Key Points
Mucin-type O-glycans are required for terminal differentiation of megakaryocytes and platelet production.
The expression of GPIbα protein is strongly reduced in O-glycans–defective megakaryocytes and platelets.
Abstract
C1galt1 is essential for synthesis of the core 1 structure of mucin-type O-glycans. To clarify the physiological role of O-glycans in adult hematopoiesis, we exploited the interferon-inducible Mx1-Cre transgene to conditionally ablate the C1galtflox allele (Mx1-C1). Mx1-C1 mice exhibit severe thrombocytopenia, giant platelets, and prolonged bleeding times. Both the number and DNA ploidy of megakaryocytes in Mx1-C1 bone marrow were similar to those in wild-type (WT) mice. However, there were few proplatelets in Mx1-C1 primary megakaryocytes. Conversely, bone marrow transplanted from Mx1-C1 to WT and splenectomized Mx1-C1 mice gave rise to observations similar to those described above. The expression of GPIbα messenger RNA was unchanged in Mx1-C1 bone marrow, whereas flow cytometric and western blot analyses using megakaryocytes and platelets revealed that the expression of GPIbα protein was significantly reduced in Mx1-C1 mice. Moreover, circulating Mx1-C1 platelets exhibited an increase in the number of microtubule coils, despite normal levels of α- and β-tubulin. Our observations suggest that O-glycan is required for terminal megakaryocyte differentiation and platelet production and that the decrease in GPIbα in cells lacking O-glycan might be caused by increased proteolysis.
Introduction
Megakaryocytes develop from hematopoietic stem cells in the bone marrow via several sequential steps. During the promegakaryoblast stage, cell size and nuclear DNA ploidy increase by the process of endomitosis. The final differentiation stage of the maturation process is characterized by platelet release from the ends of long, thin cytoplasmic processes called proplatelets.1 In addition, platelets are released from proplatelets protruding into the vascular lumen of the bone marrow sinus under the force of shear stress.2 The mechanism of proplatelet formation is still not well understood.
The megakaryocyte-/platelet-specific membrane glycoprotein GPIbα is highly glycosylated with mucin-type O-glycans and forms the GPIb-IX-V complex.3 The binding of the GPIb-IX-V complex on circulating platelets to subendothelial von Willebrand factor (vWF) is essential for hemostasis. The physiological role of the GPIbα O-glycan moieties remains poorly understood.
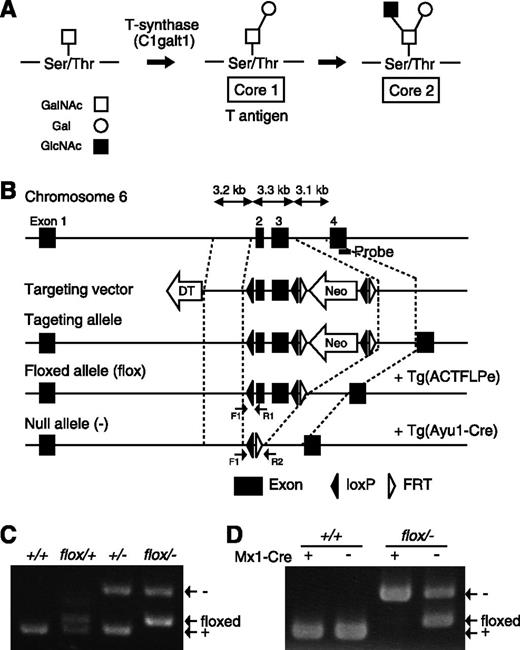
Mucins are typically expressed on the surface epithelium of the oral cavity, gastrointestine, nasal cavity, and synovial fluid and maintain a barrier against invading pathogens. They are high-molecular-weight proteins that contain multiple tandem repeats of Muc peptides modified by large numbers of mucin-type O-glycans. The addition of N-acetylgalactosamine (GalNAc) to serine or threonine residues on proteins by UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases initiates the biosynthesis of mucin-type O-glycans (Figure 1A). The GalNAc residue attached to the peptide is usually modified by further glycosylation extending from the core 1 and core 2 structures by the action of multiple glycosyltransferases. Galβ1-3GalNAcα-serine/threonine (core 1) is the major constituent of the mucin-type O-glycan core structure in many cells. The core 1 structure is also called the Thomsen-Friedenreich antigen (T antigen), which is a major cancer-associated antigen found in clinical specimens of colorectal carcinomas,4 breast tumors,5 and other types of cancer. T synthase encoded by the C1galt1 gene6,7 and its molecular chaperon, Cosmc (core 1 β3-Gal-T [C1galt1]-specific molecular chaperon), are required for the generation of T antigen and core 1–derived O-glycan.8 C1galt1 is a unique enzyme involved in core 1 synthesis, and homozygous knockout embryos exhibit embryonic lethality, defective angiogenesis, and fetal embryonic hemorrhage.9 These phenotypes indicate that O-glycosylation is essential for normal development and angiogenesis.
Generation of C1galt1 conditional knockout mice. (A) Schematic showing the mucin-type O-glycan biosynthetic pathway and relevant glycosyltransferases. (B) Targeting strategy for conditional deletion of the C1galt1 gene. The targeting construct contains the second and third exons of C1galt1 and the PGK-neo cassette (Neo) flanked by three loxP sites and Neo flanked by FRT sites. Neo was removed by crossing with mice expressing FLPe under control of the human β-actin promoter. C1galt1 exons were deleted by mating C1galt1flox/+ mice with Ayu1-Cre mice, which ubiquitously express the cre recombinase. The position of the Southern hybridization probe is indicated by the bold line. Arrows, F1, R1, and R2 indicate the position of primers for genotyping. PCR performed on genomic DNA extracted from (C) tails and (D) bone marrow of WT and Mx1-cre::C1galt1flox/− mice injected with pIpC.
Generation of C1galt1 conditional knockout mice. (A) Schematic showing the mucin-type O-glycan biosynthetic pathway and relevant glycosyltransferases. (B) Targeting strategy for conditional deletion of the C1galt1 gene. The targeting construct contains the second and third exons of C1galt1 and the PGK-neo cassette (Neo) flanked by three loxP sites and Neo flanked by FRT sites. Neo was removed by crossing with mice expressing FLPe under control of the human β-actin promoter. C1galt1 exons were deleted by mating C1galt1flox/+ mice with Ayu1-Cre mice, which ubiquitously express the cre recombinase. The position of the Southern hybridization probe is indicated by the bold line. Arrows, F1, R1, and R2 indicate the position of primers for genotyping. PCR performed on genomic DNA extracted from (C) tails and (D) bone marrow of WT and Mx1-cre::C1galt1flox/− mice injected with pIpC.
In this study, we generated mice conditionally knocked out for C1galt1 to show that O-glycosylation is critically required for the terminal differentiation of megakaryocytes and platelet production.
Materials and methods
Mice
Mice were maintained in specific pathogen-free conditions in a laboratory animal resource center at the University of Tsukuba. All experiments were performed according to the Guide for the Care and Use of Laboratory Animals at the University of Tsukuba. The targeting vector for C1galt1 gene disruption was constructed by ligation of three polymerase chain reaction (PCR) fragments into a conditional targeting vector cassette. The targeting vector was linearized and transfected into C57BL/6J mouse embryonic stem (ES) cells.10 The resulting cells were selected in medium containing 0.3 mg/mL G418 (Nacalai Tesque). The anticipated homologous recombination was subsequently confirmed by PCR and Southern hybridization. Targeted ES cells were injected into ICR mouse blastocysts to generate chimeric mice. Male mice chimeric for the targeted allele were mated with female Ayu1-Cre (a general deleter Cre recombinase–expressing transgenic line) mice11 in order to remove the center arm containing an exon encoding the translational start codon and transmembrane domain of C1galt1.
Cre deletion.
Male mice chimeric for the targeted allele were mated with female Flpe-transgenic mice to remove the neo cassette and generate a floxed C1galt1 allele (C1galt1flox/+). C1galt1flox/+ and C1galt1+/− mice were crossed with Mx1-Cre transgenic mice12 to generate inducible conditional C1galt1-knockout mice (Mx1::C1galt1flox/−). Conditional excision of the floxed allele was achieved by intraperitoneal injection of 10 μg/g body weight of polyinosinic/polycytidylic acid (pIpC; Sigma-Aldrich) 3 times at 2-day intervals. Thrombocytopenia developed approximately 2 weeks after pIpC treatment. Excision of the floxed allele and nearly complete absence of the C1galt1 transcript in bone marrow were confirmed by real-time PCR (RT-PCR).
Hematologic analysis of peripheral blood
Peripheral blood samples from adult mice were obtained from retro-orbital venous plexus sampling in polypropylene tubes containing EDTA. Blood counts were determined with an automated hemocytometer (Nihon Kohden). Blood samples were smeared onto microscope slides and stained with May-Grünwald Giemsa stain, and then photographed with a KEYENCE BIOREVO BZ-9000 microscope (KEYENCE).
Bleeding time assay
Mice were anesthetized with isoflurane via a facemask throughout the experiment. The tail was cut 3 mm from the tip and immediately immersed in saline at 37°C. Tail bleeding times were determined as the time point at which all visible signs of bleeding from the incision had stopped or at 10 minutes.13
Histologic analysis.
Dissected mouse tissues were fixed in Mildform 10N (Wako Pure Chemical Industries) after two washes in phosphate-buffered saline (PBS) and then embedded in paraffin. Hard tissues were decalcified in Morse’s solution (10% sodium citrate and 22.5% formic acid) for 24 hours after fixing in 4% formalin before embedding in paraffin. Four micrometer-thick sections were prepared from spleen, humerus, and tibia and were subjected to staining with hematoxylin and eosin. Megakaryocytes in the sections were photographed and analyzed with Adobe Photoshop software (Adobe Systems, Inc.).
Ploidy analysis of megakaryocytes
Bone marrow cells were collected from femurs and tibias in 2% fetal bovine serum/PBS. The cells were preincubated with an FcγR-blocking monoclonal antibody (mAb; 2.4G2, BD) and then stained with fluorescein isothiocyanate (FITC) –conjugated anti-mouse CD41 antibody (FITC-CD41, eBioMWReg30; BioLegend). After permeabilization in 70% ethanol, DNA from the permeabilized cells was stained with 6 μg/mL propidium iodide (Invitrogen), and treated with 25 μg/mL RNase. The ploidy of CD41+ cells was analyzed by a FACSCalibur/CellQuest system (BD).
PPF assay
Megakaryocytes were separated as described previously.14 Briefly, bone marrow in 0.1% bovine serum albumin (BSA)/CATCH buffer (Hanks' balanced salt solution with 1.3 mM sodium citrate, 1 mM adenosine, 2 mM theophylline, and 2% fetal calf serum) was laid over 50% Percoll solution and centrifuged at 200g for 20 minutes at room temperature. The cells were harvested from the interface and purified further by gravity sedimentation, using discontinuous BSA gradients. To analyze proplatelet formation (PPF) onto different adhesive substrates, eight-well chamber slides (Nunc Lab-Tek) were coated with 100 μg/mL fibrinogen (Sigma-Aldrich) for 2 hours at room temperature. Enriched megakaryocytes were cultured in eight-well chamber slides for 14 hours at 37°C and 5% CO2. PPF was observed by staining with FITC-CD41 mAb and observed under a BIOREVO BZ-9000 microscope system. CD41+ cells with cytoplasmic processes longer than the diameter of the cytoplasm were defined as proplatelet-forming megakaryocyte cells.
Immunofluorescence microscopy
Megakaryocytes cultured in eight-well chamber slides were fixed in 3% paraformaldehyde for 10 minutes, washed with Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl, pH 7.4), permeabilized in 0.1% Triton X-100 in Tris-buffered saline for 5 minutes, and washed again. After blocking with 3% BSA, cells were stained with anti-vWF or an Alexa Fluor 546 conjugate of Helix pomatia (HPA) lectin for 2 hours at room temperature. Megakaryocytes were detected by staining with FITC-CD41. After fixation with Vectashield mounting medium containing 4,6 diamidino-2-phenylindole (Vector), cells were observed with a BIOREVO BZ-9000 microscope system using a Plan Apo 40×/0.95 objective (Nikon).
