Key Points
Mitochondrial heat shock protein, mortalin, is essential for the maintenance of HSCs via the control of oxidative stress.
Mortalin directly interact with DJ-1 to regulate ROS levels in the mitochondria of HSCs.
Abstract
Hematopoietic stem cells (HSCs) maintain stemness through various mechanisms that protect against stressful conditions. Heat shock proteins (HSPs) preserve cell homeostasis during stress responses through protein quality control, suggesting that HSPs may safeguard HSCs against numerous traumas. Here, we show that mortalin, a mitochondrial HSP, plays an essential role in maintaining HSC properties by regulating oxidative stress. Mortalin is primarily localized in hematopoietic stem and progenitor cell (HSPC) compartments. In this study, the inhibition of mortalin function caused abnormal reactive oxygen species (ROS) elevation in HSCs and reduced HSC numbers. Knockdown (KD) of mortalin in HSPCs impaired their ability to repopulate and form colonies. Moreover, mortalin-KD HSCs could not maintain quiescence and showed severe downregulation of cyclin-dependent kinase inhibitor– and antioxidant-related genes. Conversely, HSCs that overexpressed mortalin maintained a high reconstitution capacity and low ROS levels. Furthermore, DJ-1, one of the genes responsible for Parkinson’s disease, directly bound to mortalin and acted as a negative ROS regulator. Using DJ-1–deficient mice, we demonstrated that mortalin and DJ-1 coordinately maintain normal ROS levels and HSC numbers. Collectively, these results indicate that the mortalin/DJ-1 complex guards against mitochondrial oxidative stress and is indispensable for the maintenance of HSCs.
Introduction
Hematopoietic stem cells (HSCs) are capable of constant self-renewal and generate multiple blood cell lineages during postnatal life. Under steady-state conditions, most adult HSCs remain quiescent within their stem cell niche.1,2 Previous studies show that the level of reactive oxygen species (ROS) in HSCs must be controlled to preserve quiescence and the capacity to self-renew.3-5 Low ROS levels are required for the proper functioning of HSCs, yet the mechanism underlying the regulation and amelioration of oxidative stress in these cells remains unclear. What is widely known, however, is that ROS are mainly generated in the mitochondria of eukaryotic cells. Changes in mitochondrial function increase ROS production, resulting in various oxidative stress-associated diseases and accelerated aging.6-8 Thus, to reduce ROS generation, the number of mitochondria in HSCs is lower than that in differentiated cells.9 These observations suggest that modulating the number and function of mitochondria is critical to avoid ROS accumulation in HSCs.
Heat shock proteins (HSPs) comprise a set of chaperone proteins that maintain cell homeostasis via diverse mechanisms, including protein folding, protein trafficking, and signal transduction. HSPs are upregulated on cellular exposure to environmental stressors such as infection, radiation, starvation, inflammation, hypoxia, chemical toxins, and oxidants.10-12 Interestingly, a number of HSPs are highly expressed in embryonic stem cells, mesenchymal stem cells, neural stem cells, and different types of cancer cells.13,14 One member of the HSP70 family genes, HSC70, contributes to the maintenance of HSC quiescence by regulating the localization of cyclin D,11 whereas another HSP70 family gene, GRP78, contributes to the preservation of HSC self-renewal by regulating glycolytic metabolism.12 Thus, HSP70 genes may play important roles in HSC maintenance.
Among all the HSP70 family genes, mortalin plays a unique role in the upkeep of mitochondria, maintaining mitochondrial homeostasis by acting as an essential component of the translocational machinery in the inner mitochondrial membrane complex.15,16 Loss of mortalin in yeast and Caenorhabditis elegans causes lethal dysfunction of mitochondria, characterized by alterations in morphology, impaired membrane potential, decreased ATP production, and increased ROS generation.17,18 Furthermore, mortalin-defective mutant zebrafish show myelodysplastic syndrome–like symptoms.19 Mitochondrial dysfunction and increased ROS levels were also observed in hematopoietic precursors and erythroblasts in these zebrafish. In humans, the mortalin gene is localized to 5q31; thus, it may be responsible, at least in part, for the development of myeloid leukemia and myelodysplastic syndrome.20 However, it is not known whether mortalin plays a specific role in HSCs.
We hypothesized that mortalin plays an essential role in regulating HSC function by preventing cellular exposure to excessive amounts of ROS. Our current observations show that mortalin is critical for HSC maintenance. First, mortalin knockdown (KD) in HSCs led to severe defects in their long-term ability to reconstitute due to increased ROS generation and the promotion of cell cycle progression. Conversely, mortalin overexpression (OE) in HSCs improved their ability to maintain stemness and suppressed ROS production. Furthermore, DJ-1, a gene responsible for Parkinson’s disease, directly associated with mortalin in hematopoietic cells. Loss of DJ-1 resulted in both HSC exhaustion and increased ROS accumulation in vivo. These findings provide insights into the physiological function of the mortalin/DJ-1 complex in the ROS regulatory mechanisms involved in HSC maintenance.
Methods
Mice
C57BL/6 (B6-Ly5.2) and C57BL/6 mice congenic for the CD45 locus (B6-Ly5.1) were purchased from Sankyo-Laboratory Service. Loss-of-function DJ-1 mutant mice21 were obtained from Dr Hiroyoshi Ariga (Hokkaido University, Saporro, Japan). Animal care was in accordance with the guidelines for animal and recombinant DNA experiments of Keio University.
Reagents
MKT-077 (formerly known as FJ-776), a cationic rhodacyanine dye analog inhibitor of HSP70 family chaperons,22 was kindly provided by Dr Sunil Kaul (AIT, Tsukuba, Japan). The antioxidant agent N-acetyl-l-cysteine (NAC; Sigma-Aldrich) was added to the culture medium and used at a final concentration of 300 μM.
Quantitative reverse transcription polymerase chain reaction analysis
Methods for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis and TaqMan Gene Expression Assay Mixes used in this study are described in the supplemental Data, available on the Blood Web site.
Flow cytometry
Monoclonal antibodies (mAbs) used for fluorescence-activated cell sorter (FACS) analysis are listed in the supplemental Data. To analyze cell cycle status, cells were first stained with cell surface mAbs and permeabilized with 4% paraformaldehyde in phosphate-buffered saline. The cells were then stained with Ki67 (Becton, Dickinson and Company) and Hoechst 33342 (Molecular Probes). To analyze intracellular ROS levels, cells were first stained with cell surface mAbs and then incubated with 300 nM 2′7′-dichlorfluorescein-diacetate (DCF-DA) (Invitrogen) at 37°C for 5 minutes or with 5 μM Mitosox (Invitrogen) at 37°C for 10 minutes. To analyze mitochondrial membrane potential, cells were first stained with cell surface mAbs and then incubated with 40 nM MitoTracker red CMXRos solution (Invitrogen) at 37°C for 30 minutes in an atmosphere containing 5% CO2. For apoptosis analysis, cells were first stained with cell surface mAbs and then suspended for 15 minutes in Annexin V binding buffer containing an Annexin V Ab (Invitrogen) and 7-Amino-Actinomycin D (Invitrogen). Stained cells were analyzed and sorted using FACSAria (BD Biosciences).
Culture of HSCs and colony-forming assays
Sorted Lin− Sca-1+ c-Kit+ (LSK) cells were cultured in 96-well plates with S-clone SF-O3 medium (Sankyo Junyaku) containing 0.5% bovine serum albumin, 50 μM 2-mercaptoethanol, 50 ng/mL stem cell factor, and 50 ng/mL thrombopoietin. Colony-forming assays were performed in MethoCult M3434 incomplete medium (Stem Cell Technologies) using 500 green fluorescent protein (GFP)+ LSK cells. Colony numbers were counted on day 7 of culture.
Immunocytofluorescence analysis
Methods for immunocytofluorescence analysis are described in the supplemental Data.
Immunoelectron microscopy
Methods for immunoelectron microscopy analysis are described in the supplemental Data.
Transplantation assay
GFP+ LSK cells (5 × 103) were isolated from GFP-expressing B6-Ly5.1 LSK cells transduced with scrambled (sh)RNA (control), sh-mortalin#1, or sh-mortalin#2. The cells were transplanted into lethally irradiated (9.5 Gy) recipient B6-Ly5.2 mice along with 2 × 105 B6-Ly5.2 bone marrow (BM) mononuclear cells (MNCs). The percentage of donor cells in the recipients’ peripheral blood (PB) was analyzed at 1 to 4 months, whereas that in the recipients’ BM was analyzed at 4 and 10 months posttransplantation.
Western blotting and immunoprecipitation
Methods for western blotting and immunoprecipitation analysis are described in the supplemental Data.
sh-Mortalin retrovirus production and transduction
Methods for sh-mortalin retrovirus production and transduction are described in the supplemental Data.
Statistical analysis
All P values were calculated using the unpaired Student t test.
Results
Mortalin is predominantly expressed in HSPCs
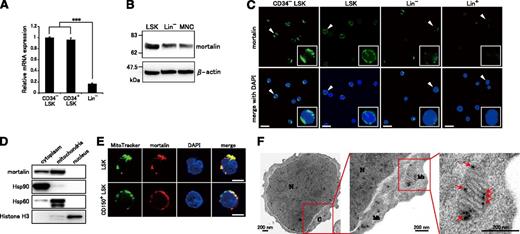
To examine how HSP70 family proteins contribute to the regulation of hematopoiesis, we first investigated the expression levels of HSP70 family genes in various hematopoietic cell populations using qRT-PCR (supplemental Figure 1). All investigated HSP70 family genes were highly expressed in relatively undifferentiated cells. Notably, mortalin was significantly up-regulated in immature LSK cells, suggesting that the protein might play a unique role in hematopoietic progenitor/stem cells (HSPC) regulation. The expression levels of mortalin in CD34− LSK HSCs and CD34+ LSK HPSCs were equally high (Figure 1A). Western blot analysis also showed that mortalin expression was stronger in LSKs than in Lin− cells or MNCs (Figure 1B). Immunofluorescence analysis demonstrated that only LSK and CD34− LSK cells contained abundant extranuclear mortalin (Figure 1C), supporting the qRT-PCR results (supplemental Figure 1). LSK cells show heterogeneous express of mortalin, whereas most CD34− LSK cells show strong expression, indicating HSCs highly express mortalin.
Mortalin is abundantly localized in the mitochondria of HSPCs. (A) qRT-PCR analysis of mortalin in CD34− LSK, CD34+ LSK, and Lin− cell populations. β-actin was used as an endogenous control. Data represent the mean ± standard deviation(SD) (n = 4; ***P < .001). (B) Western blot analysis of mortalin and β-actin in total cell extracts derived from freshly isolated LSK, Lin−, and MNC populations. (C) Fluorescence microscopy images of freshly isolated CD34− LSK, LSK, Lin−, and Lin+ cells stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) and an anti-mortalin Ab (green). The smaller boxes show higher power views of the areas denoted by arrowheads. Scale bar, 20 μm. (D) Western blot analysis of mortalin in cytoplasmic, mitochondrial, and nuclear fractions prepared from Lin− BM cells. Hsp90, Hsp60, and Histone H3 were used as cytoplasmic, mitochondrial, and nuclear markers, respectively, to monitor the purity of the subcellular fractions. (E) Fluorescence microscopy images of freshly isolated CD150+ LSK and LSK cells stained with an anti-mortalin Ab (red), Mito Tracker (a cell-permeable membrane marker, green), and DAPI (blue). Scale bar, 5 μm. (F) Electron microscopy images of ultrathin sections of Lin− BM cells. N, nucleus; C, cytoplasm; Mt, mitochondria. Arrows indicate immunogold-labeled mortalin. Magnified images show details in single mitochondria. Scale bar, 200 nm.
Mortalin is abundantly localized in the mitochondria of HSPCs. (A) qRT-PCR analysis of mortalin in CD34− LSK, CD34+ LSK, and Lin− cell populations. β-actin was used as an endogenous control. Data represent the mean ± standard deviation(SD) (n = 4; ***P < .001). (B) Western blot analysis of mortalin and β-actin in total cell extracts derived from freshly isolated LSK, Lin−, and MNC populations. (C) Fluorescence microscopy images of freshly isolated CD34− LSK, LSK, Lin−, and Lin+ cells stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) and an anti-mortalin Ab (green). The smaller boxes show higher power views of the areas denoted by arrowheads. Scale bar, 20 μm. (D) Western blot analysis of mortalin in cytoplasmic, mitochondrial, and nuclear fractions prepared from Lin− BM cells. Hsp90, Hsp60, and Histone H3 were used as cytoplasmic, mitochondrial, and nuclear markers, respectively, to monitor the purity of the subcellular fractions. (E) Fluorescence microscopy images of freshly isolated CD150+ LSK and LSK cells stained with an anti-mortalin Ab (red), Mito Tracker (a cell-permeable membrane marker, green), and DAPI (blue). Scale bar, 5 μm. (F) Electron microscopy images of ultrathin sections of Lin− BM cells. N, nucleus; C, cytoplasm; Mt, mitochondria. Arrows indicate immunogold-labeled mortalin. Magnified images show details in single mitochondria. Scale bar, 200 nm.
Mortalin is primarily localized in the mitochondria of HSPCs
We next investigated the subcellular localization of mortalin in HSPCs (Figure 1D-F). Lin− c-Kit+ BM cells were fractionated into cytoplasmic, mitochondrial, and nuclear fractions. Mortalin expression was then analyzed by western blotting. Mortalin was detected in the cytoplasmic and the mitochondrial fractions but not in the nuclear fraction (Figure 1D). Costaining with an anti-mortalin antibody and Mito Tracker, a mitochondrial marker, showed that mortalin was mainly localized to the mitochondria in both LSK and CD150+ LSK HSCs (Figure 1E).
To confirm that mortalin is primarily expressed within the mitochondria, we next performed immunoelectron microscopy analysis to establish the subcellular localization of the protein in Lin− c-Kit+ cells (Figure 1F). Mortalin, labeled with immunogold particles, was localized on the mitochondrial inner membrane and matrix but not on the outer membrane. Collectively, these results show that mortalin is specifically expressed in HSPCs, particularly within the mitochondria.
HSP70 family inhibitor MKT-077 impairs HSC properties by augmenting mitochondrial ROS production in vitro
To determine the effect of mortalin in HSPCs, we cultured LSK cells with MKT-077, a cationic rhodacyanine dye that inhibits HSP70 family proteins, including mortalin.22 Ten days after the initiation of MKT-077 treatment, there was no difference in the absolute number of cultured cells; however, a drastic dose-dependent decrease of CD150+ LSK HSCs was observed (Figure 2B). To determine why the inhibition of HSP70 family proteins reduced the number of HSCs, ROS levels in MKT-077–treated HSCs were measured by FACS using DCF-DA (Figure 2A) because increased ROS content might act to negatively regulate HSCs. Interestingly, ROS production in MKT-077–treated HSCs increased in a dose-dependent manner, whereas MKT-077 did not appear to affect ROS production in CD150− LSK HPC cells (Figure 2A,C). Addition of an antioxidant, NAC, partially rescued the MKT-077–induced decrease in the number of HSCs (Figure 2B) and completely abolished the increase in ROS levels (Figure 2C). In addition, MKT-077 suppressed the colony-forming ability of LSK cells, which was also rescued in part by NAC (Figure 2D). Taken together, these findings suggest that inhibiting the activity of mitochondrial HSP70 protein, mortalin, using MKT-077 specifically disrupts HSC function because the control of mitochondrial oxidative stress is no longer regulated appropriately in HSCs.
MKT-077, an inhibitor of HSP70 family, reduces HSC numbers and increases ROS production in vitro. (A) Representative FACS profiles of CD150+ LSK fractions (upper) and ROS levels in CD150+ LSKs (lower) treated with MKT-077 for 10 days. (B) Absolute cell numbers (upper) and CD150+ LSK cell numbers (lower) in cultures treated with MKT-077 ± NAC for 10 days. Data represent the mean ± SD (n = 4; *P < .05, **P < .01, ***P < .001). (C) Intensity of DCF-DA fluorescence in CD150+ LSK cells (upper) and CD150− LSK cells (lower) treated with MKT-077 ± NAC for 10 days. Data represent the mean ± SD (n = 4; **P < .01, ***P < .001). (D) Colony numbers originating from 300 LSK cells treated with MKT-077 ± NAC for 10 days. Data represent the mean ± SD (n = 3; **P < .01, ***P < .001).
MKT-077, an inhibitor of HSP70 family, reduces HSC numbers and increases ROS production in vitro. (A) Representative FACS profiles of CD150+ LSK fractions (upper) and ROS levels in CD150+ LSKs (lower) treated with MKT-077 for 10 days. (B) Absolute cell numbers (upper) and CD150+ LSK cell numbers (lower) in cultures treated with MKT-077 ± NAC for 10 days. Data represent the mean ± SD (n = 4; *P < .05, **P < .01, ***P < .001). (C) Intensity of DCF-DA fluorescence in CD150+ LSK cells (upper) and CD150− LSK cells (lower) treated with MKT-077 ± NAC for 10 days. Data represent the mean ± SD (n = 4; **P < .01, ***P < .001). (D) Colony numbers originating from 300 LSK cells treated with MKT-077 ± NAC for 10 days. Data represent the mean ± SD (n = 3; **P < .01, ***P < .001).
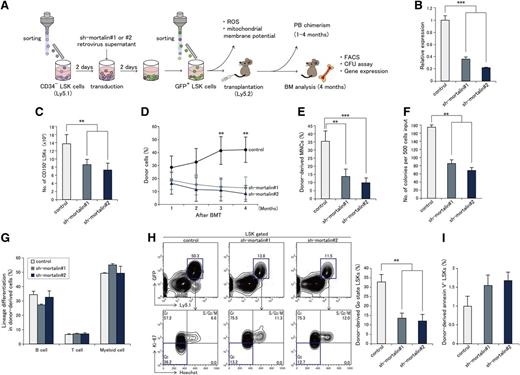
KD of mortalin causes severe defects in HSC reconstitution capability and the maintenance of quiescence
We next evaluated the impact of mortalin on HSC properties in vivo (see the experimental scheme outlined in Figure 3A). Two mortalin-specific shRNAs, sh-mortalin#1 and sh-mortalin#2, were generated to examine the effect of mortalin downregulation. LSK cells were infected with retroviral shRNAs coexpressing GFP. We confirmed that mortalin levels were effectively knocked down in sh-mortalin#1– or sh-mortalin#2–transduced LSK cells at 10 days after viral infection (Figure 3B). The number of CD150+ LSK cells was also significantly reduced in mortalin shRNA-transduced LSK cells at 10 days after the transduction (Figure 3C). As expected, mortalin KD led to a marked increase in mitochondrial ROS levels in CD150+ LSK cells (supplemental Figure 2A). We next investigated whether downregulating mortalin affects the mitochondrial survival in transducted cells by FACS using MitoTracker red CMXRos, which accumulates in mitochondria with an intact membrane potential. We found that there was no difference in the percentage of MitoTracker-negative Lin− cells within the mortalin KD and control populations, indicating that mitochondrial ROS accumulation in mortalin KD HSC is not caused by severe mitochondrial defects accompanied by a reduction in membrane potential (supplemental Figure 2B).
Mortalin KD leads to a severe deficiency in long-term HSC repopulation. (A) The experimental scheme is shown. (B) Mortalin expression in sh-mortalin–transduced LSK cells at 10 days after transduction. Data represent the mean ± SD (n = 3; ***P < .001). (C) Numbers of CD150+ LSK cells 1 week after the culture of sorted sh-mortalin–transduced GFP+ LSK cells. Data represent the mean ± SD (n = 4; **P < .01). (D) PB chimerism of donor-derived cells analyzed at each month posttransplantation. Data represent the mean ± SD (n = 7/group; **P < .01). (E) Absolute number of donor-derived MNCs in the BM at 4 months posttransplantation. Data represent the mean ± SD (n = 7/group; **P < .01, ***P < .001). (F) Number of colony forming cells originating from 500 donor-derived LSK cells at 4 months posttransplantation. Data represent the mean number of colonies (±SD) formed per 500 cells (n = 3/group; **P < .01). (G) Percentage (±SD) of donor-derived B220+ B cells, CD3+ T cells, and Mac-1+/Gr-1+ myeloid cells in the recipient BM (n = 7/group). (H) Representative cell cycle FACS profiles (left) of GFP+ donor-derived LSK cells at 4 months posttransplantation. Graphs (right) show the mean percentages (±SD) of donor-derived LSK cells in the Go state (n = 7/group; **P < .01). (I) Percentages (±SD) of donor-derived Annexin V+ LSK cells in the recipient BM (n = 7/group).
Mortalin KD leads to a severe deficiency in long-term HSC repopulation. (A) The experimental scheme is shown. (B) Mortalin expression in sh-mortalin–transduced LSK cells at 10 days after transduction. Data represent the mean ± SD (n = 3; ***P < .001). (C) Numbers of CD150+ LSK cells 1 week after the culture of sorted sh-mortalin–transduced GFP+ LSK cells. Data represent the mean ± SD (n = 4; **P < .01). (D) PB chimerism of donor-derived cells analyzed at each month posttransplantation. Data represent the mean ± SD (n = 7/group; **P < .01). (E) Absolute number of donor-derived MNCs in the BM at 4 months posttransplantation. Data represent the mean ± SD (n = 7/group; **P < .01, ***P < .001). (F) Number of colony forming cells originating from 500 donor-derived LSK cells at 4 months posttransplantation. Data represent the mean number of colonies (±SD) formed per 500 cells (n = 3/group; **P < .01). (G) Percentage (±SD) of donor-derived B220+ B cells, CD3+ T cells, and Mac-1+/Gr-1+ myeloid cells in the recipient BM (n = 7/group). (H) Representative cell cycle FACS profiles (left) of GFP+ donor-derived LSK cells at 4 months posttransplantation. Graphs (right) show the mean percentages (±SD) of donor-derived LSK cells in the Go state (n = 7/group; **P < .01). (I) Percentages (±SD) of donor-derived Annexin V+ LSK cells in the recipient BM (n = 7/group).
Next, transplantation assays were performed to examine the reconstitution capability of mortalin KD HSCs. GFP+ mortalin KD LSK cells (n = 5 × 103 cells) were transplanted into lethally irradiated recipient mice. Mortalin KD recipients showed markedly lower levels of donor-derived cell chimerism in the PB than the controls at 3 months posttransplantation, and the extent of the chimerism decreased thereafter (Figure 3D). Thus, downregulating mortalin significantly diminished the reconstitution ability of HSCs.
There was a marked reduction in the absolute number of donor-derived MNCs (Figure 3E) and the number of colony-forming cells (Figure 3F) in the BM of the mortalin KD recipients relative to that in the controls at 4 months posttransplantation. However, the percentage of donor-derived differentiated cells in mortalin KD BM and control BM at 4 weeks after transplantation was unchanged (Figure 3G). We presumed that the defect in mortalin KD HSC function resulted from changes in cell cycle distribution. Therefore, we examined the cell cycle kinetics of the donor-derived GFP+ LSK cells in the recipients at 4 months posttransplantation. Notably, mortalin KD HSCs demonstrated a greater than twofold decrease in the frequency of Ki67− quiescent cells (Figure 3H). On the other hand, the ratio of annexin V+ apoptotic cells was slightly increased in mortalin KD HSCs compared with the control HSCs, suggesting that a reduction of mortalin levels did not affect cell survival intensely (Figure 3I).
KD of mortalin significantly alters the expression of cell cycle regulatory genes and antioxidant-related genes in HSPCs
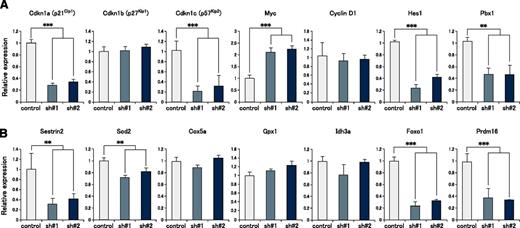
To explore the mechanism underlying the defect in quiescence maintenance in mortalin KD HSCs, we next performed gene expression analysis using mortalin KD and control LSK cells at 4 months posttransplantation. qRT-PCR analysis revealed that mortalin KD LSK cells showed significantly lower expression of several cell cycle regulatory genes, particularly the cyclin-dependent kinase inhibitors p21 and p57 (Figure 4A). On the other hand, the cells showed significantly higher expression of c-Myc, which promotes cell cycle progression (Figure 4A). Additionally, the expression of Hes1 and Pbx1, which maintain HSC quiescence,23-25 was significantly decreased. Furthermore, the expression of mitochondrial antioxidant-related genes (sestrin2 and Sod2)26-29 and transcription factors (Foxo1 and Prdm16) that control oxidative stress5,30,31 were attenuated (Figure 4B), suggesting that the regulation of mitochondrial ROS levels was disrupted in mortalin KD LSK cells. Taken together, these data suggest that mortalin is a critical molecule that maintains HSC quiescence by regulating mitochondrial ROS production.
Cyclin-dependent kinase inhibitor genes and antioxidant-related genes are markedly downregulated in mortalin-KD HSCs. Expression of (A) cell cycle–related genes and (B) oxidative stress regulatory genes in GFP+ donor-derived LSK cells at 4 months posttransplantation, as assessed by qRT-PCR. β-actin was used as an endogenous control. Data represent the mean ± SD (**P < .01, ***P < .001).
Cyclin-dependent kinase inhibitor genes and antioxidant-related genes are markedly downregulated in mortalin-KD HSCs. Expression of (A) cell cycle–related genes and (B) oxidative stress regulatory genes in GFP+ donor-derived LSK cells at 4 months posttransplantation, as assessed by qRT-PCR. β-actin was used as an endogenous control. Data represent the mean ± SD (**P < .01, ***P < .001).
Overexpression of mortalin in LSK cells suppresses ROS levels and markedly increases their ability to repopulate
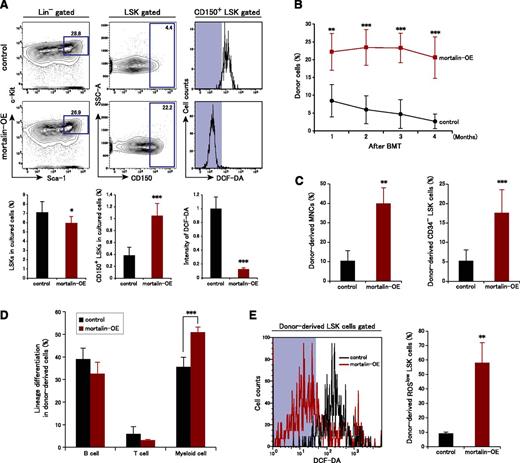
HSC defects caused by mortalin suppression prompted us to examine whether mortalin overexpression affects the properties of HSCs. We infected LSK cells with a retroviral mortalin expression vector coexpressing Kusabira Orange and then examined mortalin functions by culturing Kusabira Orange+ mortalin-overexpressing LSK cells and control cells in vitro (Figure 5A). After 10 days, the number of LSK cells within the total populations of mortalin-overexpressing (mortalin-OE) and control cells was unchanged. However, the CD150+ HSC population within the control LSK cell population was markedly reduced relative to that in the mortalin-OE cell population. Moreover, ROS production in the control CD150+ LSK cells increased after 10 days in culture, whereas the majority of mortalin-OE CD150+ LSK cells maintained low ROS levels (Figure 5A). Also, mortalin overexpression suppressed ROS levels in differentiated cells, although not significantly (data not shown).
Mortalin overexpression promotes HSC maintenance by decreasing ROS production. (A) Representative FACS profiles (upper) and population analyses (lower) of LSK cells (left) and CD150+ LSK cells (center), and the fluorescence intensity of DCF-DA in CD150+ LSK cells (right) after 10 days of culture. Sorted mortalin-overexpressing, retrovirus-transduced Kusabira-Orange+ LSK cells were used in these experiments. Data represent the mean ± SD (n = 7/group; *P < .05, ***P < .001). (B) PB chimerism of donor-derived cells analyzed at each month posttransplantation. Data represent the mean ± SD (n = 5/group; **P < .01, ***P < .001). BMT, bone marrow transplantation. (C) Absolute numbers of donor-derived MNCs (left) and CD34− LSK cells (right) in the BM at 4 months posttransplantation. Data represent the mean ± SD (n = 5/group; **P < .01, ***P < .001). (D) Percentage of donor-derived B220+ B cells, CD3+ T cells, and Mac-1+/Gr-1+ myeloid cells in the recipient BM. Data represent the mean ± SD (n = 5/group; ***P < .001). (E) Intensity of DCF-DA fluorescence and the percentage of DCF-DAlow donor-derived LSK cells at 4 months posttransplantation. Data represent the mean ± SD (n = 5/group; **P < .01).
Mortalin overexpression promotes HSC maintenance by decreasing ROS production. (A) Representative FACS profiles (upper) and population analyses (lower) of LSK cells (left) and CD150+ LSK cells (center), and the fluorescence intensity of DCF-DA in CD150+ LSK cells (right) after 10 days of culture. Sorted mortalin-overexpressing, retrovirus-transduced Kusabira-Orange+ LSK cells were used in these experiments. Data represent the mean ± SD (n = 7/group; *P < .05, ***P < .001). (B) PB chimerism of donor-derived cells analyzed at each month posttransplantation. Data represent the mean ± SD (n = 5/group; **P < .01, ***P < .001). BMT, bone marrow transplantation. (C) Absolute numbers of donor-derived MNCs (left) and CD34− LSK cells (right) in the BM at 4 months posttransplantation. Data represent the mean ± SD (n = 5/group; **P < .01, ***P < .001). (D) Percentage of donor-derived B220+ B cells, CD3+ T cells, and Mac-1+/Gr-1+ myeloid cells in the recipient BM. Data represent the mean ± SD (n = 5/group; ***P < .001). (E) Intensity of DCF-DA fluorescence and the percentage of DCF-DAlow donor-derived LSK cells at 4 months posttransplantation. Data represent the mean ± SD (n = 5/group; **P < .01).
To evaluate the functional role of mortalin in HSCs in vivo, we next examined the reconstitution ability of mortalin-OE HSCs. Kusabira Orange+ mortalin-OE, or control LSK cells (n = 2 × 103 cells) were transplanted into lethally irradiated recipient mice. At 1 month posttransplantation, the mortalin-OE recipients showed significantly higher levels of donor-derived cell chimerism in the PB than the controls, and this difference was maintained (Figure 5B). Thus, mortalin up-regulation has a stimulatory effect on HSC reconstitution capacity.
The numbers of mortalin-OE recipient donor-derived MNCs and CD34− LSK HSC cells were also markedly higher than those in the controls at 4 months posttransplantation (Figure 5C). Furthermore, the percentage of donor-derived cells of a myeloid lineage increased in the mortalin-OE recipient BM relative to that in the control at 4 months posttransplantation; however, the percentages of donor-derived cells of the T and B lineages were normal (Figure 5D). We hypothesized that increased mortalin expression strongly protected HSCs against oxidative stress, resulting in increased stemness. When ROS levels were examined in donor-derived LSK cells, we found that mortalin-OE cells contained a larger ROSlow population than the control cells (Figure 5E). These observations suggest that mortalin contributes to the maintenance of HSC quality by suppressing the accumulation of ROS.
Identification of DJ-1 as a mortalin-binding protein in hematopoietic cells
We next examined mortalin binding factors to identify the molecular mechanism underlying ROS regulation. A recent proteomics analysis study reported that DJ-1, a gene involved in the development of Parkinson’s disease, acts as a binding partner for mortalin in neural cells.32,33 We focused on DJ-1 in the current study because it also functions as an antioxidant by directly scavenging ROS.34,35
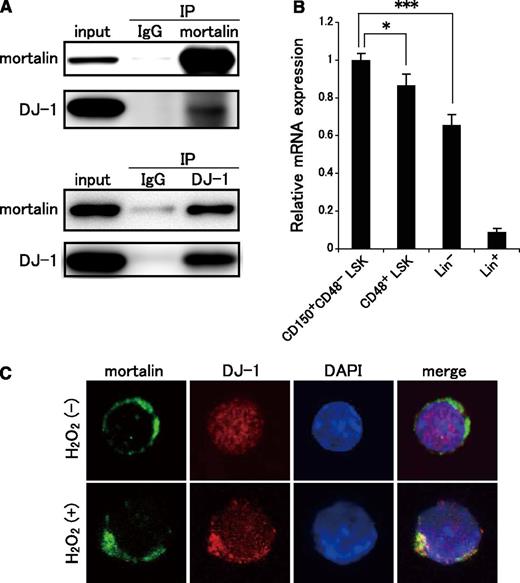
Previous studies report that DJ-1 localizes in the cytosol and nucleus under steady-state conditions. However, in the presence of elevated ROS levels, DJ-1 is primarily translocated from the cytosol into the mitochondria, where it functions as a cytoprotective intracellular redox sensor.36,37 Nonetheless, the mechanism of mitochondrial translocation remains to be determined. We hypothesized that mortalin participates in the translocation of DJ-1 into the mitochondria to protect HSCs from excessive ROS content. To confirm whether DJ-1 binds to mortalin, we performed an immunoprecipitation assay using a murine hematopoietic progenitor cell line, EML cells. Immunoprecipitation of Lin− EML cell lysates with either mortalin- or DJ-1–specific antibodies confirmed that DJ-1 acted as an endogenous mortalin-binding protein (Figure 6A). Interestingly, the expression pattern of DJ-1 was remarkably similar to that of mortalin, ie, more highly expressed in CD150+CD48− LSK HSCs than in more differentiated hematopoietic cell populations (CD48+ LSK, Lin−, and Lin+ cell fractions; Figure 6B).
DJ-1 binds to mortalin in the mitochondria of HSCs under conditions of oxidative stress. (A) Lin− EML cell lysates were immunoprecipitated with mortalin- or DJ-1–specific antibodies, and the bound complexes were subjected to western blot analysis for mortalin and DJ-1. (B) qRT-PCR analysis of DJ-1 in CD150+CD48− LSK, CD48+ LSK, Lin−, and Lin+ cell populations. β-actin was used as an endogenous control. Data represent the mean ± SD (n = 4; *P < .05, ***P < .001). (C) LSK cells were stained with mortalin Ab (green), DJ-1 pAb (red), and DAPI (blue), either with or without H2O2 (300 μM) treatment, for 2 hours.
DJ-1 binds to mortalin in the mitochondria of HSCs under conditions of oxidative stress. (A) Lin− EML cell lysates were immunoprecipitated with mortalin- or DJ-1–specific antibodies, and the bound complexes were subjected to western blot analysis for mortalin and DJ-1. (B) qRT-PCR analysis of DJ-1 in CD150+CD48− LSK, CD48+ LSK, Lin−, and Lin+ cell populations. β-actin was used as an endogenous control. Data represent the mean ± SD (n = 4; *P < .05, ***P < .001). (C) LSK cells were stained with mortalin Ab (green), DJ-1 pAb (red), and DAPI (blue), either with or without H2O2 (300 μM) treatment, for 2 hours.
We next examined whether increased ROS levels affected the colocalization of DJ-1 and mortalin in LSK cells. DJ-1 showed diffused localization pattern in the cytosol and nucleus of LSK cells under steady-state conditions; however, most of the DJ-1 protein colocalized with mortalin in the mitochondria after 2 hours of treatment with H2O2 (Figure 6C). Therefore, after oxidative stress, DJ-1 binds to mortalin and undergoes massive translocation into the mitochondria, where it binds to mortalin.
Complex formation by mortalin and DJ-1 is a key molecular mechanism underlying ROS regulation in HSCs in vivo
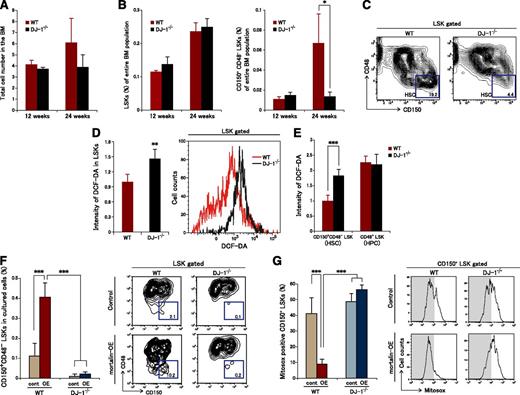
We next used 12- and 24-week-old DJ-1 homozygous knockout (DJ-1−/−) mice21 to investigate the roles of DJ-1 in HSC maintenance in vivo. DJ-1−/− mice showed a normal PB count (supplemental Figure 3A). There was no clear difference between the absolute number of MNCs or the LSK and HSC ratios in the BM between DJ-1−/− and WT mice at 12 weeks of age (Figure 7A-B). On the other hand, although no significant changes in the total MNC number or in the LSK ratio in the BM were observed at 24 weeks of age (Figure 7A-B), there was a marked decrease in the HSC ratio; also, the HPSC ratio showed a tendency to increase in DJ-1−/− mouse BM (Figure 7B-C). No distinct changes were observed in the ratios of early progenitors or in terminal erythroid differentiation (supplemental Figure 3B-D).
Mortalin/DJ-1 complex formation is a key molecular mechanism underlying ROS regulation in HSCs. (A) Total number of BM cells derived from 12- and 24-week-old WT and DJ-1 knockout mice. Data represent the mean ± SD (12-week-old mice, n = 4; 24-week-old mice, n = 6). (B) The population of LSK cells (left) and CD150+CD48- LSK cells (right) within the entire population of BM cells. Data represent the mean ± SD (12-week-old mice, n = 4; 24-week-old mice, n = 6; *P < .05). (C) Representative FACS profiles of LSK CD150+CD48− fractions derived from 24-week-old WT and DJ-1 knockout mice (n = 4). (D) Representative FACS profiles (right) and the mean (±SD) DCF-DA intensity in LSK cells derived from 24-week-old WT and DJ-1 knockout mice (left) (n = 4; **P < .01). (E) Intensity of DCF-DA fluorescence in CD150+CD48− LSK or CD48+ LSK cells derived from 24-week-old WT and DJ-1 knockout mice. Data represent the mean ± SD (n = 4; ***P < .001). (F) Percentage (left) and representative FACS profiles (right) of CD150+CD48− LSK cells in the mortalin-overexpressing DJ-1−/− and WT cell populations after 10 days of culture. Sorted mortalin-overexpressing retrovirus-transduced GFP+ LSK cells derived from DJ-1−/− and control mice were used in these experiments. Data represent the mean ± SD (n = 8/group; ***P < .001). (G) Percentage (left) and representative FACS profiles (right) of Mitosox+CD150+ LSK cells in the mortalin-overexpressing DJ-1−/− and WT cell populations after 10 days of culture. Sorted mortalin-overexpressing retrovirus-transduced GFP+ LSK cells derived from DJ-1−/− and control mice were used in these experiments. Data represent the mean ± SD (n = 8/group; ***P < .001).
Mortalin/DJ-1 complex formation is a key molecular mechanism underlying ROS regulation in HSCs. (A) Total number of BM cells derived from 12- and 24-week-old WT and DJ-1 knockout mice. Data represent the mean ± SD (12-week-old mice, n = 4; 24-week-old mice, n = 6). (B) The population of LSK cells (left) and CD150+CD48- LSK cells (right) within the entire population of BM cells. Data represent the mean ± SD (12-week-old mice, n = 4; 24-week-old mice, n = 6; *P < .05). (C) Representative FACS profiles of LSK CD150+CD48− fractions derived from 24-week-old WT and DJ-1 knockout mice (n = 4). (D) Representative FACS profiles (right) and the mean (±SD) DCF-DA intensity in LSK cells derived from 24-week-old WT and DJ-1 knockout mice (left) (n = 4; **P < .01). (E) Intensity of DCF-DA fluorescence in CD150+CD48− LSK or CD48+ LSK cells derived from 24-week-old WT and DJ-1 knockout mice. Data represent the mean ± SD (n = 4; ***P < .001). (F) Percentage (left) and representative FACS profiles (right) of CD150+CD48− LSK cells in the mortalin-overexpressing DJ-1−/− and WT cell populations after 10 days of culture. Sorted mortalin-overexpressing retrovirus-transduced GFP+ LSK cells derived from DJ-1−/− and control mice were used in these experiments. Data represent the mean ± SD (n = 8/group; ***P < .001). (G) Percentage (left) and representative FACS profiles (right) of Mitosox+CD150+ LSK cells in the mortalin-overexpressing DJ-1−/− and WT cell populations after 10 days of culture. Sorted mortalin-overexpressing retrovirus-transduced GFP+ LSK cells derived from DJ-1−/− and control mice were used in these experiments. Data represent the mean ± SD (n = 8/group; ***P < .001).
We speculated that this marked age-related decrease in HSC numbers associated with DJ-1 deficiency was caused by increased ROS levels. Therefore, we examined ROS levels in LSK cells derived from DJ-1−/− mice (Figure 7D-E). The results revealed that LSK cells from DJ-1−/− mice accumulated high levels of ROS (Figure 7D). In particular, markedly increased ROS levels were observed in CD150+CD48− LSK HSCs derived from DJ-1−/− mice but not in CD48+ LSK HPSCs (Figure 7E).
Furthermore, to examine the effect of increased mortalin on HSC maintenance in the lack of DJ-1, we transfected DJ-1−/− LSK cells with a mortalin expression vector coexpressing GFP. After 10 days of culture, mortalin-OE DJ-1−/− CD150+CD48− LSK cells could not maintain stemness in the presence of low ROS levels, suggesting that DJ-1 is essential to maintain the function of HSC via oxidative stress control by mortalin (Figure 7F-G). These findings indicate that the mortalin/DJ-1 complex is a key modulator of oxidative stress in HSCs.
Discussion
Recent studies show that HSPs play a particularly important role in HSC properties.11,12 It has been shown that mortalin KD impairs erythroid differentiation and reduces the number of colony-forming progenitor cells in the murine BM.38 However, the precise role of mortalin in HSC maintenance has yet to be clarified. Here, we examined mortalin KD and mortalin-OE HSCs, and showed that mortalin is critical for the preservation of HSC faculties under both ex vivo culture and in vivo transplantation conditions. Furthermore, we identified an interaction between a mitochondrial chaperone (mortalin) and a ROS negative regulator (DJ-1) in hematopoietic cells for the first time. We used DJ-1 knockout mice to demonstrate that this complex is essential for the conservation of HSC numbers, as well as for maintaining normal ROS levels. Therefore, mortalin cooperates with DJ-1 to maintain HSCs via mitochondrial quality control.
We previously reported that elevated ROS levels disrupted the quiescence and self-renewal ability of HSCs.3,4 We also found that mortalin is highly expressed in HSPC mitochondria, especially within the mitochondrial inner membrane and matrix areas. This is consistent with earlier work indicating that mortalin may make a crucial contribution to protein translocation systems in the mitochondrial matrix of HSPCs.39 The current study showed that HSP70 family protein inhibitor MKT-077 caused a marked augmentation of ROS levels in ex vivo–cultured HSCs, suggesting that mortalin inhibition causes mitochondrial dysfunction. Additionally, MKT-077 led to severe defects in HSC maintenance, which were partially restored by the administration of NAC, a compound with the capacity to eliminate ROS. Because MKT-077 is not a mortalin-specific inhibitor, dysfunction in other HSP70 family protein may also affect HSC maintenance.
Previous studies suggest that mortalin has an antiapoptotic function40,41 ; however, we found that reduced mortalin levels had only a small effect on HSC survival. Meanwhile, reduced mortalin expression in vivo led to severe defects in the ability of HSCs to repopulate because of the failure to maintain HSC quiescence. This finding was supported by the marked downregulation of cell cycle–negative regulators observed in mortalin KD HSPCs. Gene expression analysis also revealed that a lack of mortalin led to alterations in the expression of antioxidant genes, including sestrin2, SOD2, Foxo1, and Prdm16, suggesting that mortalin suppresses ROS in HSCs by improving their ROS scavenging ability. Forced mortalin expression may therefore protect HSCs from numerous stressors, including transplant- and age-related oxidative stress, thereby improving the efficiency of ex vivo HSC expansion.
Similar to the mammalian system, the planarian mortalin-like gene, Djmot, is essential for the proper functioning of stem cells and their progeny but not for that of differentiated cells.42 Likewise, we observed that the downregulation or up-regulation of mortalin expression affected HSPC maintenance but scarcely altered their terminal differentiation capacity. The myeloid skew observed in transplanted mortalin-OE LSK BM cells might be because this population contains a high proportion of myeloid-biased HSCs, which have an extensive capacity for self-renewal.
To identify the mortalin-mediated quality control pathway in HSCs, we established DJ-1 as a mortalin client protein, which plays an influential role in ROS regulation. As both DJ-1 and mortalin share common cytoprotective functions, including ROS regulation, it is reasonable to hypothesize that mortalin may function in HSC maintenance by directly interacting with DJ-1. Hence, mortalin may associate with DJ-1, acting as a molecular chaperone to transport it into the mitochondria under conditions of oxidative stress. In support of this hypothesis, a previous study shows that DJ-1 is translocated to the mitochondria of nerve cells in response to H2O2.43 By analogy, we confirmed DJ-1 accumulation in the mitochondria of LSK cells after H2O2 treatment. Colocalization of mortalin and DJ-1 in the mitochondria of HSCs was strongly promoted under conditions of oxidative stress. These results demonstrate that DJ-1 is translocated into the mitochondria together with mortalin when ROS accumulate, or alternatively, that mortalin sequesters DJ-1 in the mitochondria after its translocation.
Analysis of DJ-1 KO mice revealed that a lack of DJ-1 results in ROS accumulation in vivo and a subsequent reduction in HSC numbers, similar to the effects of mortalin KD. The ratios of early progenitors or terminal erythroid differentiation were normal in DJ-1−/− mice, consistent with the HSC-dominant expression of DJ-1. Interestingly, the impact of DJ-1 deprivation became more pronounced with age like Parkinson’s disease, an aging-related neurodegenerative disease, mediated in part by DJ-1 malfunction. DJ-1−/− HSCs could not maintain low ROS levels even with forced mortalin expression, suggesting that DJ-1 plays important roles in HSC maintenance by interacting with mortalin. These findings indicate that the mortalin/DJ-1 complex is a key modulator of oxidative stress in HSCs.
In conclusion, mortalin/DJ-1 complex are essential participants in a molecular mechanism that safeguards HSCs against environmental damage, including that caused by age-related ROS accumulation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Shuji Yamashita (Keio University) for instruction in immunoelectron microscopy techniques.
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science. The research leading to these results received funding from the European Union's Seventh Framework Programme (FP7/2007-2013) under grant 306240 (SyStemAge).
Authorship
Contribution: I.T.-N. and S.M. designed and performed experiments and analyzed and interpreted data; H.A. provided DJ-1−/− mice; and I.T.-N., S.M., and T.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sahoko Matsuoka, Department of Cell Differentiation, The Sakaguchi Laboratory of Developmental Biology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: sahoko@z7.keio.jp; or Toshio Suda, Department of Cell Differentiation, The Sakaguchi Laboratory of Developmental Biology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: sudato@z3.keio.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal