produce humanized mice include hematopoietic stem (HSC)/progenitor cell injection to produce human immune system (HIS) mice,4-8 transplantation of human thymus and liver under the kidney capsule to produce Thy/Liv mice,9 or a combination of these methods to produce bone marrow/liver/thymus (BLT) mice.10,11 In BLT mice, injected HSCs repopulate the previously irradiated bone marrow niche and produce high-level systemic reconstitution of all human leukocyte lineages. The implantation of thymus and liver tissue under the kidney capsule, to produce a thymic organoid, provides a thymic environment for T-cell precursors to be selected in the context of human leukocyte antigens (HLAs) to produce HLA-restricted functional T cells in the periphery.
Currently favored mouse strains for BLT humanization are NOD/SCID-based strains, which have multiple immunological defects including a lack of B and T cells, reduced natural killer functionality, absence of complement activity, and a xenotransplantation-tolerant phagocytic compartment. This strain’s receptiveness to human xenografts can be further increased by the disruption of the common γ chain (γc) gene,4,7 which eradicates multiple cytokine signaling pathways.12 Deletion of the γc gene has the added benefits of preventing development of thymomas common in NOD mice13 and of delaying the onset of graft-versus-host disease (GVHD), which remains a shortcoming in this model.14
Developing a BLT model on the C57BL/6 background is attractive because of the wide availability of transgenes and gene inactivations in these mice, its relative radiation resistance, and its intact complement system. However, previous efforts to humanize the immunodeficient C57BL/6 Rag2−/−γc−/− (DKO) strain have proven it to be nonpermissive to xenotransplantation.15 In contrast to NOD mice, C57BL/6 mice express a form of the signal recognition protein α (SIRPα) receptor that does not recognize human CD47.16,17 SIRPα-CD47 recognition transmits antiphagocytic signals necessary to prevent engulfment and clearance of transplanted human cells by macrophages.18,19 Various methods have been used to surmount the problem of mouse SIRPα-human CD47 incompatibility to produce humanized mice in non-NOD strains. Legrand et al20 showed that transgenic expression of mouse CD47 on human HSC facilitated engraftment in a BALB/c Rag2−/−γc−/− HIS model. Strowig et al21 addressed this same issue by introducing transgenic human SIRPα onto a mixed 129J/BALB/c Rag2−/−γc−/− background, and recently Yamauchi et al17 successfully surmounted this obstacle in a HIS model using DKO mice expressing a NOD SIRPα transgene. These studies indicate that the lack of tolerization of the phagocytic compartment in C57BL/6 mice is an important barrier to successful humanization.
In the current study, we took a different approach based on results demonstrating that phagocytes developing in a CD47-negative environment become tolerized to cells that do not express CD47.22 Phagocytic tolerance to xenotransplantation was induced by disrupting endogenous CD47 expression to create C57BL/6 Rag−/−γc−/−CD47−/− (TKO) mice. We show that these triple knockout BLT-humanized (TKO-BLT) mice have excellent long-term HIS reconstitution with little or no GVHD. Furthermore, TKO-BLT mice were susceptible to HIV infection and developed virus-specific immune responses. These results indicate that the TKO-BLT mouse has advantages over current humanized mouse models and is a valuable tool for studying human pathogens.
Materials and methods
Mice
C57BL/6 Rag2−/−γc−/− mice have been described previously.23-25 CD47-null B6.129-CD47tm1Fpl/J mice (The Jackson Laboratory, Bar Harbor, ME) were crossed with C57BL/6 Rag2−/−γc−/− females, and F1Rag2+/−γc-/YCD47+/− males were backcrossed with Rag2−/−γc−/− females. Mating of F2Rag2−/−γc−/−CD47+/− females and Rag2−/−γc-/YCD47+/− males produced the Rag2−/−γc−/−CD47−/− (TKO) strain. Animals were housed under specific pathogen-free conditions. Experiments were performed in accordance with the regulations and guidelines of the Animal Care and Use Committee of the Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Humanization of mice
Six- to 10-week-old mice were BLT-humanized10,11 using 17- to 22-week gestation human thymus and liver (Advanced Bioscience Resource, Alameda, CA). To prevent damage to the gut,26 mice were gavaged with 4 mg ascorbic acid in water prior to 5 or 7.50 Gy whole-body irradiation (5 Gy was found empirically to provide successful niche conditioning with lower lethality than 7.5 Gy). Approximately 1-mm3 sections of liver and thymus were implanted under the kidney capsule. The remaining liver was digested for 1 hour in RPMI-1640 (Invitrogen, Grand Island, NY) with 10% fetal bovine serum (FBS; JR Scientific, Woodland, CA), 1 mg/mL collagenase/dispase (Roche, Basel, Switzerland), and 0.5 U/mL DNase I (New England Biolabs, Ipswich, MA). Liver HSCs were purified using a human CD34 Microbead Kit (Miltenyi, Cologne, Germany) and between 1 and 2 × 106 CD34+ cells (we used the maximum number of cells depending on the amount of donor tissue) injected intravenously into mice along with 5 × 105 TKO mouse bone marrow cells.
Isolation of human leukocytes
Blood leukocytes were purified using RBC Lysis Buffer (BioLegend). Thymic organoid and mesenteric lymph node leukocytes were obtained by passage through 70-μm filters. Spleen cells were similarly obtained followed by residual blood cell lysis with ammonium chloride potassium lysis buffer (NH4Cl 0.15 M, KHCO3 10 mM, EDTA 0.1 M). Gut leukocytes were harvested by combining supernatants obtained from phosphate-buffered saline–flushed intestines that had been incubated in 3% FBS, 1 mM dithiothreitol, and 1 mM EDTA phosphate-buffered saline solution with gentle rocking at 37°C for 30 minutes, followed by incubation in RPMI-1640 with 10% FBS, 60 μg/mL collagenase D (Roche), and 10 U/mL DNase I for an additional hour.
Phenotypic analysis
Directly conjugated anti-human CD45-V500 (BD Biosciences, San Jose, CA) or CD45-eFluor450 (eBioscience, San Diego, CA) antibody (clone HI30) was used to specifically identify human leukocytes within samples. Additional antibodies used for immunophenotyping included anti-human CD8-APC eFluor780, CD14-PE-Cy7, CD127-PE-Cy7, Foxp3-eFluor 660, B220-FITC, CD4-PE-Cy7, and CD45RA-PE (all from eBioscience); CD33-PE (Miltenyi); CD56-A700, HLA-DR-A700, CD4-PB, and CD38-A700 (all from BioLegend); and CD3-V450, CD4-APC, CD19-PerCP-Cy5.5, CD11c-APC, lineage FITC, CD123-PE, CD25-FITC, CD34-APC, CD38-PE, CCR7-PE-Cy7, and Ki67-FITC (all from BD Biosciences).
Immunohistochemistry
Spleen and gut tissues were fixed in 10% formalin and processed with a Sakura VIP-5 Tissue Tek using a graded series of ethanol, xylene, and Paraplast X-tra (Sigma-Aldrich, St Louis, MO). Embedded tissues were sectioned at 5 μm and dried overnight at 42°C prior to staining. Antibodies used were rabbit anti-human CD20 (RB-9013; Thermo Scientific, Waltham, MA), rabbit anti-human CD3 (Ventana Medical Systems, Tucson, AZ), rabbit anti-human CD4 (eBioscience), and rabbit anti-human HLA class I (Epitomics, Burlingame, CA). Tissues were then processed for immunohistochemistry using the Discovery XT automated processor with a DAPMap kit (Ventana Medical Systems). Organoids were processed as previously described27 and stained with mouse anti-HIV p24 (M085701; Dako), rabbit anti-Iba1 immunoglobulin G (IgG) (018-19741; Wako), A555-conjugated goat anti-mouse IgG, and A488-conjugated donkey anti-rabbit IgG (Invitrogen). Slides were mounted with ProLong Gold antifade reagent with 4,6 diamidino-2-phenylindole (Invitrogen).
HIV infection
HIV-1JR-CSF stocks were prepared by Lipofectamine-2000 transfection of 293FT cells (Invitrogen) with the infectious molecular clone pYK-JRCSF obtained from Drs Irvin S. Y. Chen and Yoshio Koyanagi through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID. TZM-bl (JC53-bl) reporter cells (NIH AIDS Research and Reference Reagent Program from Drs John Kappes and Xiaoyun Wu and Tranzyme, Inc.) were used to determine stock concentrations. Prior to mucosal infection, mice received a 100-μL saline enema and were swabbed with a miniature cotton-tipped applicator (Puritan, Guilford, ME) before deposition of approximately 1 × 105 tissue culture infectious units (TCIU) of HIV-1JR-CSF in a volume of 15 to 40 μL. Mice were inoculated daily over a 4-day period. Intraperitoneal infections were 1-time, 100-μL injections. Infection levels in plasma were determined by p24 enzyme-linked immunosorbent assay (ELISA; Advanced Bioscience Laboratories, Rockville, MD) or Cobas Amplicor reverse transcription-polymerase chain reaction assay (Roche).
Anti-HIV antibody ELISA
Immulon 2 HB ELISA plates were coated with 2 μg/mL HIVJR-CSF gp120 (eEnzyme, Gaithersburg, MD) overnight at room temperature and blocked before the addition of diluted TKO-BLT plasma. Bound antibodies were detected using goat anti-human IgA/M/G horseradish peroxidase (AbD Serotec, Raleigh, NC) or goat anti-human IgG horseradish peroxidase (Millipore, Billerica, MA). All buffers were from Immunochemistry Technologies (Bloomington, MN). Assays were developed using SureBlue TMB and Stop solution (KPL, Gaithersburg, MD).
Generation of B-cell lines
Isogenic splenocytes, harvested from a humanized mouse, were infected in the presence of cyclosporine A with Epstein-Barr virus–containing supernatants of the B95-8 cell line.28
IFN-γ ELISpot assay
Isogenic B-cell lines were resuspended at 2 × 106 cells/mL in RAB-10 (RPMI-1640, 10% human AB serum [Invitrogen], 2mM l-glutamine, and penicillin-streptomycin [Lonza, Basel, Switzerland]) and pulsed for 1 hour at 37°C with pools of HIV-1 peptides (Sigma-Aldrich) at 25 μg/mL. Each pool contained 8 consecutive peptides from a set of 18-mer peptides overlapping by 11 amino acids that encompassed the HIV-1JR-CSF proteome. ACK-lysed splenocytes were pooled from 3 mice and depleted of murine leukocytes and immature erythrocytes using CD45 and Ter-119 microbeads over a LD depletion column (Miltenyi). B-cell lines and purified human splenocytes were combined at a ratio of 2:1 in RAB-10 on an anti–interferon-γ–coated Immobilon-P filter plate (Millipore) and incubated at 37°C for 48 hours before colorimetric development. Capture and detection antibodies were from the Human IFN-γ ELISPOT Ready-SET-Go kit (eBioscience). Positive wells had >50 spots/1 × 106 cells and >2× the spots in vehicle-only wells.
Statistical analysis
Statistical calculations were performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
The methods for antibody detection, mitogenic stimulation, mixed lymphocyte reaction, and the complement fixation assay are described in the supplemental Methods on the Blood Web site.
Results
Reconstitution of TKO-BLT mice
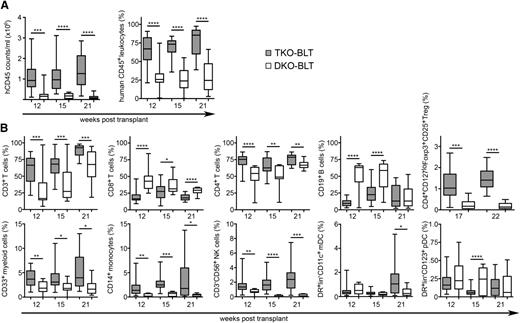
To determine whether inactivation of the CD47 gene would provide immunological tolerance and allow reconstitution of human hematopoietic systems in immunodeficient mice, we compared reconstitution of C57BL/6 Rag2−/−γc−/−CD47−/− triple knockout (TKO) mice with C57BL/6 Rag2−/−γc−/−CD47+/− double knockout (DKO) mice. The mice were BLT humanized,10,11 and blood was analyzed for reconstitution of human cells by flow cytometric analysis from 12 to 21 weeks after transplantation. As expected from previous studies, the reconstitution of DKO-BLT mice as measured by expression of human CD45 was quite poor even at 21 weeks after transplantation (Figure 1A). In contrast, the TKO-BLT mice averaged approximately 1 × 106 human leukocytes/mL blood by as early as 12 weeks after transplantation (Figure 1A). Levels remained stable or improved through 21 weeks, when an average of >80% of the leukocytes in the peripheral circulation were of human origin (Figure 1A).
TKO-BLT mice have significantly better human peripheral blood reconstitution than DKO-BLT mice. Peripheral blood was collected and analyzed by flow cytometry at the indicated time points to assess the effect of CD47 expression on (A) overall levels of human CD45+ cell reconstitution and (B) the frequencies of the major human leukocyte subsets in the blood. Representative batches of ≥9 mice are shown at each time point. Boxes indicate median and interquartile range. Whiskers are minimum and maximum values. Unpaired 2-tailed t tests were performed between groups at each time point. ****P < .0001; ***P < .001; **P < .01; *P < .05.
TKO-BLT mice have significantly better human peripheral blood reconstitution than DKO-BLT mice. Peripheral blood was collected and analyzed by flow cytometry at the indicated time points to assess the effect of CD47 expression on (A) overall levels of human CD45+ cell reconstitution and (B) the frequencies of the major human leukocyte subsets in the blood. Representative batches of ≥9 mice are shown at each time point. Boxes indicate median and interquartile range. Whiskers are minimum and maximum values. Unpaired 2-tailed t tests were performed between groups at each time point. ****P < .0001; ***P < .001; **P < .01; *P < .05.
The human cells were also analyzed with markers defining distinct cell subsets. By 12 weeks after transplantation, the ratio of total T cells (CD3+) to B cells (CD19+) in the TKO-BLT mice had normalized to values similar to human blood, and all subsets were easily detectable (Figure 1B). In contrast, total T-cell frequencies were low in the DKO-BLT mice until 21 weeks after transplantation, when the ratio of T cells to B cells finally normalized (Figure 1B), albeit at very low absolute numbers (Figure 1A). In addition, several cell subsets were very low or absent in the poorly reconstituted DKO-BLT mice. The robust long-term reconstitution of circulating human cells in the TKO-BLT mice compared with the DKO-BLT mice indicated that genetic inactivation of CD47 had tolerized the mice to xenotransplantation with human cells and tissue. Furthermore, the results indicated that SIRPα-CD47–negative signaling, normally required to prevent macrophage phagocytosis, was not required for innate tolerance in CD47−/− mice.
Reduced multipotent phenotype HSC/multipotent progenitor engraftment in DKO-BLT bone marrow
To determine whether the low-level reconstitution in the DKO-BLT mice was a result of poor engraftment of the human thymus and liver tissues in the kidney capsules, the human thymic organoids were harvested and analyzed. Gross examination showed highly vascularized, healthy organoids in both TKO-BLT and DKO-BLT mice (Figure 2A). Additionally, flow cytometric analysis revealed no differences in the levels of CD4 or CD8 single or double positive thymocytes in DKO-BLT and TKO-BLT organoids (Figure 2B). Next, bone marrow was evaluated for hematopoietic stem/progenitor cells. Compared with DKO-BLT mice, the TKO-BLT mice had significantly higher levels of human leukocytes (hCD45+) in the bone marrow (Figure 2C), with more than fourfold higher levels of CD45loB220−CD34+CD38− cells, which include HSCs and multipotent progenitors (MPPs) (Figure 2C). Thus, although the transplanted organoids appeared equivalent between DKO-BLT and TKO-BLT mice, only the TKO-BLT mice had high levels of the multipotent bone marrow HSC/MPPs required for robust reconstitution.
CD47-expressing mice have normal thymic organoid engraftment but reduced pluripotent HSCs/MPPs in bone marrow. (A) Representative implants and (B) flow cytometric analysis of single- and double-positive thymocytes in TKO-BLT and DKO-BLT mice at 20 to 22 weeks posttransplant (wpt). (C) Frequency of human CD45+ cells within the FSC/SSC live gate and CD38− HSCs/MPPs (gated on CD45loB220−CD34+) in bone marrow of 22 wpt DKO-BLT mice compared with TKO-BLT mice at 17 to 25 wpt. Boxes indicate median and interquartile range. Whiskers are minimum and maximum values. Unpaired 2-tailed t tests were performed between groups. ****P < .0001; *P < .05.
CD47-expressing mice have normal thymic organoid engraftment but reduced pluripotent HSCs/MPPs in bone marrow. (A) Representative implants and (B) flow cytometric analysis of single- and double-positive thymocytes in TKO-BLT and DKO-BLT mice at 20 to 22 weeks posttransplant (wpt). (C) Frequency of human CD45+ cells within the FSC/SSC live gate and CD38− HSCs/MPPs (gated on CD45loB220−CD34+) in bone marrow of 22 wpt DKO-BLT mice compared with TKO-BLT mice at 17 to 25 wpt. Boxes indicate median and interquartile range. Whiskers are minimum and maximum values. Unpaired 2-tailed t tests were performed between groups. ****P < .0001; *P < .05.
Development of peripheral lymphoid tissues in TKO-BLT mice
Unreconstituted TKO mice fail to develop peripheral lymph nodes, and their spleens and peripheral tissues are devoid of lymphocytes (data not shown). Once reconstituted as BLT mice, they developed extensive, sustained levels of multilineage hematopoietic reconstitution in bone marrow, spleen, and mesenteric lymph nodes. Similar to peripheral blood, all major cell subsets including CD4+ and CD8+ T cells, CD19+ B cells, CD14+ monocytes, and both myeloid and plasmacytoid dendritic cells (DCs) were detected in peripheral tissues (Figure 3A). Additionally, T cells in the spleen displayed a primarily naïve phenotype (Figure 3B). All peripheral tissues examined displayed multilineage human leukocyte reconstitution at frequencies comparable to those found in other highly reconstituted BLT models.10,11,29 Immunohistochemical analysis of the spleens revealed white pulp areas organized into follicles comprised of human cells (HLA class I+) with mature B cells (CD20+) and T cells (CD3+) (Figure 3C). As in other xenoreconstituted mice, the T and B cells are intermixed rather than divided into periarteriolar T-cell sheaths with adjacent B-cell primary follicles. Thus, the tissues of TKO-BLT mice not only contained appropriate human immune cell subsets, but the cells were able to organize into immunological structures including mesenteric lymph nodes and splenic follicles.
Peripheral tissue reconstitution and splenic structure in TKO-BLT mice. (A) Representative flow cytometry plots with means ± standard deviations illustrate the major human immune cell subset frequencies (from left to right: CD4+ and CD8+ T cells; CD3+ T cells and CD19+ B cells; CD33+ myeloid cells and CD14+ monocytes; CD11c+ myeloid DC and CD123+ plasmacytoid DCs) in bone marrow (n = 6), mesenteric lymph node (n = 4), and spleen (n = 6) and (B) the splenic T-cell subsets (n = 6) (naïve CD45RA+CCR7+; Tcm CD45RA−CCR7+; Tem CD45RA−CCR7−; TEMRA CD45RA+CCR7−) in TKO-BLT mice. Parental gates are listed at the top of each column. (C) Histological staining depicting the characteristic morphology of TKO-BLT spleens, the presence of HLA class I–expressing human cells, and follicle-like structures containing mature CD20+ B cells and CD3+ T cells. Olympus DP72 camera and BX51 microscope. Hematoxylin and eosin ×40, ×200, and immunostaining ×200 magnification; numerical aperture, 0.75. Acquisition software: Olympus cell Sens Dimension 1.4.1.
Peripheral tissue reconstitution and splenic structure in TKO-BLT mice. (A) Representative flow cytometry plots with means ± standard deviations illustrate the major human immune cell subset frequencies (from left to right: CD4+ and CD8+ T cells; CD3+ T cells and CD19+ B cells; CD33+ myeloid cells and CD14+ monocytes; CD11c+ myeloid DC and CD123+ plasmacytoid DCs) in bone marrow (n = 6), mesenteric lymph node (n = 4), and spleen (n = 6) and (B) the splenic T-cell subsets (n = 6) (naïve CD45RA+CCR7+; Tcm CD45RA−CCR7+; Tem CD45RA−CCR7−; TEMRA CD45RA+CCR7−) in TKO-BLT mice. Parental gates are listed at the top of each column. (C) Histological staining depicting the characteristic morphology of TKO-BLT spleens, the presence of HLA class I–expressing human cells, and follicle-like structures containing mature CD20+ B cells and CD3+ T cells. Olympus DP72 camera and BX51 microscope. Hematoxylin and eosin ×40, ×200, and immunostaining ×200 magnification; numerical aperture, 0.75. Acquisition software: Olympus cell Sens Dimension 1.4.1.
Development of gut-associated lymphoid tissue in TKO-BLT mice
A critical feature of the immune system necessary to study natural transmission of agents such as HIV is gut-associated lymphoid tissue (GALT). To determine whether GALT developed in TKO-BLT mice, histological sections of large and small intestines were analyzed for the presence of HLA class I+ human cells and for human CD4+ cells. Human cells were rarely observed in sections from DKO-BLT mice (Figure 4A, top). In contrast, both large and small intestines from 3 of 3 TKO-BLT mice tested had human cell reconstitution including CD4+ cells, which are targets for HIV infection (Figure 4A, bottom). We obtained sufficient numbers of cells from the GALT of a TKO-BLT mouse at 19 weeks after transplantation for flow cytometric subset analysis, which revealed the presence of human T cells of both CD4+ and CD8+ subsets (Figure 4B). As in human GALT,30,31 most of the T cells appeared activated (HLA-DRhi) and had either an effector (CCR7−/CD45RA−) or central memory (CCR7+/CD45RA−) phenotype (Figure 4B), although the CCR7 populations were not distinct. CD11c+ myeloid DCs were also observed as well as a small proportion of CD123+ plasmacytoid DCs (Figure 4B).
Human GALT reconstitution in TKO-BLT mice. (A) Representative sections of 22 wpt small and large intestine from DKO-BLT (n = 3) and TKO-BLT (n = 3) mice were stained for the presence of human cells (HLA class I+) and for HIV-susceptible CD4+ cells. Magnified ×200; numerical aperture, 0.75; Olympus DP72 camera and BX51 microscope. Acquisition software: Olympus cell Sens Dimension 1.4.1. (B) Flow cytometry plots showing cellular subsets and T-cell activation status in GALT from a TKO-BLT mouse at 19 wpt (left to right: CD3+ T cells; CD4+/CD8+ T cells; Tcm [CD45RA−CCR7+] and Tem [CD45RA−CCR7−] subsets; activated HLA-DR+ T cells; CD11c+ myeloid and CD123+ plasmacytoid DCs). Parent gates are listed above each panel. Gating was based on distinct populations in mesenteric lymph nodes that were run on the same day.
Human GALT reconstitution in TKO-BLT mice. (A) Representative sections of 22 wpt small and large intestine from DKO-BLT (n = 3) and TKO-BLT (n = 3) mice were stained for the presence of human cells (HLA class I+) and for HIV-susceptible CD4+ cells. Magnified ×200; numerical aperture, 0.75; Olympus DP72 camera and BX51 microscope. Acquisition software: Olympus cell Sens Dimension 1.4.1. (B) Flow cytometry plots showing cellular subsets and T-cell activation status in GALT from a TKO-BLT mouse at 19 wpt (left to right: CD3+ T cells; CD4+/CD8+ T cells; Tcm [CD45RA−CCR7+] and Tem [CD45RA−CCR7−] subsets; activated HLA-DR+ T cells; CD11c+ myeloid and CD123+ plasmacytoid DCs). Parent gates are listed above each panel. Gating was based on distinct populations in mesenteric lymph nodes that were run on the same day.
No evident GVHD in TKO-BLT mice
To determine whether GVHD developed in TKO-BLT mice, individual mice reconstituted from 5 different human donors were followed over time. At 20 weeks after transplantation, there was no evidence of GVHD (conjunctivitis, blepharitis, alopecia, wasting disease, ruffled fur, or death) in any of the 75 mice (Table 1). At 25 weeks after transplantation, 42 mice remained under study, and 1 mouse had died for undetermined reasons. Although this animal never displayed clinical signs, the animal was not recovered for postmortem analysis so the possibility that it died of GVHD cannot be excluded. However, none of the 17 mice remaining under observation at 29 weeks after transplantation displayed any clinical signs of GVHD. By comparison, by 29 weeks after transplantation, approximately one-third of NOD/SCID γc−/− mice died from lethal GVHD.14 Thus, the TKO-BLT mice are highly resistant to development of GVHD.
Incidence of GVHD clinical signs including conjunctivitis, blepharitis, alopecia, wasting disease, ruffled fur, or death
| Group . | 20 wpt . | 23 wpt . | 25 wpt . | 26 wpt . | 27 wpt . | 28 wpt . | 29 wpt . |
|---|---|---|---|---|---|---|---|
| 1 | 0/17 | 0/9* | 0/5* | 0/5 | 0/5 | 0/5 | 0/5 |
| 2 | 0/19 | 0/10* | 0/10 | * | |||
| 3 | 0/6 | 0/6 | 0/6 | * | |||
| 4 | 0/21 | 0/21 | 0/17* | 0/17 | 0/14* | 0/14 | 0/12* |
| 5 | 0/12 | 0/8* | 1†/4 | 1/4 | * | ||
| Total | 0/75 | 0/54 | 1†/42 | 1†/26 | 0/19 | 0/19 | 0/17 |
| Group . | 20 wpt . | 23 wpt . | 25 wpt . | 26 wpt . | 27 wpt . | 28 wpt . | 29 wpt . |
|---|---|---|---|---|---|---|---|
| 1 | 0/17 | 0/9* | 0/5* | 0/5 | 0/5 | 0/5 | 0/5 |
| 2 | 0/19 | 0/10* | 0/10 | * | |||
| 3 | 0/6 | 0/6 | 0/6 | * | |||
| 4 | 0/21 | 0/21 | 0/17* | 0/17 | 0/14* | 0/14 | 0/12* |
| 5 | 0/12 | 0/8* | 1†/4 | 1/4 | * | ||
| Total | 0/75 | 0/54 | 1†/42 | 1†/26 | 0/19 | 0/19 | 0/17 |
Numbers in groups decrease or groups are dropped when animals are killed for analysis or infected with HIV.
Death due to undetermined cause; GVHD not positively excluded as cause.
GALT reconstitution supports mucosal HIV infection
Natural HIV transmission occurs primarily via the mucosa, so it was important to determine whether TKO-BLT GALT would support mucosal infection. As expected, none of the 8 DKO-BLT control mice in the experiment supported mucosal infection. Forty-eight TKO-BLT mice were inoculated intrarectally with ∼105 TCIU of HIVJR-CSF. Twenty-six (54%) of the mice became infected with p24 plasma titers peaking between 2 and 4 weeks postinfection (wpi) and then declining over the next several weeks (Figure 5A). In HIV+ mice, analysis of peripheral blood CD4+ and CD8+ T-cell proportions revealed a loss of CD4+ T cells with a concomitant rise in CD8+ T cells (Figure 5B), consistent with HIV infections in humans. Additionally, a significantly higher proportion of CD8+ T cells from HIV-infected mice appeared activated (CD38+) compared with uninfected mice (Figure 5B). Thus, mucosal infection of TKO-BLT mice showed the classical immunological hallmarks associated with acute HIV infection in humans.
HIV infection of TKO-BLT mice. (A-B) Data from mucosally and (C-F) intraperitoneally infected mice. (A) Viral load levels (HIV p24 ±standard error of the mean [SEM]) in plasma from HIV-infected mice collected at the indicated time points. Mice were challenged at 19 to 24 wpt. (B) CD4/CD8 T-cell ratios and frequency of activated CD8 T cells (CD38+) in mucosally challenged mice that became HIV+ or remained HIV− 4 wpi compared with prechallenge values. Unpaired 2-tailed t tests were performed between the groups at 4 wpi. (C) Viral loads in plasma as measured by p24 ELISA (±SEM) at the indicated intervals after intraperitoneal infection with 3000 or 7000 TCIU of HIVJR-CSF. Mice were challenged at 17 wpt. (D) Immunostaining for HIV p24 (red) and Iba1+ macrophages (green) in thymic organoids from representative HIV+ and HIV− TKO-BLT mice. Magnified 20×. The images were captured from a Nikon Digital Sight DS-Ri1 camera on a Nikon Eclipse 55i with a Plan Fluor 20X/0.05 objective at 20°C with Prolong gold with 4,6 diamidino-2-phenylindole and AlexaFluor 488 and 555 using NIS Elements software. Hue on the red channel was changed from 0 to 60 to make the color visible to red/green colorblinds using ACD Canvas 14 software. (E) Significant reductions in the plasma CD4 T-cell count over 4 weeks of infection (1-way analysis of variance with Tukey post-test) and the correlation between plasma p24 level and peripheral CD4 T-cell frequency and activation (CD38+) at 4 wpi. (F) Positive correlations between plasma p24 levels at 4 wpi and CD8 T-cell proliferation (Ki67+), activation (HLA-DR+), and expansion of the Tem subset. ***P < .001; **P < .01.
HIV infection of TKO-BLT mice. (A-B) Data from mucosally and (C-F) intraperitoneally infected mice. (A) Viral load levels (HIV p24 ±standard error of the mean [SEM]) in plasma from HIV-infected mice collected at the indicated time points. Mice were challenged at 19 to 24 wpt. (B) CD4/CD8 T-cell ratios and frequency of activated CD8 T cells (CD38+) in mucosally challenged mice that became HIV+ or remained HIV− 4 wpi compared with prechallenge values. Unpaired 2-tailed t tests were performed between the groups at 4 wpi. (C) Viral loads in plasma as measured by p24 ELISA (±SEM) at the indicated intervals after intraperitoneal infection with 3000 or 7000 TCIU of HIVJR-CSF. Mice were challenged at 17 wpt. (D) Immunostaining for HIV p24 (red) and Iba1+ macrophages (green) in thymic organoids from representative HIV+ and HIV− TKO-BLT mice. Magnified 20×. The images were captured from a Nikon Digital Sight DS-Ri1 camera on a Nikon Eclipse 55i with a Plan Fluor 20X/0.05 objective at 20°C with Prolong gold with 4,6 diamidino-2-phenylindole and AlexaFluor 488 and 555 using NIS Elements software. Hue on the red channel was changed from 0 to 60 to make the color visible to red/green colorblinds using ACD Canvas 14 software. (E) Significant reductions in the plasma CD4 T-cell count over 4 weeks of infection (1-way analysis of variance with Tukey post-test) and the correlation between plasma p24 level and peripheral CD4 T-cell frequency and activation (CD38+) at 4 wpi. (F) Positive correlations between plasma p24 levels at 4 wpi and CD8 T-cell proliferation (Ki67+), activation (HLA-DR+), and expansion of the Tem subset. ***P < .001; **P < .01.
HIV infection of TKO-BLT mice via the intraperitoneal route
Groups of TKO-BLT mice were infected by the intraperitoneal route with either 3000 or 7000 TCIU of HIVJR-CSF and were analyzed biweekly for infection by plasma p24 assay. All 21 of the challenged mice became infected. At the higher HIV dose, plasma levels of p24 peaked early and then declined, whereas at the lower dose, p24 levels rose more gradually and no decline in levels was observed over 8 weeks (Figure 5C). In addition to plasma virus, HIV infection was also detected by p24 immunostaining in the human thymic organoids. Of the main CD4+ HIV-susceptible cell types present in the organoid, thymocytes appeared to be the major targets for infection, as limited p24 costaining with the myeloid marker Iba1 was observed (Figure 5D). Cumulative data showed reductions in CD4+ T-cell counts in the blood of HIV-infected mice that were negatively correlated with HIV p24 levels (Figure 5E). CD4+ T-cell activation (CD38+) levels correlated with HIV p24 (Figure 5E) as did CD8+ T-cell proliferation (Ki67), activation (HLA-DR+), and effector memory (Tem) differentiation (CCR7−CD45RA−) (Figure 5F). These characteristics of CD4+ T-cell depletion and general T-cell activation are consistent with HIV infection in humans and appeared to be driven by viral burden.
TKO-BLT mice have functional immune systems
The immune systems of naive TKO-BLT mice appeared functional as evidenced by complement fixation activity, circulating IgG and IgM, and T-cell responses to both mitogenic and allogeneic stimulation (supplemental Figure 1). More importantly, we wanted to determine whether HIV-infected animals could mount virus-specific immune responses. To assess for HIV-specific humoral immunity, plasma samples from HIV-infected and uninfected mice were assayed for the presence of gp120JR-CSF-specific antibodies. HIV-specific antibodies were detected in mice using both a broadly reactive anti-human immunoglobulin and an IgG class-specific secondary antibody (Figure 6A). Thus, HIV infection provoked not only HIV-specific antibody responses but also T cell–dependent class switching to IgG.
TKO-BLT mice mount specific humoral and cellular immune responses to HIV infection. (A) Plasma collected at 8 wpi from 6 intraperitoneally infected mice injected with 3000, 7000, or 20 000 TCIU of HIVJR-CSF or 2 uninfected vehicle control injected mice was assayed by ELISA for combined IgM, IgA, or IgG antibodies or only IgG antibodies specific for HIVJR-CSF gp120 protein. Each line represents data from a single mouse. (B) At 7 wpi, 3 pooled spleens from HIV+ mice and 3 control HIV− spleens were depleted of murine leukocytes prior to ELISpot assay to determine specific cellular responses to pools of 8 overlapping HIV peptides that covered the HIVJR-CSF proteome. Positive responses (denoted by an asterisk) had >50 spots/106 splenocytes and were twofold over vehicle-only (dimethylsulfoxide) controls.
TKO-BLT mice mount specific humoral and cellular immune responses to HIV infection. (A) Plasma collected at 8 wpi from 6 intraperitoneally infected mice injected with 3000, 7000, or 20 000 TCIU of HIVJR-CSF or 2 uninfected vehicle control injected mice was assayed by ELISA for combined IgM, IgA, or IgG antibodies or only IgG antibodies specific for HIVJR-CSF gp120 protein. Each line represents data from a single mouse. (B) At 7 wpi, 3 pooled spleens from HIV+ mice and 3 control HIV− spleens were depleted of murine leukocytes prior to ELISpot assay to determine specific cellular responses to pools of 8 overlapping HIV peptides that covered the HIVJR-CSF proteome. Positive responses (denoted by an asterisk) had >50 spots/106 splenocytes and were twofold over vehicle-only (dimethylsulfoxide) controls.
To test for HIV-specific T-cell responses, splenocytes from HIV-infected and uninfected mice were stimulated by HIV peptide pools and assayed by IFN-γ ELISPOT. HIV-specific T-cell responses were observed against a broad range of peptide pools (Figure 6B). Thus, the T cells from the TKO-BLT mice were competent to respond in an antigen-specific manner to HIV infection. Because T-cell responses are dependent on antigen presentation, these results also indirectly indicated that antigen presentation in the mice was functional.
Discussion
Inhibitory signaling through the SIRPα receptor on macrophages has been shown to be critical for preventing the phagocytosis of human cell transplants in immunodeficient mice.16 However, a study using chimeric mice showed that macrophages could be tolerized by exposure to nonhematopoietic cells null for CD47 expression22 and led us to test CD47-null mice as recipients for xenotransplantation. We now demonstrate that signaling via SIRPα-CD47 is not absolutely necessary to prevent phagocytosis of xenotransplants when the macrophages develop and mature in mice with genetic inactivation of CD47. Furthermore, signaling through CD47 on transplanted human cells does not appear to be required for the homing and survival of HSCs, T-cell homeostasis, or the development of secondary lymphoid structures20 in this model.
The use of the C57BL/6 genetic background for hosting xenotransplantation with human cells has advantages over the NOD/SCID background, which is currently the most widely used mouse strain for humanization. One of the most severe drawbacks of BLT-humanized NOD/SCID γc−/− (NSG) mice is their high incidence of GVHD. Clinical signs of GVHD are apparent in 35% of NOD/SCID mice by 22 weeks after transplantation and are delayed a few weeks on the NSG background.14 There was a significant correlation between the degree of human cell reconstitution and GVHD incidence, although some mice with poor engraftment also developed the condition. We found that TKO-BLT mice reconstitute with human cells as well as NSG-BLT mice, but interestingly, TKO-BLT mice observed out to 29 weeks after transplantation developed no clinical signs of GVHD (Table 1). The lack of GVHD in TKO-BLT mice could be related to genetic inactivation of CD47 but may also be due to other genetic factors in the C57BL/6 background that differ from the NOD mouse. The DKO-BLT mice in our studies also did not develop GVHD, but they did not reconstitute well with human cells. Regardless of the reason why TKO-BLT mice do not develop GVHD, their lack of GVHD makes them an exceptional model for long-term studies.
There are additional reasons that make the C57BL/6 genetic background attractive as hosts for humanization. For example, the C57BL/6 strain is significantly more radiation resistant than NOD/SCID mice, and higher irradiation provides better conditioning to open niches for transplant engraftment with less morbidity and mortality.32,33 Also, the wide variety of gene inactivations and transgenes available on the C57BL/6 background provides a resource for genetic studies not available in other strains. Another important advantage of using C57BL/6 hosts is that their complement system is intact (supplemental Figure 1), whereas the NOD/SCID mouse has a C5 deficiency.34 Complement is a critical component of innate immunity that has antiviral activity against HIV and HIV-infected cells through the classical pathway. It has been shown that non-neutralizing antibodies that exhibit antiviral activity may mediate protection at least partially through complement activation.35 In contrast, incorporation of complement components into HIV virions enhances infection in vitro and may play important roles in pathogenesis (reviewed in Yu et al36 ). This complex role of complement in HIV infection illustrates how the TKO-BLT mouse could be a valuable model for in vivo investigations where complement is a factor.
Susceptibility to infection and immune responses to HIV in the TKO-BLT mice appeared comparable to reports of other BLT-humanized models.11,29,37 The observance of classic human hallmarks of HIV infection suggests that this model could be useful in studying various aspects of HIV pathogenesis including CD4+ T-cell depletion and immune hyperactivation. In addition, virus-specific immune responses by both B cells and T cells were detected in HIV-infected animals. Although it is difficult to compare between different assays, the antibody responses appeared to be ≥10-fold lower than in humans.38 However, the antibody responses class-switched indicating the presence of T-cell help and B-cell affinity maturation. Furthermore, T-cell responses developed toward a broad range of peptides encoded by open reading frames across the HIV genome. Whether these responses are of sufficient magnitude to allow studies of anamnestic responses in vaccinated mice is under investigation. Of course this model is not restricted to HIV studies and could be quite useful in studying or testing therapeutics or vaccines to additional human pathogens.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Suzette Priola and Leonard Evans for critical review of the paper, Dan Long and Rebecca Rosenke for expert histology, and Siddartha Jaiswal for crossing the CD47 knockout mouse with C57BL/6 Rag2−/−yc−/−.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health HIVRAD grant P01 AI104715 (to T.M.A.), the Intramural Research Program of the Medical Faculty of the University Duisburg-Essen, and funding from the Ludwig Foundation.
Authorship
Contribution: K.J.L. designed and performed experiments, analyzed and interpreted data, and wrote the paper; W.W.P. developed the TKO mouse strain and advised; R.J.M. and A.K.D. performed experiments and surgical procedures; D.S. and K.E.P. performed and interpreted immunohistology; U.D., B.R., and K.P. performed surgical procedures; C.K.C. advised on surgical procedures; T.D. and T.M.A. contributed vital reagents and information; I.L.W. developed the humanized TKO model; and K.J.H. proposed experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kim J. Hasenkrug and Kerry J. Lavender, Rocky Mountain Labs, 903 S 4th St, Hamilton, MT 59875; e-mail: khasenkrug@nih.gov and kerry.lavender@nih.gov.



![Figure 4. Human GALT reconstitution in TKO-BLT mice. (A) Representative sections of 22 wpt small and large intestine from DKO-BLT (n = 3) and TKO-BLT (n = 3) mice were stained for the presence of human cells (HLA class I+) and for HIV-susceptible CD4+ cells. Magnified ×200; numerical aperture, 0.75; Olympus DP72 camera and BX51 microscope. Acquisition software: Olympus cell Sens Dimension 1.4.1. (B) Flow cytometry plots showing cellular subsets and T-cell activation status in GALT from a TKO-BLT mouse at 19 wpt (left to right: CD3+ T cells; CD4+/CD8+ T cells; Tcm [CD45RA−CCR7+] and Tem [CD45RA−CCR7−] subsets; activated HLA-DR+ T cells; CD11c+ myeloid and CD123+ plasmacytoid DCs). Parent gates are listed above each panel. Gating was based on distinct populations in mesenteric lymph nodes that were run on the same day.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/25/10.1182_blood-2013-06-506949/4/m_4013f4.jpeg?Expires=1765918738&Signature=ih5Y1S3tKlq9X1t9Pa1fMN-lfusNJKcAXMmSwirEqhPtLhU0-LhNKry-n7qu~CMg8UOyyBN6SWzzRcW5WeHlfiUJnTNpO1X1JuAbU~M0RisfZpflNQ5WiLHeQm0UA01bt24tUR-BDzIXmX1aXUiHswtmufIzfxao3ANFMApbtVjhTLcxFyTSHD9hwFlq0HVEAKcvBNwXjqG2qVHjZ3yAWCYRxPgssLjddWfM59AbzsPjVj0IUjDA1VfeX9fXpsYJOrr8dmEGkd9anmAu5QG9RCVpASzvqOw9OfaStAGGvAc6GzM0ZHrAoyA3B8vhESYJF55F18A32oBtT9MMemjmiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. HIV infection of TKO-BLT mice. (A-B) Data from mucosally and (C-F) intraperitoneally infected mice. (A) Viral load levels (HIV p24 ±standard error of the mean [SEM]) in plasma from HIV-infected mice collected at the indicated time points. Mice were challenged at 19 to 24 wpt. (B) CD4/CD8 T-cell ratios and frequency of activated CD8 T cells (CD38+) in mucosally challenged mice that became HIV+ or remained HIV− 4 wpi compared with prechallenge values. Unpaired 2-tailed t tests were performed between the groups at 4 wpi. (C) Viral loads in plasma as measured by p24 ELISA (±SEM) at the indicated intervals after intraperitoneal infection with 3000 or 7000 TCIU of HIVJR-CSF. Mice were challenged at 17 wpt. (D) Immunostaining for HIV p24 (red) and Iba1+ macrophages (green) in thymic organoids from representative HIV+ and HIV− TKO-BLT mice. Magnified 20×. The images were captured from a Nikon Digital Sight DS-Ri1 camera on a Nikon Eclipse 55i with a Plan Fluor 20X/0.05 objective at 20°C with Prolong gold with 4,6 diamidino-2-phenylindole and AlexaFluor 488 and 555 using NIS Elements software. Hue on the red channel was changed from 0 to 60 to make the color visible to red/green colorblinds using ACD Canvas 14 software. (E) Significant reductions in the plasma CD4 T-cell count over 4 weeks of infection (1-way analysis of variance with Tukey post-test) and the correlation between plasma p24 level and peripheral CD4 T-cell frequency and activation (CD38+) at 4 wpi. (F) Positive correlations between plasma p24 levels at 4 wpi and CD8 T-cell proliferation (Ki67+), activation (HLA-DR+), and expansion of the Tem subset. ***P < .001; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/25/10.1182_blood-2013-06-506949/4/m_4013f5.jpeg?Expires=1765918738&Signature=wH~NFhjvkrNR0C~LOZTbwodUgiVQpOJgpr0~SKUnyYJSDYNChuHQTLlkfz0xQ3EZZ5rVlWqF0K451Gav-AEFk8LZogrzFO0asn7Zh0PsgzoBvoWsCqYf7C0S5hNTt9tqHa3RrEUeiHqwo-yn9Jzqohlx~UgzbxoGTAo~itGwX8KCsPsq0iHmCHnM~lpeimieecqB7pOVSZ2FNdfl3EKN-Z1UJBrrqkXG2EXH4nPV5NlFmQrLeX9Wp4FmEcuC74WCbxvKe6ilWHcV6ntM47LXkgkzN9DXubiVwCzqk7RG5Mn3KOm3YxxvtJPWkAuE8bEmyDzZIg~Q5FwG1KTgbC6NEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal