In this issue of Blood, Garcia-Santos et al advance the field of heme regulation, a highly complex process involving iron and globin metabolism. They focus on a key enzyme involved in heme catabolism, heme oxygenase 1 (HO-1), which, ironically, has been poorly investigated in erythroid cells, the largest pool of heme-containing cells.1
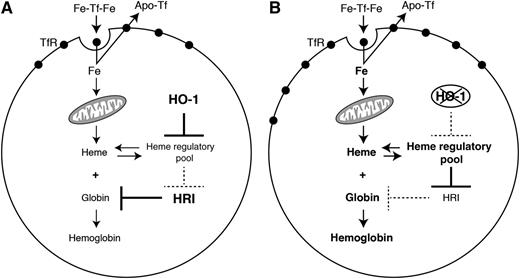
This figure illustrates the role of HO-1 on hemoglobinization by acting on intracellular heme level, TfR expression, and iron uptake. See Figure 7 in the article by Garcia-Santos et al that begins on page 2269.
This figure illustrates the role of HO-1 on hemoglobinization by acting on intracellular heme level, TfR expression, and iron uptake. See Figure 7 in the article by Garcia-Santos et al that begins on page 2269.
They address for the first time the role of the inducible HO-1 during murine erythroid differentiation.
Hemoproteins are involved in a broad spectrum of crucial biological functions, including oxygen binding, oxygen metabolism, and electron transfer.2 Hemoglobin is the most abundant hemoprotein, and the highest amount is found in circulating red blood cells. A fine balance between heme synthesis and catabolism is pivotal because non–protein-bound heme results in cell damage and tissue injury. Heme oxygenases are the initial and rate-limiting enzymes in the breakdown of heme.3
HO-1 has been given much attention in the literature on nonerythroid cells and hepatocytes. Here, Garcia-Santos and colleagues first explore the expression of HO-1 in mouse erythroid cells isolated from bone marrow. Next, they address the role of HO-1 through a series of in vitro experimental investigations that explore both the effect of HO-1 overexpression and suppression in different murine cell models. Their final step is to challenge their results in vivo with an HO-1 knockout mouse model.
The authors first demonstrate that HO-1 is highly expressed in murine erythroid cells and upregulated during in vitro erythroid differentiation. Furthermore, they demonstrate in a cell model of erythroid differentiation that intracellular heme concentrations and globin production are negatively impacted by HO-1 overexpression. They additionally show that this decrease in globin production most likely results from less heme, because it was associated with the activation of the globin suppressor heme-regulated eIF2 α kinase, which is normally negatively regulated by heme. Overexpression of HO-1 also leads to a decrease in transferrin receptor (TfR), both at the messenger RNA and surface protein levels, and subsequently to a decreased transferrin-dependent iron uptake (see figure). This decrease in iron uptake is partially responsible for reduced heme levels because supplying the cells with a different source of iron that bypasses the TfR pathway leads to incorporation into heme and normal heme biosynthesis.
Conversely, in fetal liver cells derived from HO-1 knockout mice, the authors show a significant increase in hemoglobin levels during erythroid differentiation. This finding is associated with increased TfR messenger RNA and surface protein as well as globin.
Finally, the authors confirm the in vitro cellular model data by performing direct measurements in circulating reticulocytes from HO-1+/+ and HO-1−/− mice. They show, in HO-1−/− mice, higher expression of TfR at the cell surface, increased iron uptake and globin expression, and a decreased level of active heme-regulated eIF2 α kinase.
Altogether these experiments point to the role of HO-1 as a coregulator of hemoglobinization, suggesting that under physiological conditions, appropriate levels of HO-1 guarantee optimal hemoglobinization rates (see figure).
Garcia-Santos and colleagues suggest a potential consequence of clinical relevance in thalassemia syndromes, notably β-thalassemia, which is characterized by an imbalance of globin chains with an excess of unpaired α-globin chains.4 It has previously been demonstrated that entrapment of purified α-hemoglobin chains within normal human erythrocytes significantly enhanced oxidant stress and resulted in pathological changes characteristic of thalassemic cells in vivo.5 The degree of ineffective erythropoiesis also correlates with the degree of imbalanced globin chain synthesis.6 Garcia-Santos and colleagues propose that unpaired α-globin chains may liberate their heme, thereby inducing HO-1. This abnormal and sustained HO-1 overexpression may further contribute to oxidative damage and to erythroid apoptosis, as evidenced by a significantly higher level of reactive oxygen species and elevated apoptosis found in differentiating MEL cells overexpressing HO-1 compared with control.
Future investigations of HO-1 effect in human erythroid cells and human thalassemic progenitors are warranted in order to extend these findings to human pathology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal