Key Points
hGH has a distinct capacity to promote the differentiation, especially the terminal differentiation of human primary megakaryocytes.
hGH exerts a complementary and synergistic effect with c-Mpl ligands on thrombopoiesis.
Abstract
Human growth hormone (hGH) is known to play a functional role in regulating hematopoiesis, although its direct effect on thrombopoiesis is unclear. In this study, we show for the first time that hGH has a distinct capacity to promote the differentiation of human primary megakaryocytes derived from umbilical cord blood CD34+ cells. In particular, hGH is potent in facilitating proplatelet formation and platelet production from cultured megakaryocytes. The stage- and time-specific activations of extracellular signal-regulated kinase 1/2 and protein kinase B signaling pathways are involved in the action of hGH. Fusion with hGH enhances the effect of a tandem dimer of thrombopoietin mimetic peptide (dTMP) on thrombopoiesis, manifested by a significant acceleration and increase of platelet production, indicating that hGH may exert a complementary and synergistic effect with c-Mpl ligands on thrombopoiesis. Accordingly, the administration of dTMP-growth hormone fusion protein led to a rapid platelet recovery in mice with severe thrombocytopenia induced by 6.5 Gy total body irradiation, thereby markedly abridging the duration of thrombocytopenia crisis (platelets <150 × 109/L), in comparison with high doses of dTMP. These findings demonstrate the functional role of growth hormone in promoting thrombopoiesis and provide a promising avenue for the treatment of severe thrombocytopenia.
Introduction
Growth hormone (GH), a pleiotropic cytokine, has the ability to promote the proliferation and/or differentiation of various kinds of cells through receptor-mediated activation of certain signal transduction pathways.1 The functional role of GH in regulating hematopoiesis has been recognized. Murphy et al primarily found that DW/J dwarf mice deficient in GH exhibit a significant decrease in peripheral blood cell counts, including leukocytes, erythrocytes, and platelets.2 Thereafter, increasing studies reported that a high dose of GH could restore hematopoietic functions, including platelet recovery in mice and rats, after radiation, chemotherapy, or bone marrow (BM) transplantation.3-7 An in vitro study further revealed that human growth hormone (hGH) has a distinct ability to stimulate the proliferation and differentiation of multilineage hematopoietic cells.8 Later, a clinical investigation showed that hematologic patients with recombinant human growth hormone (rhGH) treatment underwent a faster recovery of platelets to 25 × 109/L after intensive chemotherapy.9 All the published data imply that GH may have a functional role in regulating thrombopoiesis. However, the direct effect of GH on thrombopoiesis remains unclear.
Platelets are produced by megakaryocytes that originate from hematopoietic stem cells (HSCs) through several consecutive stages of differentiation. Therefore, the progress of megakaryocyte differentiation affects the schedule and output of platelet production. Thrombopoietin (TPO), the natural ligand of c-Mpl, is the primary regulator of thrombopoiesis and considered the most effective factor used for increasing the platelet count.10 However, TPO takes at least 5 days to increase the platelet count, and the peak platelet level is reached at approximately 12 days later, after TPO injection in vivo.11-13 Thus, TPO treatment results in a relatively delayed recovery of platelets, and it is therefore inefficient in reducing the duration of thrombocytopenia crisis when used against severe thrombocytopenia.13,14 The time-lagged increase of platelet level is most likely due to the inability of TPO to promote the terminal differentiation of megakaryocytes, including proplatelet formation and platelet shedding.15-17
More importantly, due to the undesired immunogenicity, the development of recombinant protein drugs derived from TPO for the treatment of thrombocytopenia has failed.18-20 Therefore, increased efforts have focused on the development of novel thrombopoietic agents based on TPO mimetics. Among them, a 14-mer TPO mimetic peptide (TMP), especially in the form of a covalent dimer, is of interest due to its high affinity to the TPO receptor and similar megakaryocytopoiesis activity as TPO in vitro.21 Several methods such as PEGylation and being grafted with Fab or Fc fragments, have been adopted to prolong the half-life of TMP for in vivo application.22-26 However, the aforementioned strategies are still unable to improve the characteristic of time-lagged promotion of platelet recovery of TMP.27,28 Therefore, how to endow TMP with the capacity to promote the terminal differentiation of megakaryocytes is worth pursuing.
In this study, we demonstrate that hGH is efficient in promoting the differentiation, especially the terminal differentiation, of human megakaryocytes through stage- and time-specific activation of extracellular signal-regulated kinase (ERK1/2) and protein kinase B (Akt), and it has a complementary and synergistic effect with a tandem dimer of thrombopoietin mimetic peptide (dTMP) on thrombocytopoiesis.
