Key Points
Histopathologic variants of nodular lymphocyte–predominant Hodgkin lymphoma are associated with advanced stage and increased relapse rate.
A prognostic score combining histopathologic and clinical features can allocate patients to 3 defined risk groups.
Abstract
Nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) accounts for approximately 5% of all Hodgkin lymphoma cases. The aim of this study was to evaluate the prognostic implication of histopathologic NLPHL variants. Biopsies of 423 NLPHL patients treated within 9 prospective clinical trials performed by the German Hodgkin Study Group were classified as tumor cell–rich cases (n = 10), typical NLPHL (n = 308), or histopathologic variants (n = 105). Histopathologic variants were characterized by the presence of lymphoma cells outside the B-cell nodules or B-cell depletion of the microenvironment. Compared with typical NLPHL, histopathologic variants were associated with advanced disease (29.5% vs 14.6%, P = .0012) and a higher relapse rate (18.1% vs 6.5% at 5 years, P = .0009). Variant histology represented an independent prognostic factor (odds ratio = 2.955) in a multivariate model of progression/relapse. A prognostic score, including the risk factors variant histopathologic growth pattern, low serum albumin, and male gender, was derived from this model and allowed the definition of 3 distinct risk groups. NLPHL patients presenting with histopathologic variants have a poorer outcome compared with those showing typical histology. The newly developed prognostic score combining histologic and clinical features allows allocating NLPHL patients to defined risk groups.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 4292.
Disclosures
Associate Editor Laurie Sehn served as an advisor or consultant for Roche/Genentech, Janssen, Lundbeck, Amgen, and Celgene, and she received grants for clinical research from Roche/Genentech. The authors and Laurie Barclay, freelance writer and reviewer, Medscape LLC, declare no competing financial interests.
Learning objectives
Outline clinical and epidemiologic features of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), based on a study report.
Describe the association of histopathologic variants of NLPHL with outcomes and prognosis.
Identify a prognostic score for NLPHL including histopathologic growth pattern.
Release date: December 19, 2013; Expiration date: December 19, 2014
Introduction
Nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) accounts for ∼5% of all Hodgkin lymphoma (HL) cases.1 NLPHL more commonly affects males (male-female ratio, 3:1). The median age at initial diagnosis is ∼40 years.2,3 In contrast to classical HL (cHL), the majority of NLPHL patients are diagnosed in the early favorable stages. The long-term remission rate among these patients exceeds 90% when they are treated with current standard approaches.2 In early unfavorable and advanced NLPHL, late relapses occur more frequently than in cHL.2 However, most relapses can be treated successfully, and overall survival (OS) is not impaired.
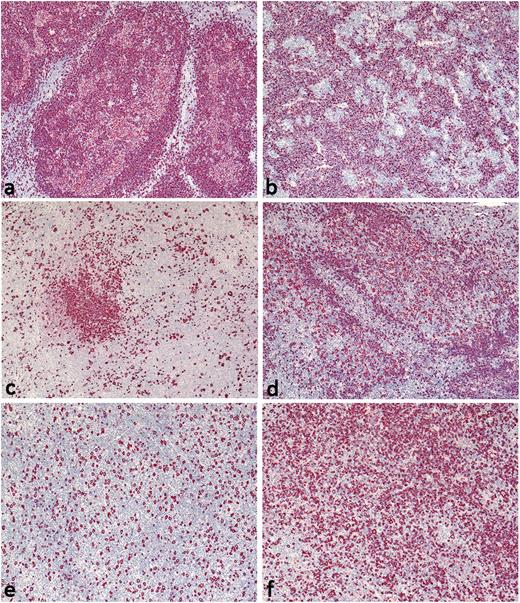
Histologically, NLPHL is characterized by large nodules of reactive small B lymphocytes. The blastic tumor cells, termed lymphocyte predominant (LP) cells, are located within these nodules. LP cells are derived from germinal center B cells, show ongoing somatic hypermutation,4-6 and express B-cell markers at varying intensities.7-9 Typical histopathologic growth patterns in NLPHL, described by Fan and colleagues,10 include B cell–rich nodular (pattern A) or serpiginous/interconnected (pattern B) subtypes. Both of these typical patterns show a predominantly nodular growth, with non-neoplastic B cells and LP cells located within the nodules. However, LP cells may rarely be found outside of the nodules. In contrast, histopathologic variants are characterized by either LP cells located outside of the nodules and/or reduced numbers of non-neoplastic B cells within the nodules. According to Fan and associates,10 therefore, histopathologic NLPHL variants are defined by prominent extranodular LP cells (pattern C), a T cell–rich (B cell–depleted) nodular pattern (pattern D), a diffuse T cell/histiocyte–rich large B-cell lymphoma (THRLBCL)-like pattern (pattern E), or a diffuse moth-eaten pattern (pattern F) with a B cell–rich background (Figure 1). Different growth patterns can be found in one lymph node sample.
Histopathologic NLPHL variants according to Fan et al. (A) Pattern A: B cell–rich nodular, tumor cell–rich case with high numbers of LP cells in the nodules; (B) pattern B: serpiginous/interconnected; (C) pattern C: an infiltrate with prominent extranodular LP cells; (D) pattern D: a T cell–rich nodular pattern; (E) pattern E: a diffuse T cell/histiocyte–rich B-cell lymphoma–like pattern; and (F) pattern F: a diffuse, moth-eaten pattern. CD20-immunostaining, original magnification ×40.
Histopathologic NLPHL variants according to Fan et al. (A) Pattern A: B cell–rich nodular, tumor cell–rich case with high numbers of LP cells in the nodules; (B) pattern B: serpiginous/interconnected; (C) pattern C: an infiltrate with prominent extranodular LP cells; (D) pattern D: a T cell–rich nodular pattern; (E) pattern E: a diffuse T cell/histiocyte–rich B-cell lymphoma–like pattern; and (F) pattern F: a diffuse, moth-eaten pattern. CD20-immunostaining, original magnification ×40.
Although higher relapse rates among NLPHL patients presenting with histopathologic variants were reported previously, the prognostic value of different histopathologic growth patterns remains undefined because studies published thus far were either limited in case numbers or were biased toward patients with histopathologic NLPHL variants.10,11 Thus, the aim of the present study was to assess the frequency and potential prognostic implications of the different histopathologic NLPHL growth patterns as defined by Fan et al.10 To this end, we reviewed 423 lymph node biopsies obtained from NLPHL patients treated within 9 prospective clinical trials conducted by the German Hodgkin Study Group (GHSG) and correlated the histopathologic growth patterns with baseline characteristics and clinical outcomes.
Materials and methods
Patients
All patients with newly diagnosed NLPHL enrolled within the prospective GHSG trials HD10-HD15, LPHD, LP, and RIPL (n = 558) between 1998 and 2010 were identified in the GHSG database.12-19 After exclusion of cases lacking documented clinical baseline or follow-up data (n = 65) or sufficient biopsy size/quality (n = 70), lymph node samples of 423 NLPHL patients were selected for review. Detailed information on the treatment regimens administered is provided in supplemental Table 1. Consent of the institutional review board of the University Cologne was obtained through the GHSG for this study, which was conducted in accordance with the Declaration of Helsinki.
Histopathologic review
A joint meeting of all participating pathologists was held to allocate the lymph node samples according to the histopathologic NLPHL growth patterns as defined by Fan and colleagues.10 Full slides of 423 specimens comprising a basic set of stainings (hematoxylin and eosin, CD20, CD30, CD3, LMP1, CD15) were first reviewed by one expert pathologist. A major (dominating) and, if present, a minor pattern component (a variant detectable in <50% of the lymphoma area) were assigned to each case. All NLPHL cases with at least a minor component not being typically nodular (not corresponding to pattern A or B) were selected and reviewed with a multihead microscope by a panel of 10 expert pathologists to confirm the variant histopathologic growth pattern. The expert panel reached a unanimous decision in all cases.
Patients were categorized according to their NLPHL histology: (1) Typical NLPHL—all cases presenting only with patterns A and/or B; (2) histopathologic variants—all cases presenting with at least one variant pattern (C, D, E, F) as a major or minor component; (3) tumor cell–rich—cases presenting with a high number of tumor cells forming focal sheets, not effacing the architecture.
Statistical analysis
All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC). Demographics and disease characteristics were summarized and compared between groups using the Fisher exact test. Survival curves were analyzed according to the Kaplan-Meier method and were compared between groups using the log-rank test. Explorative logistic regression analyses of the binary response (no vs yes)—variable progression or relapse within 5 years after diagnosis—in univariate and multivariate fashion were performed to assess the predictive value of histopathologic variants and baseline risk factors for treatment failure.20 Covariates included in the univariate analyses were: stage of disease according to GHSG staging criteria (early vs advanced), gender (female vs male), age at diagnosis (<45 years vs >45 years), serum albumin level (>4 g/dL vs <4 g/dL), hemoglobin level (>10.5 g/dL vs <10.5 g/dL), leukocyte count (<15 000/mm3 vs >15 000/mm3), lymphocyte count (normal vs <600/mm3 or <8% of the white cell count or both), splenic involvement (no vs yes), and histopathologic variant (typical vs variant). Risk factors with P < .1 were included as predictor variables of interest in a multivariate logistic regression analysis. Using backward selection, effects not meeting the .05 significance level were removed from the model. The final model was used to calculate odds ratio (OR) estimates and 95% confidence intervals (CI), as well as 2-sided probability values based on the Wald test.21
Results
The morphologic spectrum of NLPHL
Of 413 cases analyzed, 331 (80.1%) exhibited a single growth pattern (Table 1). In 82 cases (19.9%), a minor pattern was present in addition to the major pattern; in 6 of these cases (1.4%), an apparent third component was seen but was ignored in further analyses. Typical NLPHL histology as the major pattern was observed in 350 of the 413 patients who were ultimately considered (pattern A: n = 332, pattern B: n = 18). Among these 350 patients, 308 did not show any variant histology as a minor component. Thus the group of patients with typical NLPHL histology comprised 308 patients (74.6%). A major nodular growth pattern with extranodular expansion of LP cells (pattern C) or with B cell–depleted nodules (pattern D) was observed in 27 (6.5%) and 21 cases (5.1%), respectively (Table 1). A major diffuse growth pattern of LP cells was observed in 3.6% (7 cases and 8 cases with pattern E and pattern F, respectively). Thus, 63 cases (15.3%) had a variant histopathologic growth pattern as the major component. In 42 cases (10.2%), histopathologic variants were found as a minor component; thus 105 patients (25.4%) were included in the variant NLPHL histology group. Ten of 423 cases were categorized as tumor cell–rich and were not included in any statistical analysis because the low number of cases precluded drawing statistically valid conclusions. We did not identify cases with an atypical T-cell proliferation.22
Distribution of histopathologic variant patterns in NLPHL cases
| . | Minor component† . | ||||||
|---|---|---|---|---|---|---|---|
| Major component* . | A . | B . | C . | D . | E . | F . | Total‡ . |
| A | 294 (71%) | — | 10 (2%) | 24 (6%) | 1 (0%) | 3 (1%) | 332 (80%) |
| B | 2 (0%) | 12 (3%) | 1 (0%) | 3 (1%) | — | — | 18 (4%) |
| C | 5 (1%) | 1 (0%) | 15 (4%) | 3 (1%) | 3 (1%) | — | 27 (7%) |
| D | 7 (2%) | 1 (0%) | — | 3 (1%) | 7 (2%) | 3 (1%) | 21 (5%) |
| E | — | — | 1 (0%) | 5 (1%) | — | 1 (0%) | 7 (2%) |
| F | — | — | 1 (0%) | — | — | 7 (2%) | 8 (2%) |
| Total | 308 (75%) | 14 (3%) | 28 (7%) | 38 (9%) | 11 (3%) | 14 (3%) | 413 (100%) |
| . | Minor component† . | ||||||
|---|---|---|---|---|---|---|---|
| Major component* . | A . | B . | C . | D . | E . | F . | Total‡ . |
| A | 294 (71%) | — | 10 (2%) | 24 (6%) | 1 (0%) | 3 (1%) | 332 (80%) |
| B | 2 (0%) | 12 (3%) | 1 (0%) | 3 (1%) | — | — | 18 (4%) |
| C | 5 (1%) | 1 (0%) | 15 (4%) | 3 (1%) | 3 (1%) | — | 27 (7%) |
| D | 7 (2%) | 1 (0%) | — | 3 (1%) | 7 (2%) | 3 (1%) | 21 (5%) |
| E | — | — | 1 (0%) | 5 (1%) | — | 1 (0%) | 7 (2%) |
| F | — | — | 1 (0%) | — | — | 7 (2%) | 8 (2%) |
| Total | 308 (75%) | 14 (3%) | 28 (7%) | 38 (9%) | 11 (3%) | 14 (3%) | 413 (100%) |
Cases with a single growth pattern are bolded.
Pattern A: “Classic” nodular pattern, B cell–rich; pattern B: serpiginous/interconnected nodular pattern; pattern C: nodular with prominent extranodular LP cells; pattern D: nodular with T cell–rich background; pattern E: diffuse pattern (T cell/histiocyte–rich large B-cell lymphoma–like); pattern F: diffuse, “moth-eaten,” with B cell–rich background
Major component: Dominating histopathologic growth (found in >50% of lymphoma tissue).
Minor component: Histopathologic growth pattern observed in <50% of the lymphoma tissue.
Ten cases with tumor cell–rich histology are excluded.
Patient characteristics
Baseline characteristics and follow-up data of all 493 NLPHL patients with documented clinical information available in the GHSG database were compared with the group of 413 patients included in the final analysis to minimize the risk of bias. There were no significant differences between these 2 groups with respect to the proportion of patients diagnosed with advanced stages (20.3% vs 18.4%), male gender (74.8% vs 74.1%), age <45 years (64.7% vs 63.7%), International Prognostic Score (IPS) (IPS 0-2: 93.3% vs 93.9%), splenic involvement (4.7% vs 4.1%), or the rate of patients with progress/relapse within the first 5 years after enrollment into the study (9.7% vs 9.4%).
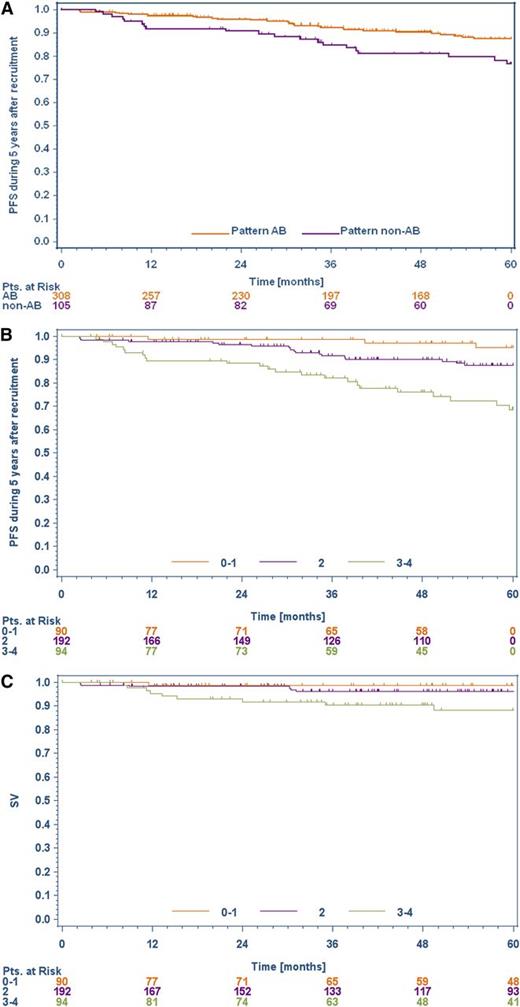
Patients with histopathologic NLPHL variants were more frequently diagnosed with advanced stages (clinical stage IIB with the risk factors of a large mediastinal mass and/or extranodal disease or clinical stages III/IV) than were patients with typical NLPHL histology (29.5% vs 14.6%, P = .002, Table 2). A low IPS (0-2) was more common in patients with typical histology than in patients with a variant histology (96.7% vs 85.7%, P = .0005). Progression-free survival (PFS) at 5 years was significantly better for patients with typical NLPHL histology than for those presenting with a histopathologic variant (P = .0158; Figure 2A). However, this advantage in PFS did not translate into a superior 5-year OS for patients presenting with typical NLPHL histology (P = .1751). Eight patients (1.9%) presented with bone marrow involvement. Interestingly, seven of them had a variant NLPHL histology.
Clinical parameters of typical NLPHL patients compared with histopathologic NLPHL variants
| . | Typical NLPHL (pattern A/B), n = 308% . | NLPHL variant pattern (non-A/B), n = 105% . | Fisher exact test . |
|---|---|---|---|
| Clinical stages III/IV or IIB with a large mediastinal mass and/or extranodal disease | 14.6 | 29.5 | P = .0012 |
| International Prognostic Score (IPS) ≥3* | 3.3 | 14.3 | P = .0005 |
| Male gender | 72.4 | 79.0 | n.s. |
| Stage IV | 1.9 | 11.4 | P = .0002 |
| Age ≥45 y | 38.6 | 29.5 | n.s. |
| Albumin <4 g/dL | 10.7 | 18.8 | n.s. |
| Hemoglobin <10.5 g/dL | 0.7 | 4.8 | P = .0136 |
| Leukocytes >15 000/mm3 | 0.3 | 1.0 | n.s. |
| Lymphocytes <600/mm3 | 0.7 | 1.0 | n.s. |
| Splenic involvement | 6.7 | 3.2 | n.s. |
| Disease progression or relapse in the first 5 years after study enrollment in the GHSG | 6.5 | 18.1 | P = .0009 |
| . | Typical NLPHL (pattern A/B), n = 308% . | NLPHL variant pattern (non-A/B), n = 105% . | Fisher exact test . |
|---|---|---|---|
| Clinical stages III/IV or IIB with a large mediastinal mass and/or extranodal disease | 14.6 | 29.5 | P = .0012 |
| International Prognostic Score (IPS) ≥3* | 3.3 | 14.3 | P = .0005 |
| Male gender | 72.4 | 79.0 | n.s. |
| Stage IV | 1.9 | 11.4 | P = .0002 |
| Age ≥45 y | 38.6 | 29.5 | n.s. |
| Albumin <4 g/dL | 10.7 | 18.8 | n.s. |
| Hemoglobin <10.5 g/dL | 0.7 | 4.8 | P = .0136 |
| Leukocytes >15 000/mm3 | 0.3 | 1.0 | n.s. |
| Lymphocytes <600/mm3 | 0.7 | 1.0 | n.s. |
| Splenic involvement | 6.7 | 3.2 | n.s. |
| Disease progression or relapse in the first 5 years after study enrollment in the GHSG | 6.5 | 18.1 | P = .0009 |
n.s., not significant.
In 9% of the patients, information on the IPS was missing.
PFS and OS in patients with typical/variant NLPHL and patients allocated to 3 different risk groups, based on a newly developed prognostic score. (A) Kaplan-Meier curve for relative PFS in typical NLPHL (A/B, n = 308) and NLPHL variant (non-A/B, n = 105) (P = .0158). (B) Kaplan-Meier curve for the relative PFS of the 3 prognostic risk groups in NLPHL patients (P < .0001). (C) Kaplan-Meier curve for the relative OS of the 3 prognostic risk groups in NLPHL patients (P = .0173).
PFS and OS in patients with typical/variant NLPHL and patients allocated to 3 different risk groups, based on a newly developed prognostic score. (A) Kaplan-Meier curve for relative PFS in typical NLPHL (A/B, n = 308) and NLPHL variant (non-A/B, n = 105) (P = .0158). (B) Kaplan-Meier curve for the relative PFS of the 3 prognostic risk groups in NLPHL patients (P < .0001). (C) Kaplan-Meier curve for the relative OS of the 3 prognostic risk groups in NLPHL patients (P = .0173).
Univariate and multivariate analyses of progression/relapse
Within the first 5 years after study enrollment, 39 of 413 patients (9.4%) had progressed or relapsed. Univariate regression analyses revealed several factors that were associated with an increased risk for progression/relapse. These included a variant histopathologic growth pattern (P = .0007; OR = 3.182), advanced stage (P = .137; OR = 2.468), stage IV disease (P = .0001; OR = 7.220), male gender (P = .0124; OR = 4.622), splenic involvement (P = .0080; OR = 4.439), low serum albumin (P = .0049; OR = 3.189), and low serum hemoglobin level (P = .0001; OR = 26.819).
Because only 7 patients presented with a low serum hemoglobin level, this factor was excluded from the multivariate analysis. All other factors with univariate significance were included as covariates in a multivariate regression analysis of progression/relapse risk. However, 37 patients were not included in this analysis because of missing hemoglobin values. In the final model, male gender (P = .0119; OR = 6.653), low serum albumin (P = .0055; OR = 3.437), and a variant histopathologic growth pattern (P = .0038; OR = 2.955) were identified as significant independent risk factors for progression/relapse within the first 5 years after study enrollment.
Combined histopathologic and clinical prognostic score
To further assess the prognostic value of the multivariate model, a score composed of the following 3 relevant risk factors was developed: histopathologic pattern (0 = typical NLPHL, 1 = variant growth pattern), albumin (0 = albumin ≥4 g/dL, 1 = albumin <4 g/dL), and gender (0 = female, 2 = male) (Table 3). The sum was calculated for each patient. The 5-year progression/relapse rates significantly correlated with the scores achieved. Patients achieving 0 (n = 62), 1 (n = 28), 2 (n = 192), 3 (n = 80), and 4 (n = 14) points in the score had 5-year progression/relapse rates of 1.6%, 3.6%, 6.3%, 18.8%, and 42.9%, respectively. The scores were used to define 3 risk groups (0-1 = low risk, 2 = intermediate risk, 3-4 = high risk). Among these 3 groups, there were significant differences in PFS (P < .0001) and OS (P = .0173). The 5-year PFS rate for low-risk patients was 95.2% (95% CI, 89.8-100), for intermediate risk patients, 87.5% (95% CI, 82.1-93.0), and for high-risk patients, 68.7% (95% CI, 57.7-79.7) (Figure 2B). The corresponding 5-year OS rates were 98.7% (95% CI, 96.2-100), 96.2% (95% CI, 93.3-99.2), and 88.3% (95% CI, 80.9-95.7), respectively (Figure 2C).
Composition of the prognostic score for risk groups of relapse/progress
| . | . | . | Scoring points . |
|---|---|---|---|
| Variable A | Histopathologic NLPHL subtype | Typical NLPHL pattern* | 0 |
| Morphologic NLPHL variant† | 1 | ||
| Variable B | Albumin | Albumin ≥4 g/dL | 0 |
| Albumin <4 g/dL | 1 | ||
| Variable C | Gender | Female | 0 |
| Male | 2 | ||
| Prognostic score variable A + B + C | |||
| . | . | . | Scoring points . |
|---|---|---|---|
| Variable A | Histopathologic NLPHL subtype | Typical NLPHL pattern* | 0 |
| Morphologic NLPHL variant† | 1 | ||
| Variable B | Albumin | Albumin ≥4 g/dL | 0 |
| Albumin <4 g/dL | 1 | ||
| Variable C | Gender | Female | 0 |
| Male | 2 | ||
| Prognostic score variable A + B + C | |||
Score 0-1, low risk; Score 2, intermediate risk; Score 3-4, high risk.
Typical NLPHL include patterns A and B.
Morphologic NLPHL variants include patterns C, D, E, and F defined by Fan et al.10
Discussion
This study assessed the prognostic implication of histopathologic NLPHL growth patterns and their clinical impact. A total of 423 NLPHL patients treated within 9 prospective GHSG trials were included. The major finding of the study was that histopathologic NLPHL variants are associated with more unfavorable clinical characteristics and have a poorer prognosis compared with typical histology. A prognostic score combining histopathologic and clinical features was developed that helps to predict the prognosis of patients with newly diagnosed NLPHL.
In the present analysis, patients with variant NLPHL histology more often presented with unfavorable baseline characteristics such as advanced stage and higher IPS. This is consistent with earlier studies in which NLPHL patients with T cell/histiocyte–rich nodules or other histopathologic variants more frequently presented in advanced stages. In one report, stage III/IV disease was diagnosed in 20% of patients presenting with typical NLPHL histology in contrast to 60% of patients presenting with T cell/histiocyte–rich nodules.10 Compared with patients with typical histology, we found a significantly higher relapse rate among patients presenting with histopathologic variants. This observation is in line with the findings of Fan and coworkers.10 In their analysis of 56 patients with sufficient clinical follow-up data, an atypical, more diffuse growth pattern represented an independent predictor for disease recurrence (P = .00324).
Histopathologically, 75% of patients included in the present study displayed a typical NLPHL infiltrate, whereas 25% had histopathologic variants. Thus, cases with variant NLPHL histology were less frequent than they were in the Stanford study.10 However, the majority of the 137 biopsies evaluated in their analysis were seen in consultancy and therefore a bias toward diagnostically difficult cases is likely. In a report by Boudova and coworkers,11 variants with T cell/histiocyte–rich nodules accounted for 17 of 156 NLPHL cases, but diagnostic criteria differed from those used in the present analysis and in the study by Fan and colleagues. Thus, data are difficult to compare.
Histopathologic NLPHL variants may reflect an altered homing pattern of LP cells. The common feature of all variant patterns is a T cell–rich microenvironment, either with a retained nodular growth (pattern D) or with a loss of nodular growth to varying extents (patterns C, E, F). We speculate that a T cell–dominated microenvironment supports dissemination of LP cells to distant lymph nodes and explains the occurrence of higher clinical stages in these patients. A correlation between clinical stage and the composition of the microenvironment has been observed in follicular lymphoma.23,24 It remains unclear whether the alterations in the microenvironment reflect a biological process actively induced by the LP cells or a different host response. The prognostic value of the histopathologic growth pattern might suggest a true biologic progression of NLPHL to a more aggressive disease.
In general, the prognosis for patients with NLPHL is at least as good as that for patients with cHL. Long-term survival is achieved in >90% of cases.2 Given this excellent clinical outcome, the major aim of current clinical studies of HL is to reduce treatment-related toxicity without compromising efficacy.12,17 In cHL, interim positron emission tomography (PET) is a promising tool to distinguish low-risk patients who might be sufficiently treated with reduced-intensity approaches from intermediate- or high-risk patients who might require standard or intensified treatment.25,26 In NLPHL, the value of interim PET is unclear, mainly because of the rarity of this disease. Therefore, alternative tools to predict the prognosis of NLPHL patients are needed to develop risk-adapted treatment strategies.
Based on histopathologic and clinical data, we established a prognostic score combining the factors histopathologic growth pattern, serum albumin, and gender, defining 3 risk groups. Although only 2.2% of low-risk patients and 6.3% of intermediate-risk patients relapsed within 5 years after initial diagnosis, the relapse rate among high-risk patients was 22.3% when treated with stage-adapted GHSG protocols. This poorer PFS did also translate into a worse OS for high-risk patients. Thus, current standard approaches may not represent the optimal treatment of high-risk patients. Here, the implementation of anti-CD20 antibodies into first-line approaches may improve the clinical outcome because CD20 expression represents a hallmark of NLPHL. Within 2 prospective phase 2 trials including patients with relapsed NLPHL, response rates after 4 doses of the chimeric anti-CD20 antibody rituximab given as a single agent were 94% and 100%. Long-term remission was observed in a relevant proportion of cases.27,28 However, mature data on the use of first-line protocols such as ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) or BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone) in combination with anti-CD20 antibodies in NLPHL are pending. Only one retrospective report on the efficacy of R-CHOP (rituximab, cyclophosphamide, adriamycin, vincristine, prednisone) in newly diagnosed NLPHL is currently available. Fifteen patients received R-CHOP alone and 5 patients received R-CHOP in combination with radiotherapy (RT). At a median follow-up of 42 months, no relapses had occurred.29 Despite these promising data, no general treatment recommendation should be made on the basis of this analysis because of its retrospective nature and the small number of patients included.29 Thus, more studies will be necessary to define a standard of care for newly diagnosed NLPHL.
In summary, we found that 25% of NLPHL patients enrolled in prospective GHSG trials presented with histopathologic variants. A variant histopathologic growth pattern was associated with more advanced stages and higher IPS. Variant histopathology also represented an independent risk factor for progress/relapse. A prognostic score including histopathologic growth pattern, serum albumin, and gender was developed and allows for identifying patients at higher risk of treatment failure when they are treated with current standard approaches. These patients may be candidates for novel treatment strategies including targeted drugs such as anti-CD20 antibodies.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Olivera Batic, Charlotte Botz-von Drathen, Ralf Lieberz, and Yvonne Michel for excellent technical support.
This work was supported in part by the Deutsche Forschungsgemeinschaft (grant HA 6145/1-1) (S.H.).
Authorship
Contribution: S.H. and W.K. designed the study, and acquired, analyzed, and interpreted data; and drafted the manuscript; D.A.E., A.P., and A.E. analyzed and interpreted data, drafted the manuscript, and supplied essential material; and A.M., R.B., K.K., H.-W.B., S.C., M.H., A.C.F., G.O., P.M., A.R., H.S., and M.-L.H. supplied essential material, reviewed cases, and acquired and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sylvia Hartmann, Goethe University, Theodor-Stern-Kai 7, Frankfurt, 60590 Germany; e-mail: s.hartmann@em.uni-frankfurt.de.
References
Author notes
S.H. and D.A.E. contributed equally to this study.