Tubulin staining
Primary megakaryocytes cultured in eight-well chamber slides were fixed in Mildform 10N for 20 minutes, washed with PBS, permeabilized in 0.1% Triton X-100 in PBS for 5 minutes, and washed again. After blocking with 5% normal goat serum, cells were stained with anti–β-tubulin (2-28-33; Sigma-Aldrich) and then with an Alexa Fluor 594 conjugated anti-mouse immunoglobulin G for 2 hours at room temperature. Megakaryocytes were detected by staining with FITC-CD41. The cell images were observed by using a Leica TCS SP5 confocal laser scanning microscope with an HCX PL APO CS 40.0×/1.25 oil immersion objective.
Reconstitution of the hematopoietic system and splenectomy
Donor cells for hematopoietic reconstitution were prepared from the bone marrow of Mx1::C1galt1flox/− mice (C57BL/6J, CD45.2). Cells were injected into lethally irradiated (10.5 Gy) recipient mice (SJL, CD45.1) under general anesthesia via the retro-orbital vein. Mice were maintained for 1 to 2 months before analysis. Splenectomy was carried out under ether anesthesia as previously described.15
Flow cytometric analysis of CD42 (GPIbα)
Whole blood was obtained by puncturing the right ventricle of isoflurane-anesthetized mice. Blood was anticoagulated by collecting into a 1:10 volume of a Citramin solution (Fuso Pharmaceutical, Japan) containing a 3.8% (w/v) sodium citrate solution. Platelet-rich plasma (PRP) was isolated by centrifuging whole blood at low speed (180g, 10 minutes, room temperature). After centrifugation, the supernatant was washed and resuspended in Plt-HEPES-ACD buffer (137 mM NaCl, 2 mM KCl, 0.4 mM NaH2PO4, 1 mM MgCl2, 1 mM CaCl2, and 10% [w/v] anticoagulant citrate dextrose A [ACD-A] solution) (Terumo). CD41+ cells were enriched from bone marrow by the MACS cell separation system (Miltenyi Biotec). Bone marrow cells were exposed to FITC-CD41 and mixed with anti-FITC microbeads (Miltenyi Biotec). The associated cells were separated by using a large-cell separation column (Miltenyi Biotec). However, contaminating nonmegakaryocytic cells could not be completely removed by this method. Platelets in PRP- and CD41-enriched cells were immunostained with FITC-CD41 and phycoerythrin-conjugated anti-CD42 mAbs (GPIbα, Xia.G5; Emfret). Data were analyzed by using FlowJo (Tree Star Inc.) analysis software.
Transmission electron microscopy
Platelets in PRP samples were fixed in 0.1% glutaraldehyde/0.1 M phosphate buffer for 10 minutes at room temperature, then fixed in cold glutaraldehyde/0.1 M phosphate buffer for 30 minutes at 4°C, and postfixed in 1% osmium tetroxide for 30 minutes at 4°C. After dehydration in ethanol, the samples were embedded in epon. The thin sections were double stained with uranyl acetate and lead citrate and examined under a JEM1400 transmission electron microscope (JEOL).
Quantitative RT-PCR
Total RNA was isolated from the bone marrow of adult mice by using an RNeasy Mini Kit (Qiagen), and complementary DNA templates were synthesized from the total RNA by using a QuantiTect Reverse Transcription Kit (Qiagen). PCR was carried out with a 7500 Fast Real-Time PCR System (Applied Biosystems) that used a Fast SYBR Green Master Mix (Applied Biosystems) as the reporter. TaqMan Rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Control Reagents (Cat. No.4308313; Applied Biosystems) were used for the quantitative analysis of gapdh genes as the internal control. The following primer sets were used for the SYBR Green method: C1galt1, (forward) 5′-CACCACTTAATCAAAGGTTATCTACCAA-3′, (reverse) 5′-AGGACCCTCTATGGGAGGATAATAG-3′; cd41, (forward) 5′-TGGCTTCTCAGTGGACTTTCATAA-3′, (reverse) 5′-GGCGCCCACCACGAT-3′; gpIbα, (forward) 5′-GCTAGTAGAGAGAAGGACCGAGTCA-3′, (reverse) 5′-GAGCCATATGAACAGACAGTCCTTT-3′; gpIbβ, (forward) 5′-CCTCATTTGGCGGTTTCTGT-3′, (reverse) 5′-TCCCTCAGGCCCTTTCTAGAAT-3′; and gpIX, (forward) 5′-TGTGCTGGGCCTGATTCTG-3′, (reverse) 5′-GGCTCAGTTCCTGGACTCTGTAA-3′. The relative amount of each gene was normalized to the amount of the gapdh transcript in the same complementary DNA sample.
Western blot analysis
Platelet and megakaryocyte extracts from bone marrow were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a Hybond enhanced chemiluminescence (ECL) (GE Healthcare) membrane. The membrane was exposed to the following primary Abs: anti-mouse GPIbα (Xia.G7; Emfret), anti–α-tubulin (B-5-1-2; Sigma-Aldrich), anti–β-tubulin, and anti–β-actin (A3854; Sigma-Aldrich). Signals specific to each primary Ab were developed by horseradish peroxidase conjugated to each secondary Ab to the anti-mouse immunoglobulin G by using Western Lighting Plus-ECL (PerkinElmer, Inc.) and Hyperfilm ECL (GE Healthcare).
Statistical analysis
Results are reported as the mean ± standard error of the mean. Statistical analyses were performed by using the Student t test. P values are provided in the figure legends.
Results
Generation of conditional C1galt1 knockout mice
We generated conditional C1galt1 mice by using the Cre-loxP system. A targeting vector was constructed in which exon 1, containing the 5′-untranslational region and encoding a translational start site followed by the transmembrane domain, was flanked with loxP sequences (Figure 1B). We confirmed homologous recombination between the targeting vector and the C1galt1 locus in C57BL6/J mouse ES cells by PCR and Southern hybridization (data not shown). Chimeric mice were obtained by microinjecting C1galt1flp/+ ES cells into mouse blastocysts, and the resulting animals were crossed with C57BL/6J mice to achieve germ line transmission of the floxed allele. Next, C1galt1flox/+ mice were mated with general deleter mice, ACTB-FLPe, to remove the phosphoglycerokinase (PGK) neomycin resistance (neo) cassette, which might produce unwanted side effects, by excisional recombination of the Flp/FRT system. Heterozygotes (C1galt1+/−) harboring the null allele were successfully generated by mating male C1galt1flox/+ mice with female Ayu1-Cre mice, a general deleter Cre recombinase–expressing transgenic line.11 Homozygous mice (C1galt1−/−) were generated by subsequent interbreeding of C1galt1+/− mice, which exhibited fetal hemorrhage and embryonic lethality, as was previously reported by Xia et al.9 The genotype of C1galt1 mice harboring the null allele was confirmed by PCR by using tail genomic DNA to amplify a 269-bp product from the wild-type (WT) allele, a 293-bp fragment from the C1galt1 floxed allele, and a 375-bp fragment from the C1galt1 null allele (Figure 1C). Further confirmation was obtained by Southern hybridization using tail genomic DNA (data not shown).
Next, C1galt1flox/− mice were crossed with Mx1-cre mice to generate pIpC-induced conditional knockout mice with a C1galt1 gene deletion restricted to bone marrow cells. Hayashi et al16 reported that the efficiency of pIpC-induced recombination in Mx1-cre mice was nearly complete in the bone marrow, liver, and spleen. We confirmed deletion of the C1galt1 gene by Mx1-mediated recombination in the bone marrow of pIpC-treated Mx1/C1galt1flox/− mice (Figure 1D). These results demonstrate successful disruption of the C1galt1 gene on the C57BL/6J background. Mx1-Cre/C1galt1flox/− mice treated with pIpC were designated “Mx1-C1” mice.
Mx1-C1 mice exhibit severe thrombocytopenia.
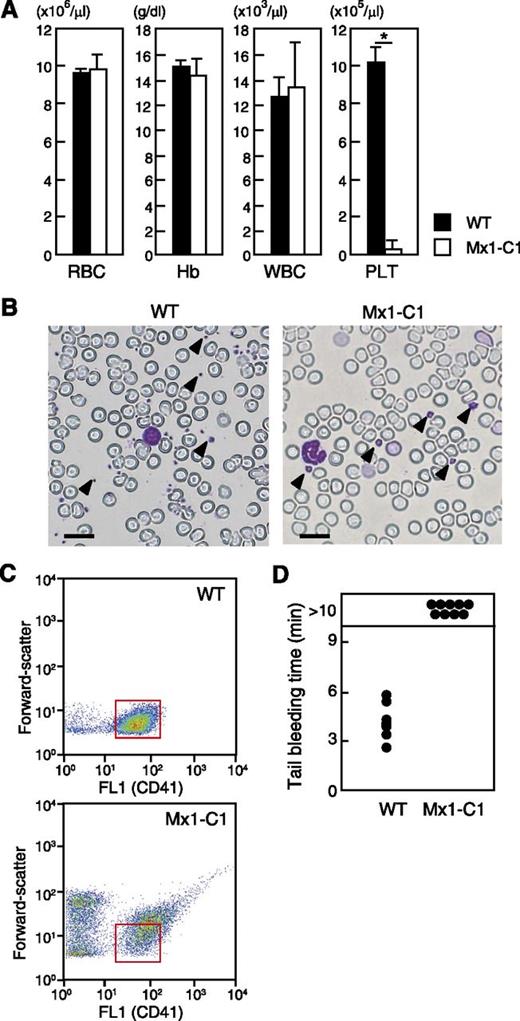
Analysis of the hematologic parameters of Mx1-C1 mice revealed a significant decrease in platelet counts but normal red and white blood cell counts and hemoglobin level (Figure 2A). Giant platelets were observed in May-Grünwald Giemsa–stained peripheral blood smears from Mx1-C1 mice, whereas the morphology of other cells was normal (Figure 2B). Platelets were identified by using fluorescence FL1 (FITC–anti-CD41), and their sizes (forward-scatter) were evaluated by using flow cytometry. These profiles showed that the size of Mx1-C1 platelets was greater than that observed in WT mice (Figure 2C). Tail bleeding time assays were performed to define the role of mucin-type O-glycans in hemostasis (Figure 2D). Bleeding in WT mice was arrested by 6 minutes after the tail tip was cut, whereas bleeding in Mx1-C1 mice was significantly prolonged (more than 10 minutes).
Blood parameters in WT and pIpC-treated Mx1-C1 mice. (A) Red blood cells (RBC), hemoglobin (Hb), white blood cells (WBC) and platelets (PLT). The results represent the median values from 5 WT vs 5 Mx1-C1 males. *P < .01. (B) May-Grünwald Giemsa stain of peripheral blood smears from WT and Mx1-C1 mice. Representative platelets are indicated by arrowheads. Scale bars, 20 μm. (C) Dot plots of forward-light scattering vs FL1 (FITC–anti-CD41) profiles of PRP display of the entire population of platelet sizes in WT and Mx1-C1 mice. Insert boxes (red) indicate the CD41+ population of WT platelets. (D) Prolonged bleeding time in Mx1-C1 mice. Tail bleeding times of WT (n = 6) and Mx1-C1 (n = 9) mice were monitored visually.
Blood parameters in WT and pIpC-treated Mx1-C1 mice. (A) Red blood cells (RBC), hemoglobin (Hb), white blood cells (WBC) and platelets (PLT). The results represent the median values from 5 WT vs 5 Mx1-C1 males. *P < .01. (B) May-Grünwald Giemsa stain of peripheral blood smears from WT and Mx1-C1 mice. Representative platelets are indicated by arrowheads. Scale bars, 20 μm. (C) Dot plots of forward-light scattering vs FL1 (FITC–anti-CD41) profiles of PRP display of the entire population of platelet sizes in WT and Mx1-C1 mice. Insert boxes (red) indicate the CD41+ population of WT platelets. (D) Prolonged bleeding time in Mx1-C1 mice. Tail bleeding times of WT (n = 6) and Mx1-C1 (n = 9) mice were monitored visually.
Normal number of bone marrow megakaryocytes in Mx1-C1 mice.
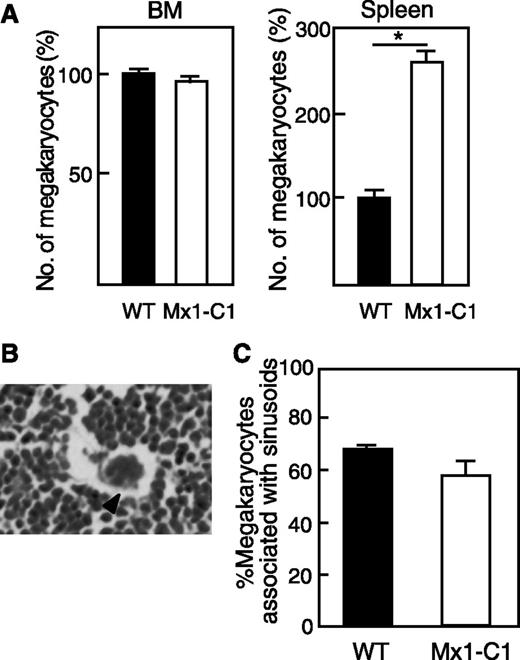
Thrombopoiesis is the process by which platelets are produced from megakaryocytes in the bone marrow and spleen. A reduced platelet number could be due to a reduced megakaryocyte number or a decrease in the breakdown of megakaryocytes to form platelets. To determine whether macrothrombocytopenia in Mx1-C1 mice was due to inefficient production of megakarocytes, we compared the number of megakaryocytes in WT and Mx1-C1 mice. The bone marrow and spleen appeared normal in Mx1-C1 mice. The number of megakaryocytes in the bone marrow also appeared normal in Mx1-C1 mice by hematoxylin and eosin staining (Figure 3A). However, the megakaryocyte counts in the spleen were significantly increased in Mx1-C1 mice. A typical megakaryocyte associated with sinosoids is shown in Figure 3B. The fraction of megakaryocytes associated with the bone marrow sinusoid microvasculature was similar in Mx1-C1 and WT mice (Figure 3C). To examine whether the decrease in peripheral platelet counts was due to an increase in platelet consumption by the spleen, we generated splenectomized Mx1-C1 mice. The splenectomized Mx1-C1 mice exhibited thrombocytopenia and extended bleeding times similar to those observed in nontreated Mx1-C1 mice (data not shown). These results suggest that the reduction in the peripheral platelet count might result from a defect in platelet production owing to a defect in megakaryocytes.
Number of megakaryocytes. (A) Comparison of the number of megakaryocytes in bone marrow and spleen of WT (n = 3) and Mx1-C1 (n = 3) mice. Values are expressed as means ± standard error (SE) relative to the WT level (100%). *P < .01. (B) Photomicrograph of a hematoxylin and eosin–stained bone marrow section illustrating a megakaryocyte intimately associated with marrow vessels. Scale bar, 10 μm. (C) Quantification of megakaryocyte number associated with sinusoidal vessels in WT (n = 3) and Mx1-C1 (n = 3) mice. Values are presented as means ± standard error of the mean (SEM).
Number of megakaryocytes. (A) Comparison of the number of megakaryocytes in bone marrow and spleen of WT (n = 3) and Mx1-C1 (n = 3) mice. Values are expressed as means ± standard error (SE) relative to the WT level (100%). *P < .01. (B) Photomicrograph of a hematoxylin and eosin–stained bone marrow section illustrating a megakaryocyte intimately associated with marrow vessels. Scale bar, 10 μm. (C) Quantification of megakaryocyte number associated with sinusoidal vessels in WT (n = 3) and Mx1-C1 (n = 3) mice. Values are presented as means ± standard error of the mean (SEM).
Abnormal morphology of terminally differentiating Mx1-C1 megakaryocytes.
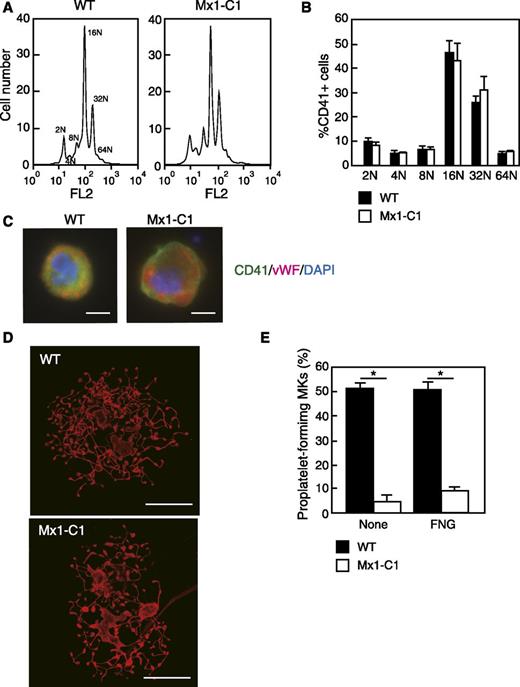
Since megakaryocyte differentiation correlates with increased DNA content, we next examined the distribution of relative DNA content in megakaryocytes derived from the bone marrow of WT and Mx1-C1 mice. Flow cytometry was performed to examine in further detail whether megakaryotic development was altered in Mx1-C1 mice. Propidium iodide ploidy analysis of bone marrow CD41+ megakaryocytes showed no significant difference between WT and Mx1-C1 cells (Figure 4A-B). We analyzed Mx1-C1 bone marrow megakaryocytes stained with CD41 and vWF to examine the expression of megakaryocyte differentiation markers in primary megakaryocytes. We observed a typical granular distribution of vWF in both WT and Mx1-C1 megakaryocytes (Figure 4C). These results indicate that commitment to polyploidization is normal in Mx1-C1 megakaryocytes.
Abnormal terminal differentiation of megakaryocytes in Mx1-C1 mice. (A) Ploidy analysis of femur bone marrow CD41+ megakaryocytes form WT and Mx1-C1 mice. (B) Bar graph of megakaryocyte ploidy analysis. Data represent the mean ± standard deviation (n = 3). (C) Megakaryocytes were stained for CD41 (green), vWF (magenta), and 4,6 diamidino-2-phenylindole (DAPI) (blue). Both WT and Mx1-C1 megakaryocytes exhibit finely granular cytoplasmic staining by anti-vWF Ab. Scale bars, 10 μm. (D) Proplatelet-forming megakaryocytes obtained from WT and Mx1-C1 mice were cultured on fibrinogen-coated glass slides for 14 hours. These proplatelet-forming megakaryocytes were stained for β-tubulin. Scale bars, 50 μm. (E) The percentage of proplatelet-forming megakaryocytes in CD41+ cells was analyzed by fluorescence microscopy. Primary megakaryocytes were plated on fibrinogen-coated (FNG) glass slides or plated in the absence of adhesive proteins (None). Values represent the means ± SEM. *P < .01.
Abnormal terminal differentiation of megakaryocytes in Mx1-C1 mice. (A) Ploidy analysis of femur bone marrow CD41+ megakaryocytes form WT and Mx1-C1 mice. (B) Bar graph of megakaryocyte ploidy analysis. Data represent the mean ± standard deviation (n = 3). (C) Megakaryocytes were stained for CD41 (green), vWF (magenta), and 4,6 diamidino-2-phenylindole (DAPI) (blue). Both WT and Mx1-C1 megakaryocytes exhibit finely granular cytoplasmic staining by anti-vWF Ab. Scale bars, 10 μm. (D) Proplatelet-forming megakaryocytes obtained from WT and Mx1-C1 mice were cultured on fibrinogen-coated glass slides for 14 hours. These proplatelet-forming megakaryocytes were stained for β-tubulin. Scale bars, 50 μm. (E) The percentage of proplatelet-forming megakaryocytes in CD41+ cells was analyzed by fluorescence microscopy. Primary megakaryocytes were plated on fibrinogen-coated (FNG) glass slides or plated in the absence of adhesive proteins (None). Values represent the means ± SEM. *P < .01.
To determine whether thrombocytopenia in Mx1-C1 mice was derived from defects in the terminal differentiation of megakaryocytes, we performed a PPF assay by using primary megakaryocytes from bone marrow. Primary megakaryocytes were plated on noncoated or fibrinogen-coated glass slides and cultured for 14 hours. Megakaryocytes were identified by immunostaining with β-tubulin and the megakaryocyte marker CD41. Figure 4D shows β-tubulin expression in megakaryocytes forming proplatelets from WT and Mx1-C1 mice. WT megakaryocytes exhibited typical formation of proplatelets appearing as extensively fragmented mature proplatelets. In contrast, Mx1-C1 CD41+ proplatelets were shorter and exhibited less branching and budding. WT megakaryocytes comprised both fragmented proplatelets and spherical immature cells, whereas Mx1-C1 megakaryocytes comprised mostly spherical immature cells. Therefore, the fraction of proplatelet forming megakaryocytes on noncoated or fibrinogen-coated glass slides was significantly lower in Mx1-C1 CD41+ cells than in WT cells (Figure 4E).
Megakaryocytes transmigrate through the sinusoidal endothelium in bone marrow and release platelets into the sinus. Because Mx1-Cre is expressed in multiple tissues, ablation of the target gene is not restricted to hematopoietic cells of the bone marrow. Therefore, to determine whether these results could be attributed to an intrinsic hematopoietic requirement for C1galt1, we transplanted bone marrow cells from Mx1::C1galt1flox/− donor mice (Ly5.1) into recipient mice (Ly5.2). Subsequent pIpC treatment resulted in thrombocytopenia and abnormal proplatelet formation in the transplants that was similar to what was observed in nontransplanted Mx1-C1 mice (data not shown). These results indicated that O-glycosylation in megakaryocytes plays an important role in megakaryocyte terminal differentiation at the stage of proplatelet formation.
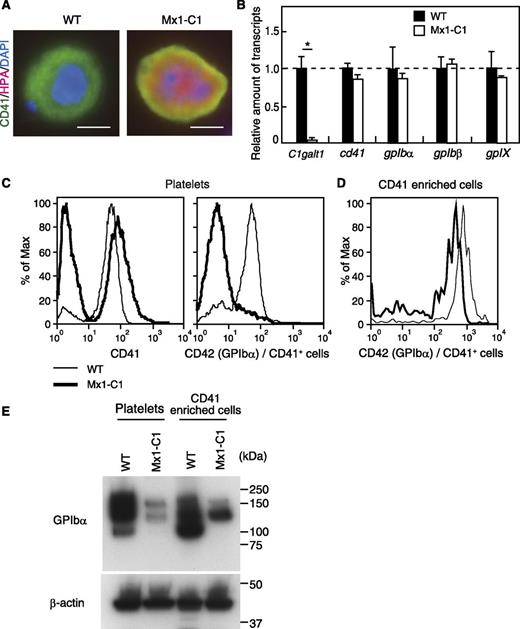
The expression of GPIbα protein is strongly reduced in Mx1-C1 platelets, despite normal expression of the GPIbα transcript in Mx1-C1 megakaryocytes.
To investigate changes in carbohydrate modification in Mx1-C1 mice, we stained WT and Mx1-C1 primary megakaryocytes with HPA lectin, which recognizes terminal α-GalNAc. CD41+ Mx1-C1 megakaryocytes exhibited HPA lectin–reactive carbohydrate epitopes both on the cell surface (CD41 [green] merged yellow) and within the cytoplasm (orange), whereas WT megakaryocytes did not (Figure 5A). The appearance of GalNAc epitope in Mx1-C1 megakaryocytes results in the disappearance of core 1 and more extended carbohydrates by ablation of the C1galt1 gene (Figure 1A). Next, to elucidate the molecular mechanism underlying the abnormal differentiation of megakaryocytes in Mx1-C1 mice, we first compared the expression of megakaryocyte and platelet-specific glycoproteins to that observed in WT mice. Bone marrow cells from WT and Mx1-C1 mice were analyzed for expression of megakaryocyte-specific transcripts by using RT-PCR. C1galt1 transcripts were essentially absent from Mx1-C1 bone marrow cells. However, the expression of megakaryocyte-specific transcripts encoding cd41, gpibα, gpIbβ, and gpIX was unchanged in Mx1-C1 bone marrow cells (Figure 5B). Next, we examined the expression of CD41 and GPIbα (CD42) protein on the surface of platelets in PRP by flow cytometry (Figure 5C). There were two peaks of CD41 expression in Mx1-C1 PRP. The CD41+ peak was similar in intensity to that observed in WT cells. We hypothesize that the increase in the intensity of the CD41− peak in Mx1-C1 cells represents an increase in cellular debris and/or machine noise owing to the analysis of a large volume of the Mx1-C1 PRP containing few platelets. Interestingly, the expression of GPIbα in Mx1-C1 CD41+ platelets was strongly decreased. Moreover, GPIbα expression on the surface of CD41-enriched cells was also decreased in Mx1-C1 mice (Figure 5D). To determine whether the reduction of GPIbα expression on the cell surface was due to protein instability or abnormal surface trafficking, GPIbα expression was examined by using western blot analysis of platelets from PRP and CD41-enriched cells from bone marrow (Figure 5E). Anti-GPIbα Ab gave rise to broad signals (100 to ∼200 kDa) in both WT platelets and WT CD41-enriched cells. In contrast, Mx1-C1 samples showed two major bands at approximately 160 and 130 kDa. Moreover, the signal intensities in the Mx1-C1 samples were only 10% to 20% of that observed in WT samples.
Expression of megakaryocytic-/platelet-specific glycoproteins in Mx1-C1 mice. (A) HPA lectin staining of WT and Mx1-C1 megakaryocytes. Merged image of anti-CD41 (green), HPA lectin (magenta), and DAPI (blue). Scale bars, 10 μm. (B) Quantitative analysis of transcription of megakaryocytic-/platelet-specific glycoproteins in bone marrow using RT-PCR. The expression of each transcript was normalized to that of the gapdh transcript. Data were obtained from triplicate experiments and presented as means ± SEM. *P < .01. (C) Flow cytometric analysis of the expression of CD41 and CD42 (GPIbα) in PRP prepared from WT and Mx1-C1 peripheral blood. The expression of CD42 was represented in a fraction of CD41+ cells within PRP. (D) Flow cytometric analysis of the expression of CD42 (GPIbα) in CD41-enriched cells prepared from WT and Mx1-C1 bone marrow cells. (E) Western blot analysis of platelet or enriched megakaryocyte extracts with anti-GPIbα and anti–β-actin Abs as internal controls.
Expression of megakaryocytic-/platelet-specific glycoproteins in Mx1-C1 mice. (A) HPA lectin staining of WT and Mx1-C1 megakaryocytes. Merged image of anti-CD41 (green), HPA lectin (magenta), and DAPI (blue). Scale bars, 10 μm. (B) Quantitative analysis of transcription of megakaryocytic-/platelet-specific glycoproteins in bone marrow using RT-PCR. The expression of each transcript was normalized to that of the gapdh transcript. Data were obtained from triplicate experiments and presented as means ± SEM. *P < .01. (C) Flow cytometric analysis of the expression of CD41 and CD42 (GPIbα) in PRP prepared from WT and Mx1-C1 peripheral blood. The expression of CD42 was represented in a fraction of CD41+ cells within PRP. (D) Flow cytometric analysis of the expression of CD42 (GPIbα) in CD41-enriched cells prepared from WT and Mx1-C1 bone marrow cells. (E) Western blot analysis of platelet or enriched megakaryocyte extracts with anti-GPIbα and anti–β-actin Abs as internal controls.
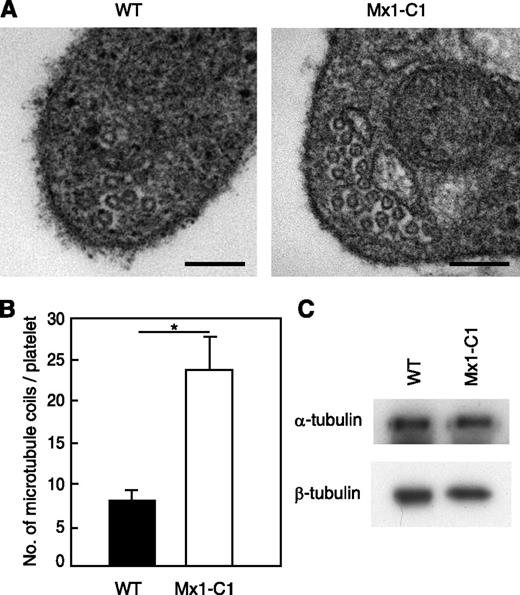
Circulating Mx1-C1 platelets contain a greater number of microtubule coils.
Platelets from WT and Mx1-C1 mice were prepared and analyzed by transmission electron microscopy. As shown in Figure 6A, the number of microtubule coils in Mx1-C1 platelets was greater than that observed in WT platelets. Mx1-C1 platelets had on average 23.9 ± 4.0 microtubule coils per cell compared with 8.0 ± 1.2 microtubule coils per cell in WT platelets (Figure 6B). We next examined tubulin expression in the platelets by western blot analysis using α- and β-tubulin Abs (Figure 6C). The total α- and β-tubulin content of platelets isolated from WT and Mx1-C1 mice was similar, suggesting that changes in tubulin expression were not responsible for the abnormal microtubule organization in Mx1-C1 mice.
Platelets from Mx1-C1 mice exhibit an abnormal microtubule ultrastructure. (A) Electron micrographs of portions of individual WT and Mx1-C1 platelets. Scale bars, 100 nm. (B) Quantification of the number of microtubule coils. Data were obtained from 8 WT and Mx1-C1 platelets each and presented as means ± SEM. *P < .01. (C) Western blot analysis of platelet extract with anti–α-tubulin and anti–β-tubulin Abs.
Platelets from Mx1-C1 mice exhibit an abnormal microtubule ultrastructure. (A) Electron micrographs of portions of individual WT and Mx1-C1 platelets. Scale bars, 100 nm. (B) Quantification of the number of microtubule coils. Data were obtained from 8 WT and Mx1-C1 platelets each and presented as means ± SEM. *P < .01. (C) Western blot analysis of platelet extract with anti–α-tubulin and anti–β-tubulin Abs.
Discussion
The physiological role(s) of mucin-type O-glycosylation of proteins remains unclear. However, the molecular cloning of the C1galt1 gene6,7 and functional analyses performed in C1galt1-deficient mice should provide insight into this question. Recent studies of the in vivo phenotypes of C1galt1-deficient mice have begun to clarify the significance of mucin-type O-glycosylation.9,17-20 Several reports have implicated O-glycosylation in platelet function, although the molecular mechanisms of these effects remain poorly understood. In this study, we demonstrated that mucin-type O-glycosylation is necessary for platelet production and megakaryocyte development in vivo using interferon-inducible conditional knockout mice. Mx1-C1 mice, which lack expression of the C1galt1 transcript in bone marrow, exhibited severe thrombocytopenia, the emergence of giant platelets, and a prolonged bleeding time. In addition, we provide evidence for a role for O-glycosylation in megakaryocyte development and the maintenance of proplatelet formation.
Alexander et al21 reported that the plt1 mutant, which contains an ENU-induced point mutation in the gene encoding C1galt1, exhibits recessive thrombocytopenia and kidney disease. Although platelet counts in plt1/plt1 mice were 40% of those in WT mice, Mx1-C1 mice exhibited a much stronger decrease (>90%) in platelet counts. In addition, bleeding times were markedly prolonged in Mx1-C1 mice but were unaffected in plt1/plt1 mice. Although platelet production is reduced in plt1/plt1 mice, they retain a number sufficient for maintaining normal hemostasis. Moreover, the residual T-synthase activity in the bone marrow of plt1/plt1 mice is approximately 20% of that in WT mice. Although these phenotypic differences between plt1/plt1 and Mx1-C1 mice could originate from residual T-synthase activity in plt1/plt1 mice, the results suggest that the abnormal hemostatic function in Mx1-C1 mice is caused by the complete loss of T-synthase activity.
The GPIb-IX receptor complex on the platelet surface comprises three polypeptides—GPIbα, GPIbβ, and GPIX—and mediates the adhesion of platelets to injured endothelial cells through an interaction with subendothelial vWF.22 A complete loss of the GPIb-IX complex due to autosomal recessive inheritance of GPIbα, GPIbβ, and GPIX gene mutations results in a hemorrhagiparous thrombocytic dystrophy called Bernard-Soulier syndrome. This rare syndrome is characterized by the tendency to bleed, the appearance of giant blood platelets, and low platelet counts. GPIbα and GPIbβ knockout mice commonly display severe thrombocytopenia, the presence of giant platelets, and a prolonged bleeding time.23-25 In GPIbα-deficient mice, the enlarged platelets may be generated by abnormal membrane development.26 GPIbα is composed of 4 structural domains: a ligand binding domain, a macroglycopeptide domain, a single transmembrane domain, and a cytoplasmic domain.3 The macroglycopeptide domain of murine GPIbα contains 73 putative O-glycosylation sites, and O-glycans make up 30% of its total molecular weight. The expression of GPIbα protein on the cell surface of, as well as its level in cell extracts prepared from Mx1-C1 platelets and megakaryocytes, was significantly reduced despite the fact that the expression of its transcript was similar to that observed in WT mice. Moreover, the phenotype of Mx1-C1 mice was very similar to that of GPIbα or GPIbβ knockout mice. In particular, the deficient megakaryocytes in each strain exhibited a reduced ability to produce proplatelets in vitro.26,27 However, there have been no reports of GPIbβ modification by O-glycosylation. Since GPIbβ-deficient platelets have no detectable GPIb-IX complex,24 the phenotype of Mx1-C1 mice is considered identical to that of both GPIbα- and GPIbβ-deficient mice. The coexpression of all three subunits—GPIbα, GPIbβ, and GPIX—is required for the cell surface expression of the GPIb-IX complex.28 Our results suggest that the phenotype of Mx1-C1 mice might be mediated by defects in GPIbα stability and/or shedding.
Very recently, Wang et al29 reported the phenotype of a targeted deletion of Cosmc in murine endothelial hematopoietic cells (EHCs; Cosmc−/y). Platelets from EHC Cosmc−/y mice lacked C1galt activity. EHC Cosmc−/y mice displayed severely prolonged bleeding times and macrothrombocytopenia. EHC Cosmc−/y platelets exhibited abnormal function of the GPIb-IX-V complex and impaired integrin αIIbβ3 activation, resulting in defective interactions with vWF and fibrinogen, respectively. Conversely, the interaction between developing megakaryocytes and fibrinogen supported the development of proplatelet and platelet release.30,31 However, the proportion of mature proplatelet-forming cells among Mx1-C1 megakaryocytes was not increased when cells were plated on fibrinogen-coated slides, suggesting that Mx1-C1 megakaryocytes had defects in their ability to interact via the integrin αIIbβ3.
The expression of GPIbα on EHC Cosmc−/y platelets was reduced compared with that observed in Mx1-C1 platelets. Full-length GPIbα (120 kDa) was not detected in extracts prepared from EHC Cosmc−/y platelets, although three shorter immunoreactive polypeptides (∼45, 40, and ∼25 kDa) were detected by western blot analysis. Because the deduced molecular weight of hypoglycosylated murine GPIbα is about 80 kDa, these truncated polypeptides detected in EHC Cosmc−/y platelets might be produced by proteolytic cleavage. The western blot analysis showed a reduced signal corresponding to full-length GPIbα (approximately 130 and 160 kDa), whereas the truncated immunoreactive peptides were not observed in Mx1-C1 platelets or CD41-enriched cells. The former discrepancy might be explained as follows. C1galt1 expression might not be completely lost in all hematopoietic cells by activation of the Cre recombinase expressed in the Mx1-C1 inducible knockout mouse model. Therefore, the residual signal might be derived from a small fraction of cells expressing C1galt1. The latter discrepancy might be explained by a difference in the specificity of the anti-GPIbα Ab. Our observations coupled with those of Wang et al suggest that mucin-type O-glycosylation of GPIbα is necessary to shield the glycoprotein from proteolytic attack.
The discoid shape of resting platelets is maintained by microtubules organized in the marginal band,32,33 and microtubules and cortical forces determine platelet size.34 To determine whether giant platelets arise in Mx1-C1 mice due to abnormal microtubule organization, we analyzed the ultrastructure of resting platelets. We observed an increased number of microtubule coils in Mx1-C1 platelets that is similar to that observed in GPIbβ-deficient mice.27 These data indicate that hypoglycosylated GPIbα protein might be unstable in megakaryocytes and platelets, resulting in thrombocytopenia due to loss of GPIb-IX complex in Mx1-C1 mice.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr K. Araki, Kumamoto University, for kindly providing the Ayu1-Cre transgenic mice, and Ms Junko Sakamoto, University of Tsukuba, for excellent technical assistance.
This work was supported by the New Energy and Industrial Technology Development Organization, by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS) (KAKENHI Grants No. 17590236, 22500383, and 24300152). This study was also supported by the World Premier International Research Center Initiative, Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Authorship
Contributions: T.K., T.S., Y.K., Y.I., M.E., N.O., H.N., and S.T. designed research; T.K., T.S., K.H., Y.K., T.Y., K.M., M.H., and S.M., performed experiments; T.K., K.H., Y.K., M.H., and S.M., analyzed data; and T.K., T.S., and S.T. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takashi Kudo, Department of Anatomy and Embryology, Faculty of Medicine, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8575, Japan; e-mail: t-kudo@md.tsukuba.ac.jp; and Satoru Takahashi, Department of Anatomy and Embryology, Faculty of Medicine, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8575, Japan; e-mail: satoruta@md.tsukuba.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal