Key Points
PKCε, regulating RhoA activity, is a critical mediator of proplatelet formation.
PKCε shut down results in RhoA expression levels that are incompatible with normal platelet generation.
Abstract
During thrombopoiesis, megakaroycytes undergo extensive cytoskeletal remodeling to form proplatelet extensions that eventually produce mature platelets. Proplatelet formation is a tightly orchestrated process that depends on dynamic regulation of both tubulin reorganization and Rho-associated, coiled-coil containing protein kinase/RhoA activity. A disruption in tubulin dynamics or RhoA activity impairs proplatelet formation and alters platelet morphology. We previously observed that protein kinase Cepsilon (PKCε), a member of the protein kinase C family of serine/threonine-kinases, expression varies during human megakaryocyte differentiation and modulates megakaryocyte maturation and platelet release. Here we used an in vitro model of murine platelet production to investigate a potential role for PKCε in proplatelet formation. By immunofluorescence we observed that PKCε colocalizes with α/β-tubulin in specific areas of the marginal tubular-coil in proplatelets. Moreover, we found that PKCε expression escalates during megakarocyte differentiation and remains elevated in proplatelets, whereas the active form of RhoA is substantially downregulated in proplatelets. PKCε inhibition resulted in lower proplatelet numbers and larger diameter platelets in culture as well as persistent RhoA activation. Finally, we demonstrate that pharmacological inhibition of RhoA is capable of reversing the proplatelet defects mediated by PKCε inhibition. Collectively, these data indicate that by regulating RhoA activity, PKCε is a critical mediator of mouse proplatelet formation in vitro.
Introduction
Defective platelet numbers can compromise proper wound healing and cause bleeding, whereas excessive platelet production or activation can lead to thrombosis. As a result, platelet homeostasis is a tightly regulated process. The process of generating thousands of platelets from a single megakaryocyte is characterized by several morphologically distinct stages that involve extensive cytoskeletal remodeling. In particular, megakaryoctyes, following polyploidization and prior to platelet release, form elongated cellular processes called proplatelets (proPLT).1 Perturbations in proPLT formation lead to alterations in platelet morphology and thrombocytopenia,2,3 emphasizing the importance of this step.
The marginal microtubule band, actin-based cytoskeleton, and spectrin-based membrane skeleton are all prominent components of the cytoskeletal remodeling that occurs during platelet formation.4 Specifically, dynamic tubulin polymerization is required for the proper platelet morphology, assembly of the marginal microtubule coil, proPLT extensions and microtubule band formation.4-8 Various mutations in genes that regulate actin polymerization and turnover cause thrombocytopenia due to altered formation and morphology of proPLT extensions, increased platelet size, and defective platelet release.9,10 The spectrin cytoskeleton membrane influences platelet formation by supporting the megakaryocyte membrane invagination that is critical to the production of proPLT extensions.11
In addition to these cytoskeletal pillars, the Rho/Rho-associated, coiled-coil containing protein kinase (ROCK) pathway regulates platelet production. Overexpression of a dominant-negative RhoA boosts proPLT formation, whereas constitutive RhoA activation abrogates this process.12 Moreover, blocking RhoA activity is necessary for microtubule-driven formation of proPLT cytoplasmic extensions.13 RhoA-deficient mice display macrothrombocytopenia, suggesting that RhoA activity must be temporally regulated for successful PLT production.14 There are implications that RhoA signaling influence platelet formation by regulating cytoskeleton components. For example, it has been demonstrated that the correlation between PLT size and changes in tubulin polymerization involves a RhoA/Rho-dependent regulation of cytoskeletal microtubule ring remodeling.2,15 Furthermore, RhoA contributes to actin function during actomyosin contractility and stress fiber formation.16 Additionally, the Rho/ROCK pathway is related to the late failure of cytokinesis responsible for the endomitotic process in megakaryocytes.17
We recently showed that expression of protein kinase Cepsilon (PKCε), a member of the protein kinase C family of serine/threonine kinases, changes throughout the course of thrombopoietin (TPO)–induced megakaryocyte (MK) differentiation from human CD34pos cells.18,19 PKCε has been implicated in both the regulation of cytoskeleton remodeling and the RhoA pathway.20 Specifically, PKCε, unlike other PKC family members, contains an F-actin-binding region that promotes the formation of F-actin filaments by preventing depolymerization and increasing the rate of actin filament elongation.21 During mitotic cytokinesis of several cell types (ie, COS-7, HeLa, MEF, HEK 293), PKCε assembles with 14-3-3zeta, and together they transiently accumulate at the actomyosin ring with RhoA and is required to dissociate both itself and RhoA from the midbody.22 In neurons, PKCε promotes neurite outgrowth, which can be blocked by forced activation of the ROCK/RhoA pathway, indicating that PKCepilson may attenuate RhoA/ROCK pathway activity in the process.23
On these bases we hypothesized that PKCε regulates proPLT formation by modulating RhoA activity. Indeed, our data show that PKCε shut down results in RhoA expression levels that are incompatible with normal PLT generation.
Methods
Cell isolation and cell cultures
Mouse fetal liver cells (FLC) were isolated from embryos at day 13.5. Single-cell suspensions were prepared as previously described24 and cultured for 4 days in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA), supplemented with 10% fetal calf serum, 2 mmol/L l-glutamine, 50 U/mL penicillin, and 50 µg/mL streptomycin at 37°C and 5% CO2 in the presence of 0.1 µg/mL purified recombinant mouse TPO.25 All experiments were complied with institutional guidelines approved by the Children’s Hospital Animal Care and Use Committee and the Institutional Animal Care and Use Committee.
Isolation of MK and proPLT
FLC cultures were layered on a single-step gradient (1.5% to 3.0% bovine serum albumin [BSA]) at different times of differentiation (days 2, 3, 4, 5), and MKs were allowed to sediment for 30 minutes. For proPLT production analysis, the isolated MKs were resuspended in fresh media and cultured for an additional 24 hours, during which proPLT production was readily observed. For some experiments, MK cultures were layered on a second single-step gradient (1.5% to 3.0% BSA) on day 5. MKs were allowed to sediment for 30 minutes, during which intermediate stages in PLT production were resolved within different fractions of the gradient.26 Round MKs (rMK) sedimented on the bottom, and proPLT-forming MKs (ppfMK) localized to the BSA fraction, whereas released proPLTs and individual PLTs remained in the top fraction.
Immunofluorescence microscopy
Mouse MKs, culture intermediates, and PLTs were purified and probed as previously described.27 In brief, samples were fixed in 4% formaldehyde, centrifuged onto 1 µg/mL poly-l-lysine–coated coverslips, permeabilized, and blocked8 before antibody labeling. Samples were incubated with mouse monoclonal antibodies against α and β tubulin (Sigma-Aldrich, St. Louis, MO) and a rabbit monoclonal antibody against PKCε (Novus Biologicals, Littleton, CO). They were treated with secondary goat anti-mouse and goat anti-rabbit antibodies, conjugated to an Alexa Fluor 568 and to an Alexa Fluor 488 (Invitrogen), respectively; 4,6 diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) was used to label the MK nucleus. Samples were examined with a microscope (Axiovert 200, Carl Zeiss, Inc.,Oberkochen, Germany) equipped with a 63× numerical aperture 1.4 oil immersion objective. Images were obtained using a charge-coupled device (CCD) camera (Hamamatsu Photonics, Hamamatsu, Japan) and analyzed using the MetaMorph image analysis software (MDS Analytical Technologies, Sunnyvale, CA).
Further images were acquired with a confocal microscope Olympus FluoView™ FV10i (Center Valley, PA), with a 60× water corrected objective. Series of x-y images were collected along the z-axis at 0.1- or 0.5-µm intervals. Three individual images were averaged to generate a representative image for each sample plane. At least 7 averaged images were collected at 0.5-µm steps along the z-axis of each sample for both fluorescence channels. Image analysis was generated using ImageJ software (National Institutes of Health, Bethesda, MD).
Cell perimeter and diameter were analyzed using the MetaMorph software thresholding, integrated morphometry analysis, and software calipers tools and by ImageJ software. Experiments represent 1 standard deviation about the mean for at least 3 independent cultures.
shRNA cell infection
For short hairpin RNA (shRNA)–based gene silencing, pLKO.1 lentiviral vector encoding shRNA against mouse PKCε (NM_011104; shRNA59 and shRNA60) were obtained from Open-Biosystem (Thermo Scientific, Waltham, MA). As control (shRNACT), we used the MISSION pLKO.1-puro Non-Target shRNA Control Plasmid, containing a shRNA insert that does not target any known genes from any species (Sigma-Aldrich). The shRNA expressing viruses were produced in 293TL cells according to standard protocols. Mouse FLCs were infected at day 0 of culture and then cultured with TPO in the presence of puromycin, to select infected, puromycin-resistant cells.
PKCε was downmodulated in primary MK progenitors infecting FLCs with specific shRNA at day 0 of culture. Infected cells were cultured with TPO in the presence of puromycin, for cell selection. At day 4 of culture, infected rMKs were isolated by BSA gradient and cultured for an additional 24 hours in the presence of TPO and puromycin. At day 5 of culture, cells were analyzed for proPLT formation by light microscopy and then fixed for immunofluorescence staining.
Aliquots from each culture were collected for western blot analysis.
Inhibition of RhoA
At day 4 of culture, infected rMKs were isolated by BSA gradient and cultured for an additional 24 hours in the presence of TPO and puromycin, with or without C3 transferase RhoA inhibitor (Cytoskeleton, Inc., Denver, CO). At day 5 of culture, cells were analyzed for proPLT formation by light microscopy and then fixed for immunofluorescence staining, and a fixed volume of culture supernatants were collected for PLT count by flow cytometry.
PLT count by flow cytometry
PLT production in culture was quantified as previously described.19,28 Briefly, fixed volumes of the culture supernatants were collected, incubated with anti-CD41-RPE and Calcein AM (Sigma-Aldrich) (to exclude fragments), and added to a fixed volume of calibration beads (DAKO, Glostrup, Denmark) (size of beads of known concentration). CD41pos/Calcein AMpos PLTs were quantified by flow cytometry and normalized to bead counts to determine absolute PLT counts.
Western blot
Cultured cells were counted, and 1.5 × 106 cells were collected on days 0, 2, 3, 4, and 5, washed in phosphate-buffered saline, and centrifuged at 200 g for 10 minutes. Pellets were resuspended in a cell lysis buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; 1 mM Na3VO4; 1 mM NaF), supplemented with fresh protease inhibitors, and protein concentration was determined using a Pierce BCA protein assay kit (Thermo Scientific). Thirty micrograms of proteins from each sample were run on 4% to 20% sodium dodecyl sulfate–acrylamide gels (Bio-Rad, Hercules, CA), blotted onto nitrocellulose membranes, blocked, and incubated with specific primary antibodies diluted as described in manufacturers’ protocols. Specifically, rabbit polyclonal anti-PKCε antibody (catalog number: 06991; Merck Millipore, Darmstadt, Germany) was used at the concentration of 1 μg/mL. Mouse monoclonal anti-PKCδ antibody (BD Pharmingen, San Jose, CA) was diluted 1:500, rabbit polyclonal anti-PKCθ antibody (Cell Signaling, Danvers, MA) was diluted 1:1000, rabbit polyclonal anti-PKCα antibody (Cell Signaling) was diluted 1:1000, and rabbit polyclonal anti-14-3-3zeta antibody (catalog number: AB9746; Merk Millipore) was diluted 1:500. A mouse monoclonal anto-RhoA antibody (Cytoskeleton, Inc.) was diluted 1:500, and mouse monoclonal anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (catalog number: MAB374; Merk Millipore), 1:5000 dilution) used as a protein-loading control.
Membranes were washed and incubated for 1.5 hours at room temperature with 1:5000 peroxidase-conjugated anti-rabbit or with 1:2000 peroxidase-conjugated anti-mouse IgG (Pierce) at room temperature and resolved by ECL Supersignal West Pico Chemiluminescent Substrate detection system (Pierce). Protein densitometric analyses from western blot assays were performed by using the Image J software system, normalized for GAPDH expression levels.
Statistical analyses were performed by using analysis of variance (ANOVA) and Tukey tests.
Results
PKCε levels are modulated during MK differentiation
In agreement with our previous studies in human megakaryocyte coltures,18 PKCε protein expression increases during the early phases of MK differentiation (Figure 1A). However, unlike human megakaryopoiesis, mouse PKCε remains highly expressed throughout the entire maturation process, including PLT release (relative densitometric measurements of the samples tested are shown in Figure 1B).
PKCε levels are modulated during MK differentiation. (A) Western blot detection of total PKCε protein expression in mouse MK cultures. GAPDH was monitored for protein loading. FLC, fetal liver cells; rMK, round MK; ppfMK, proplatelet-forming MK; PPL, proplatelets. (B) Relative PKCε protein expression during megakaryocytic differentiation from mouse FLC. Densitometric measurements of western blots from 5 replicates were performed by ImageJ software (means ± standard deviations [SD]; *P < .01 vs FLC culture day 0; #P < .05 vs rMK culture day 2). (C) Immunofluorescence analysis of PKCε and α/β-Tubulin expression and their colocalization in proPLT. Samples were examined with a confocal microscope Olympus FluoView FV10i (Center Valley, PA), with a 60× water-corrected objective. Each image represents a projection from a z-stack of 7 images collected at 0.5-µm increments. Images were obtained at 22°C and analyzed by ImageJ software. PKCε was detected with a rabbit polyclonal antibody and a secondary goat anti-rabbit antibody, conjugated to an Alexa Fluor 488; α and β tubulin were detected with mouse monoclonal antibodies and a secondary goat anti-mouse antibody, conjugated to an Alexa Fluor 568; MK nucleus was detected with DAPI.
PKCε levels are modulated during MK differentiation. (A) Western blot detection of total PKCε protein expression in mouse MK cultures. GAPDH was monitored for protein loading. FLC, fetal liver cells; rMK, round MK; ppfMK, proplatelet-forming MK; PPL, proplatelets. (B) Relative PKCε protein expression during megakaryocytic differentiation from mouse FLC. Densitometric measurements of western blots from 5 replicates were performed by ImageJ software (means ± standard deviations [SD]; *P < .01 vs FLC culture day 0; #P < .05 vs rMK culture day 2). (C) Immunofluorescence analysis of PKCε and α/β-Tubulin expression and their colocalization in proPLT. Samples were examined with a confocal microscope Olympus FluoView FV10i (Center Valley, PA), with a 60× water-corrected objective. Each image represents a projection from a z-stack of 7 images collected at 0.5-µm increments. Images were obtained at 22°C and analyzed by ImageJ software. PKCε was detected with a rabbit polyclonal antibody and a secondary goat anti-rabbit antibody, conjugated to an Alexa Fluor 488; α and β tubulin were detected with mouse monoclonal antibodies and a secondary goat anti-mouse antibody, conjugated to an Alexa Fluor 568; MK nucleus was detected with DAPI.
Both morphological and confocal immunofluorescence analyses of differentiating MKs show that progressively enlarged, multinucleated cells stably express PKCε and α/β-Tubulin, the main cytoskeletal protein comprising the marginal tubular coil,5 and that PKCε colocalizes with α/β-Tubulin dimers at the marginal tubular coil of proPLTs (Figure 1C). Indeed in the cytoplasm, PKCε staining appears diffuse, with no evident overlapping with α/β-Tubulin. On the contrary, a strong overlap of PKCε with α/β-Tubulin is present along all the proPLT extensions and the marginal tubular coils, as shown in the composite panel of Figure 1C.
PKCε expression is necessary for proPLT formation
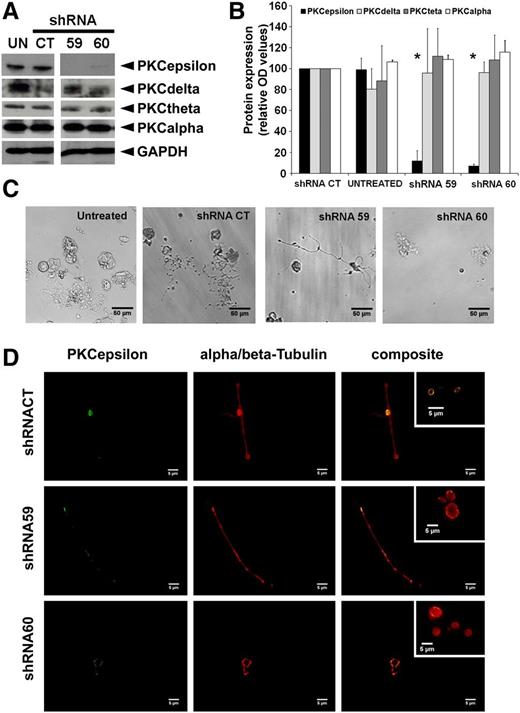
We then sought to determine whether PKCε expression was necessary for proplatelet production. Therefore we used recombinant lentiviral vectors to introduce and stably express shRNA molecules that specifically target PKCε into FLC at day 0 of culture. Analysis of megakaryocyte cultures at day 5 postinfection revealed that abrogation of PKCε was specific, because it did not modify either the expression of the other novel PKC isoforms or that of PKC α (Figure 2A-B). Morphological analyses of isolated megakaryocytes revealed that inhibition of PKCε expression resulted in the production of more aberrant proPLTs in comparison with control cultures (Figure 2C). The shRNA60 was more effective than shRNA59 in proPLT inhibition. However, although few residual branched protrusions could still be observed, proPLTs generated by both shRNA-infected cells were characterized by few abortive branches with some residual PKCε staining and virtual absence of the discoid structures. On the contrary, shRNACT proPLTs, characterized by the presence of discoid structures, expressed PKCε both in the cytoplasm and in the marginal tubular coil, where it appears particularly bright (Figure 2D). Moreover, we observed a greater than 70% reduction in platelet numbers in cultures in which we introduced PKCε shRNAs, and the remaining released PLTs were bigger than normal with a blurred tubulin-formed marginal tubular coil (Figure 2D insert), consistent with a defective cytoskeletal organization. Similar results were observed also by the inhibition of PKCε expression using specific small interfering RNA (siRNA) (supplemental data found on the Blood Website).
PKCε expression is necessary for proPLT formation. (A) Western blot detection of PKCε, PKCδ, PKC\x{03b8}θ, and PKCα in mouse MK untreated (UN) or infected with PKCε-specific shRNAs (shRNA59, shRNA60) and nontarget shRNACT. GAPDH was monitored for protein loading. (B) Densitometric analysis of PKC protein expression was performed using ImageJ software. Densitometric measurements of western blots from 3 replicates (means ± 1 SD; *P < .05 vs shRNACT ANOVA and Tukey tests). (C) Representative images of proplatelet-forming MK infected with PKCε-specific shRNAs (shRNA59, shRNA60) or control (shRNACT), isolated at day 4 by gradient and cultured for an additional 24 hours. (D) Immunofluorescence analyses of proplatelets from MK infected with PKCε-specific shRNA (shRNA59, shRNA60) and control (shRNACT), isolated at day 4 by gradient separation and cultured for a further 24 hours. Samples were labeled with specific antibodies against α/β-Tubulin and PKCε. The composite panels show the colocalization of PKCε and α/β-Tubulin at the tubular coil (particularly in released platelets, as shown in the insert) in shRNACT, and the abnormal morphology, in terms of dimension and tubular coil definition, of platelets released by PKCε-specific shRNA infected MKs. Samples were examined with microscope Axiovert 200 (Carl Zeiss, Inc., Thornwood, NY), equipped with a 63× numerical aperture 1.4 oil immersion objective. Images were obtained using a CCD camera and analyzed using the MetaMorph image analysis software. Images were obtained at 22°C and analyzed using ImageJ software. PKCε was detected with a rabbit polyclonal antibody and a secondary goat anti-rabbit antibody, conjugated to an Alexa Fluor 488; α and β tubulin were detected with rmouse monoclonal antibodies and a secondary goat anti-mouse antibody, conjugated to an Alexa Fluor 568.
PKCε expression is necessary for proPLT formation. (A) Western blot detection of PKCε, PKCδ, PKC\x{03b8}θ, and PKCα in mouse MK untreated (UN) or infected with PKCε-specific shRNAs (shRNA59, shRNA60) and nontarget shRNACT. GAPDH was monitored for protein loading. (B) Densitometric analysis of PKC protein expression was performed using ImageJ software. Densitometric measurements of western blots from 3 replicates (means ± 1 SD; *P < .05 vs shRNACT ANOVA and Tukey tests). (C) Representative images of proplatelet-forming MK infected with PKCε-specific shRNAs (shRNA59, shRNA60) or control (shRNACT), isolated at day 4 by gradient and cultured for an additional 24 hours. (D) Immunofluorescence analyses of proplatelets from MK infected with PKCε-specific shRNA (shRNA59, shRNA60) and control (shRNACT), isolated at day 4 by gradient separation and cultured for a further 24 hours. Samples were labeled with specific antibodies against α/β-Tubulin and PKCε. The composite panels show the colocalization of PKCε and α/β-Tubulin at the tubular coil (particularly in released platelets, as shown in the insert) in shRNACT, and the abnormal morphology, in terms of dimension and tubular coil definition, of platelets released by PKCε-specific shRNA infected MKs. Samples were examined with microscope Axiovert 200 (Carl Zeiss, Inc., Thornwood, NY), equipped with a 63× numerical aperture 1.4 oil immersion objective. Images were obtained using a CCD camera and analyzed using the MetaMorph image analysis software. Images were obtained at 22°C and analyzed using ImageJ software. PKCε was detected with a rabbit polyclonal antibody and a secondary goat anti-rabbit antibody, conjugated to an Alexa Fluor 488; α and β tubulin were detected with rmouse monoclonal antibodies and a secondary goat anti-mouse antibody, conjugated to an Alexa Fluor 568.
PKCε modulates RhoA in MK progenitors
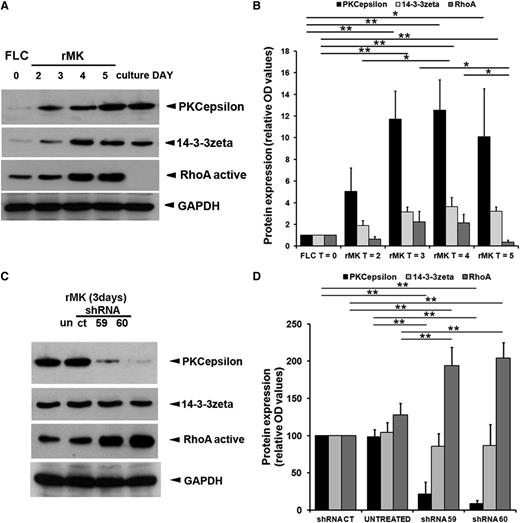
In certain cell types, PKCε cooperates with the 14-3-3zeta isoform to inhibit RhoA activity and drive cell division.22 RhoA levels are also downmodulated in the later phases of thrombopoiesis to allow MK polyploidization29 and cytoskeletal rearrangement.30 Western blot analyses of TPO-treated FLCs over the course of MK differentiation revealed that active and cleaved RhoA levels diminish at day 5 post–TPO stimulation, when rMK start producing proPLTs. On the contrary, the expression of both PKCε and 14-3-3zeta escalates within the first 2 days of TPO induction and then remains stable for the duration of MK differentiation (Figure 3A-B).
PKCε modulates RhoA in MK progenitors. (A) Western blot detection of PKCε, 14-3-3zeta, and RhoA protein expression in mouse MK cultured in the presence of TPO at indicated time points. GAPDH was monitored for protein loading. (B) Levels of PKCε, 14-3-3zeta, and RhoA protein expression during megakaryocytic differentiation of FLC. Densitometric measurements, performed using ImageJ software, of western blots from 3 replicates. Data are normalized for FLC (means ± 1 SD; *P < .05; **P < .01; ANOVA and Tukey tests). (C) Western blot detection of PKCε, 14-3-3zeta, and RhoA in mouse MK. At day 3 of culture, rMK untreated (un) or infected with PKCε-specific shRNA (shRNA59, shRNA60) and controls (shRNACT) were collected. GAPDH was monitored for protein loading. In the presence of PKCε-specific shRNA, the 14-3-3zeta protein levels remained stable at the control levels, whereas active RhoA expression levels sensibly increased. (D) Densitometric analysis of PKCε, 14-3-3zeta, and RhoA protein expression. Densitometric measurements, performed using ImageJ software, of western blots from 5 replicates. Data are normalized for shRNACT (means ± 1 SD; **P < .01 ANOVA and Tukey tests).
PKCε modulates RhoA in MK progenitors. (A) Western blot detection of PKCε, 14-3-3zeta, and RhoA protein expression in mouse MK cultured in the presence of TPO at indicated time points. GAPDH was monitored for protein loading. (B) Levels of PKCε, 14-3-3zeta, and RhoA protein expression during megakaryocytic differentiation of FLC. Densitometric measurements, performed using ImageJ software, of western blots from 3 replicates. Data are normalized for FLC (means ± 1 SD; *P < .05; **P < .01; ANOVA and Tukey tests). (C) Western blot detection of PKCε, 14-3-3zeta, and RhoA in mouse MK. At day 3 of culture, rMK untreated (un) or infected with PKCε-specific shRNA (shRNA59, shRNA60) and controls (shRNACT) were collected. GAPDH was monitored for protein loading. In the presence of PKCε-specific shRNA, the 14-3-3zeta protein levels remained stable at the control levels, whereas active RhoA expression levels sensibly increased. (D) Densitometric analysis of PKCε, 14-3-3zeta, and RhoA protein expression. Densitometric measurements, performed using ImageJ software, of western blots from 5 replicates. Data are normalized for shRNACT (means ± 1 SD; **P < .01 ANOVA and Tukey tests).
Next, we examined whether modulating PKCε expression impacted RhoA activity during proPLT formation. Though abrogation of PKCε expression in primary MK precursors did not affect 14-3-3zeta levels, we did observe a substantial increase of RhoA expression (Figure 3C). Figure 3D shows the cumulative densitometric data from 4 unrelated experiments.
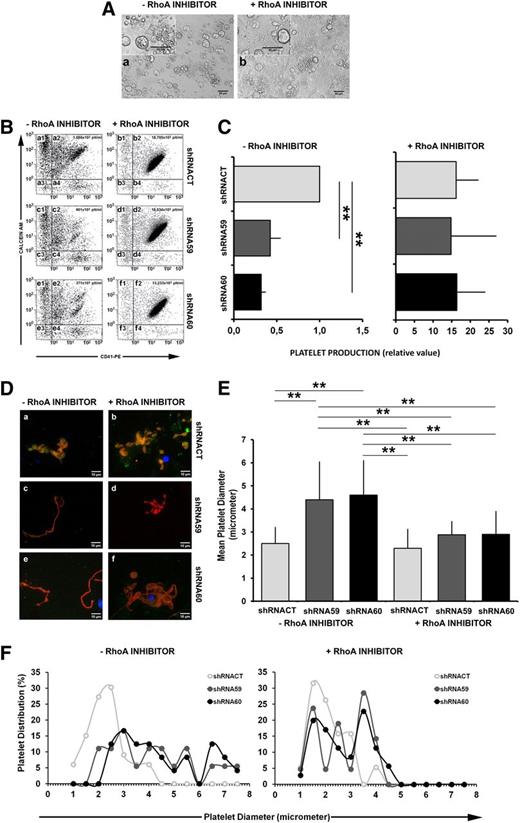
Finally, we set out to test whether the proPLT reduction mediated by PKCε inhibition was due to inappropriately high RhoA expression and activity. Therefore we analyzed the proPLT formation from PKCε knock-down and control samples in the presence or absence of a pharmacological RhoA inhibitor (Figure 4A). As shown in Figure 4B, using a well-established flow cytometric method,19,28 we also counted the absolute number of platelets in the culture medium. Flow cytometric analysis of functional platelets—positive for surface expression of CD41 and Calcein AM—showed that pharmacological inhibition of RhoA dramatically increased PLT formation and release in both control and PKCε-depleted samples (Figure 4B; a2 vs b2, c2 vs d2, and e2 vs f2; Figure 4C). Additionally, RhoA inhibition restored normal proPLT morphology in cells depleted of PKCε expression (Figure 4D-F). Figure 4F shows the size distribution within the platelet populations. Ninety-five percent of the shRNACT PLTs had a diameter smaller than 3.8 μm, whereas only 42% of the shRNA59 and 35% of the shRNA60 showed a diameter comparable to that of the control. Instead, in the presence of the RhoA inhibitor, 80% of shRNA59 and 75% of shRNA60 PLT populations returned to a size similar to that of the control.
RhoA inhibition restores proPLT formation and PLT release in MK infected with PKCε-specific shRNA. (A) Representative images of ppFMK in the absence or presence of RhoA pharmacological inhibitor. Cultures of cells treated with RhoA inhibitor presented increased quantities of proplatelets and released platelets. (B) Representative scatter plots of flow cytometric analyses of platelets released in culture. Fixed volumes of media from the different cultures were collected, labeled with CD41 and Calcein AM, and mixed with a fixed volume of calibration beads for absolute PLT count. Only cells CD41pos/Calcein AMpos were considered as preserved platelets (quadrants: a2, b2, c2, d2, e2, f2). The absolute count for each sample was reported inside the scatter plots. In the absence of RhoA inhibitor, the cells treated with PKCε-specific shRNA59 (c2) and shRNA60 (e2) released fewer platelets than did control (shRNACT; a2). In the presence of RhoA inhibitor, both control (shRNACT; b2) and PKCε-specific shRNA (shRNA59 and shRNA60; d2 and f2) samples released around 20-fold more platelets. (C) Analysis of PLT production. Data, from 4 replicates, are normalized for shRNACT (means ± 1 SD; **P < .01 ANOVA and Tukey tests). (D) Immunofluorescence analyses of proplatelets, labeled with specific antibodies against PKCε (green) and α/β-Tubulin (red), in the absence (a, c, e) or presence of RhoA inhibitor (b, d, f). In comparison with the control (shRNACT; a), proplatelets derived from PKCε-specific shRNAs infected MK (shRNA59 and shRNA60; c and e) were aberrant and lacking the typical spherical organization of PLT precursors. In the presence of the RhoA inhibitor, the shRNAs infected MK (shRNA59 and shRNA60; d and f) produced proplatelets with a morphology similar to the control (shRNACT; b). Samples were examined with microscope Axiovert 200 equipped with a 63× numerical aperture 1.4 oil immersion objective. Images were obtained using a CCD camera and analyzed using the MetaMorph image analysis software. Images were obtained at 22°C. PKCε was detected with a rabbit polyclonal antibody and a secondary goat anti-rabbit antibody, conjugated to an Alexa Fluor 488; α and β tubulin were detected with rmouse monoclonal antibodies and a secondary goat anti-mouse antibody, conjugated to an Alexa Fluor 568. (E) Analysis of PLT dimensions (means ± 1 SD; **P < .01 ANOVA and Tukey tests). For each group, several platelets were analyzed from different fields. In the absence of RhoA inhibitor the platelet counts were (1) shRNACT, 33 PLTs; (2) shRNA59, 18 PLTs; and (3) shRNA60, 23 PLTs. In the presence of RhoA inhibitor, they were (1) shRNACT, 19 PLTs; (2) shRNA59, 21 PLTs; and (3) shRNA60, 35 PLTs. The mean cell diameter was calculated with MetaMorph and Image J software. (F) Analysis of size distribution within PLT populations.
RhoA inhibition restores proPLT formation and PLT release in MK infected with PKCε-specific shRNA. (A) Representative images of ppFMK in the absence or presence of RhoA pharmacological inhibitor. Cultures of cells treated with RhoA inhibitor presented increased quantities of proplatelets and released platelets. (B) Representative scatter plots of flow cytometric analyses of platelets released in culture. Fixed volumes of media from the different cultures were collected, labeled with CD41 and Calcein AM, and mixed with a fixed volume of calibration beads for absolute PLT count. Only cells CD41pos/Calcein AMpos were considered as preserved platelets (quadrants: a2, b2, c2, d2, e2, f2). The absolute count for each sample was reported inside the scatter plots. In the absence of RhoA inhibitor, the cells treated with PKCε-specific shRNA59 (c2) and shRNA60 (e2) released fewer platelets than did control (shRNACT; a2). In the presence of RhoA inhibitor, both control (shRNACT; b2) and PKCε-specific shRNA (shRNA59 and shRNA60; d2 and f2) samples released around 20-fold more platelets. (C) Analysis of PLT production. Data, from 4 replicates, are normalized for shRNACT (means ± 1 SD; **P < .01 ANOVA and Tukey tests). (D) Immunofluorescence analyses of proplatelets, labeled with specific antibodies against PKCε (green) and α/β-Tubulin (red), in the absence (a, c, e) or presence of RhoA inhibitor (b, d, f). In comparison with the control (shRNACT; a), proplatelets derived from PKCε-specific shRNAs infected MK (shRNA59 and shRNA60; c and e) were aberrant and lacking the typical spherical organization of PLT precursors. In the presence of the RhoA inhibitor, the shRNAs infected MK (shRNA59 and shRNA60; d and f) produced proplatelets with a morphology similar to the control (shRNACT; b). Samples were examined with microscope Axiovert 200 equipped with a 63× numerical aperture 1.4 oil immersion objective. Images were obtained using a CCD camera and analyzed using the MetaMorph image analysis software. Images were obtained at 22°C. PKCε was detected with a rabbit polyclonal antibody and a secondary goat anti-rabbit antibody, conjugated to an Alexa Fluor 488; α and β tubulin were detected with rmouse monoclonal antibodies and a secondary goat anti-mouse antibody, conjugated to an Alexa Fluor 568. (E) Analysis of PLT dimensions (means ± 1 SD; **P < .01 ANOVA and Tukey tests). For each group, several platelets were analyzed from different fields. In the absence of RhoA inhibitor the platelet counts were (1) shRNACT, 33 PLTs; (2) shRNA59, 18 PLTs; and (3) shRNA60, 23 PLTs. In the presence of RhoA inhibitor, they were (1) shRNACT, 19 PLTs; (2) shRNA59, 21 PLTs; and (3) shRNA60, 35 PLTs. The mean cell diameter was calculated with MetaMorph and Image J software. (F) Analysis of size distribution within PLT populations.
Collectively, these data show that PKCε downregulates RhoA activity to promote proPLT formation and platelet release.
Discussion
The process of platelet formation from megakaryocytes is characterized by distinct morphological stages that require extensive cytoplasmic remodeling. We had previously demonstrated that PKCε levels are specifically modulated by TPO in order to drive human hematopoietic cell precursors to PLT-producing MK.18,31 We now show here that PKCε expression, which is virtually absent in mouse FLC, progressively increases in TPO-treated cells, and its levels remain high also in ppfMK and isolated proPLT. In proPLT, PKCε colocalizes with α/β-tubulin, which forms the marginal tubular coil, suggesting a functional correlation with the cytoskeleton reorganization events that accompany MK diffentiation and proplatelet formation. In support of this hypothesis, we observed that downregulation of PKCε expression by specific siRNAs resulted in lower proplatelet numbers and larger diameter platelets in culture. Because the efficiency of siRNA transfection is not homogeneous, we used recombinant lentiviral vectors that coexpress PKCε-targeting shRNAs and a puromycin-resistant cassette, thus allowing for the enrichment of PKCε-depleted cells. As expected, we detected a strong numerical reduction of proPLT formation with the remaining proPLTs lacking the typical organization in connected discoid structures.
It is well known that PKCε has a key role in neurite outgrowth,23 a process that shares aspects with proPLT formation, such as the involvement of the RhoA pathway23 which in turn is regulated by the complex PKCε/14-3-3zeta during MK polyploidization.22 For this reason, we subsequently asked whether PKCε regulates proPLT formation and PLT release via inhibition of RhoA.
Indeed, we found that the active form of RhoA was downregulated at the terminal phases of MK differentiation, just before proPLT production, whereas PKCε and 14-3-3zeta expression did not change. PKCε knockdown by specific shRNA resulted in a significant increase of the active RhoA expression, along with hampered proPLT formation and PLT production. Accordingly, PKCε-depleted precursors treated with the pharmacological RhoA inhibitor generated normally shaped proPLT, with long cytoplasmic extensions characterized by a network of discoid structures, and restored PLT generation both in terms of number and size. Pleines et al14 have previously described the effects of RhoA knockdown on PLT size, showing that RhoA−/− mice are macrothrombocytopenic with high ploidy MKs. Given the role of RhoA in a plethora of signaling pathways, the RhoA−/− mouse might well be considered as a good indicator of the relevance of RhoA in megakaryopoiesis, but probably is not ideal for assessing the impact of compensatory mechanisms that represent unpredictable variables in a highly specific setting as proPLT formation. Our data show that inhibiting RhoA specifically at day 4 (preterminal phase) of proPLT formation carefully avoids perturbations of the earlier phases of cell commitment and differentiation.
These data show for the first time that PKCε has a key role in mouse PLT formation, by modulation of RhoA activity and interaction with the marginal tubular coil. These results represent a valid model for unraveling the molecular regulation of PLT generation. In fact, though PKCε is not expressed in late human megakaryocytopoiesis and its forced expression impairs PLT generation18 and function,32 it must be considered that human—but not mouse—platelets express other novel PKC isoforms (namely PKCδ). The goal now will be to understand whether these 2 PKC isoforms parallel in the 2 species, with obvious consequences on our approach to human PLT disorders such as thrombocytopenia or thrombocytemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Matthew Devine, Luciana Cerasuolo, Vincenzo Palermo, Domenico Manfredi, and Davide Dallatana for technical support.
This work was supported by Regione Emilia-Romagna Area 1—Strategic Program 2010-2012, and Fondo per gli Investimenti per la Ricerca di Base-accordi di programma 2010 (Italian-Ministry of the University and Scientific and Technological Research/Ministry of Education, University and Research, Ministero dell'Istruzione, dell'Università e della Ricerca), RBAP10KCNS_002.
Authorship
Contribution: G.G., P.M., M.V., and J.E.I. designed the experiments and interpreted the data; C.C., G.G., E.M., F.F., and A.N. performed the experiments; J.N.T., J.E.I., and S.M.S. revised the manuscript; M.V. and G.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Vitale, Department of Biomedical, Biotechnological, and Translational Sciences, Operative Unit of Anatomy and Histology, University of Parma, Ospedale Maggiore, Via Gramsci, 14, I-43126 Parma, Italy; e-mail: marco.vitale@unipr.it.

![Figure 1. PKCε levels are modulated during MK differentiation. (A) Western blot detection of total PKCε protein expression in mouse MK cultures. GAPDH was monitored for protein loading. FLC, fetal liver cells; rMK, round MK; ppfMK, proplatelet-forming MK; PPL, proplatelets. (B) Relative PKCε protein expression during megakaryocytic differentiation from mouse FLC. Densitometric measurements of western blots from 5 replicates were performed by ImageJ software (means ± standard deviations [SD]; *P < .01 vs FLC culture day 0; #P < .05 vs rMK culture day 2). (C) Immunofluorescence analysis of PKCε and α/β-Tubulin expression and their colocalization in proPLT. Samples were examined with a confocal microscope Olympus FluoView FV10i (Center Valley, PA), with a 60× water-corrected objective. Each image represents a projection from a z-stack of 7 images collected at 0.5-µm increments. Images were obtained at 22°C and analyzed by ImageJ software. PKCε was detected with a rabbit polyclonal antibody and a secondary goat anti-rabbit antibody, conjugated to an Alexa Fluor 488; α and β tubulin were detected with mouse monoclonal antibodies and a secondary goat anti-mouse antibody, conjugated to an Alexa Fluor 568; MK nucleus was detected with DAPI.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/7/10.1182_blood-2013-04-490599/4/m_1305f1.jpeg?Expires=1767771199&Signature=1tDvqrIaLYGapBSeLKVlkChKY4E6xElNsZWaurTCE7yeXL0YE2oEfaWOFDG-0-b70Aq6S3MQc-QF13f9n2NF3n3r3gP6tppx~AK4SjqoUEJgmdFvOW~GgjdpFucm57XP~lbQMi3HGwiEmLanhtqw6xylIJBEUTC2sESK9dBVLKF7q2YkAVxJ5zBZzjmICRpwyDb6svKkzXyd828rkRFU5EhWTjlzq2TKtih25ghbVEyEPh0M3tfRfhLbSMgIuv2UmCle90rXaUx3vwndYLe9bVtw3FkiS8sXA8q7Jniin2hpTvU2SxnGWe7RWeGVGwbeXyLiRVwtmBFjjBhULW~F-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)