Materials
Human primary megakaryocyte culture
Human cord blood-derived CD34+ cells were isolated by an immunomagnetic bead separation system (EasySep, Human CD34 Positive Selection kit; Stem Cell Technologies) and cultured in serum-free medium (StemSpan SFEM; Stem Cell Technologies) supplemented with 1% penicillin/streptomycin and different growth factors, such as different concentrations of rhTPO, dTMP, rhGH, and dTMP-GH fusion protein, with or without 20 ng/mL recombinant human stem cell factor (rhSCF). These cells were seeded at particular days in 24-well plates, according to the requirements of different experimental arrangements. The density of the cells was 4 × 104/mL. For neutralizing the action of TPO, 20 μg/mL antithrombopoietin receptor (TPOR) (R&D Systems, Minneapolis, MN) was added in the culture 1 hour before rhGH or rhTPO treatment.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
The primers used for RT-PCR were as follows: human GH receptor (GHR) sense: 5′-GCCCAGGTGAGCGACATT-3′, antisense: 5′-CCAGCAGCAGTGGTAAGG-3′; globin transcription factor 1 (GATA-1) sense: 5′-CCTCAATTCAGCAGCCTATT-3′, antisense: 5′-CAGTGTCGTGGTGGTCGT-3′; nuclear factor erythroid 2 (NF-E2) sense: 5′-CCTCGTCCAGCAGTGTCA-3′, antisense: 5′-GGAAGTGGGAAGCCAGAAT-3′; β1-tubulin sense: 5′-TGTTTGCTGCCTCTATCTT-3′, antisense: 5′-CTACCCACTACCTTCTACCAT-3′; and glyceraldehyde-3-phosphate dehydrogenase sense: 5′-TCTGATTTGGTCGTATTGGG-3′, antisense: 5′-GGAAGATGGTGATGGGAT T-3′. Reactions were carried out with the One-Step RT-PCR kit (TaKaRa Bio; Otsu, Shiga Prefecture, Japan).
Immunofluorescent staining of GHR
Human cord blood-derived CD34+ cells were cultured in serum-free medium with 20 ng/mL rhTPO for 13 days. The cells were fixed and stained overnight at 4°C with the following polyclonal primary antibodies: rabbit anti-human CD34 (day 0) or CD41 (day 7 and 13) and mouse anti-human GHR (day 0, 7, and 13). After being stained with appropriate secondary antibodies for 30 minutes at room temperature (RT), the cells were washed and analyzed by a Carl Zeiss LSM 5 LIVE laser confocal microscopy.
Western blot analysis
The cell lines were plated at 5 × 105 cells/mL, treated with rhTPO, rhGH, dTMP, or dTMP-GH after 16 to 18 hours starvation, and collected at the designated times. The proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred by a Semi-Dry Transfer System (Bio-Rad, Hercules, CA), and probed with antibodies to p-STAT5 (BD Biosciences, Sparks, MD), p-ERK1/2, p-Akt, STAT5, ERK1/2, Akt (Cell Signaling Technology, Danvers, MA), GHR (Novus Biologicals, Littleton, CO), β1-tubulin, and glyceraldehyde-3-phosphate dehydrogenase (Sigma-Aldrich, St. Louis, MO). Species-specific horseradish peroxidase-conjugated secondary antibodies were used for signal detection with an ECL Plus Kit (Amersham, Pittsburgh, PA). Primary human cord blood-derived CD34+ cells plated at 2 × 106 cells/mL were also treated as described above for western blot analyses.
RhoA, Rac1, and Cdc42 activity assay
Pull-down assays were performed using the ρ Activation Assay Kit (Millipore, Billerica, MA) following the manufacturer’s instructions, and then immunoblotted with antibodies to RhoA, Rac1, and Cdc42 antibodies (Pierce Biotechnology, Rockford, IL), respectively.
Flow cytometry analysis
Analysis of human primary megakaryocytes.
A total of 1 × 105 cultured cells were labeled with allophycocyanin (APC)-conjugated anti-CD41 (eBioscience) and fluorescein isothiocyanate (FITC)-conjugated anti-CD42b antibodies (eBioscience) for 30 minutes at RT in the dark. The cells were fixed and finally analyzed using a FACSCanto II flow cytometer (Becton Dickinson).
Analysis of culture-derived platelets.
Primary megakaryocytes derived from human cord blood-derived CD34+ hematopoietic cells were harvested and centrifuged at 1000 rpm for 5 minutes. The supernatants were centrifuged at 3000 rpm for 10 minutes. The resuspended pellet containing the platelets was incubated with APC-CD41 and FITC-CD42b antibodies (eBioscience) for 30 minutes in the dark, and then with 10 μg/mL propidium iodide for 15 minutes. Conjugated cells were washed with phosphate-buffered saline, fixed with 1% paraformaldehyde, and analyzed by flow cytometry. Peripheral blood-derived human platelets were analyzed as the control for primary megakaryocyte-derived platelets. The operation was performed according to previous reports,29-31 and the details of the assay procedure are shown in supplemental Figure 1 (available on the Blood Web site).
Analysis of platelet function.
For the aggregation assay, culture-derived platelets were stimulated with 2 U/mL thrombin for 15 minutes at RT. The size distribution of the platelet aggregates was analyzed using the FSC/SSC dot plot by flow cytometry. To further determine the expression of P-selectin, culture-derived platelets were stimulated with 2 U/mL of thrombin for 15 minutes at RT, incubated with PE-CD62p antibody for 30 minutes in the dark at RT, and then measured by flow cytometry.
Cell viability assay
The growth factor-starved M07e cells were seeded into 96-well plates at a density of 5 × 104/well in the presence of 20 ng/mL rhTPO or various concentrations of rhGH or dTMP-GH. After 72 hours, 10 µL CCK-8 regent (Dojindo, Japan) was added to each well for a 6-hour incubation. The absorbance was measured at 450 nm using a 96-well spectrophotometer (MK3; Thermo Scientific).
Proplatelet-forming assay
The cells were cultured in 24-well plates in a serum-free medium with the indicated treatment for 14 days. The number of proplatelet-forming megakaryocytes was determined by phase-contrast images. For confocal microscopy, cells were spun onto glass slides, fixed with 10% formalin and permeabilized with 0.25% Triton X-100. Tubulin was stained with anti-β1-tubulin antibody and the nuclei were stained with 4′6 diamidino-2-phenylindole (DAPI). The slides were imaged by laser confocal microscope.
Transmission electron microscopy analysis
Cells were centrifuged at 1500 rpm for 5 minutes and washed twice with phosphate-buffered saline. The resulting pellets were immersed in a fixative consisting of 2.5% glutaraldehyde and 3% paraformaldehyde for 2 days at 4°C. Cell sections were viewed using a transmission electron microscope (Philips TECANAI-10; Eindhoven, The Netherlands).
Animals
Male BALB/c mice, 8 to 10 weeks old and 18 to 22 grams in weight were purchased from the Institute of Zoology, Chinese Academy of Sciences (Beijing, China). All procedures performed on the mice were approved by the Animal Care Committee of Third Military Medical University, Chongqing, China, and carried out in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health. The mice were divided randomly into 3 groups: saline control group, dTMP-GH treatment group, and dTMP treatment group.
Irradiation injury
The mice were subjected to a single dose of 6.5 Gy total body irradiation (TBI) by using a 60Co γ-ray source (Irradiation Center, Third Military Medical University, Chongqing, China). The dose rate was 92.8 to 95.5 cGy/min.
Measurement of hematologic parameters
A small amount of blood (20 µL) was collected on the indicated days from the tail vein of the mice and diluted in 1% EDTA solution. Platelets in the blood samples were counted automatically in a hematology analyzer (Sysmex XT-1800i/2000IV; Kobe, Japan).
Histology analysis
Fourteen days after irradiation, 3 mice selected randomly from each group were euthanized, and their tibias were removed and stored in 10% formaldehyde. Specimens were then embedded in paraffin, cut into 5 μm sections, stained with hematoxylin and eosin (H&E), and observed under a microscope (Olympus BX51).
Statistical analysis
All data in this report are the means ± standard deviations (SDs) of at least 3 independent experiments. Statistical analyses were performed by Student t test for data involving two groups or by analysis of variance for data involving more than two groups. P < .05 was considered statistically significant.
Results
Human GHR is expressed during megakaryocytopoiesis
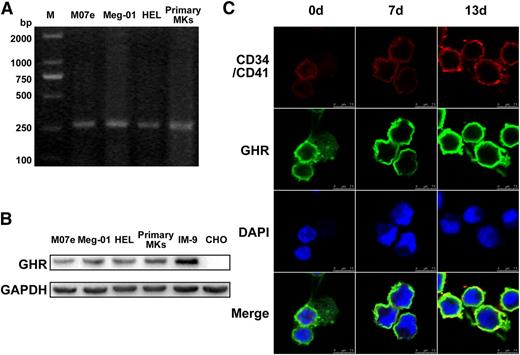
As shown in Figure 1A, the mRNA transcript of GHR was clearly detected in various megakaryocytes, including the megakaryoblastic cell line M07e, mature megakaryocyte cell line Meg-01, megakaryoblastic human erythroleukemia cell line HEL, and human primary megakaryocytes derived from umbilical cord blood CD34+ cells (Figure 1A). The expression of GHR protein in these megakaryocytes was confirmed by western blot (Figure 1B). Immunofluorescent staining revealed that GHR was expressed and accompanied by increased expression of CD41 in cord blood-derived CD34+ cells cultured with rhTPO for 0, 7, or 13 days (Figure 1C). These data indicate that GHR is expressed in the cells throughout the process of megakaryocytopoiesis.
The expression of GH receptor in megakaryocytes. (A-B) RT-PCR and western blot analysis of GHR expression in M07e, Meg-01, HEL cell lines, and human primary megakaryocytes. IM-9 and Chinese hamster ovary cells were taken as positive and negative controls, respectively. (C) Human cord blood-derived CD34+ cells were cultured in serum-free medium with 20 ng/ml TPO for 13 days. The expressions of GHR (green fluorescence), CD34, and CD41 (red fluorescence) in the cells were detected by confocal microscopy. DAPI was used for nuclear staining (blue fluorescence). All data are from 3 independent experiments.
The expression of GH receptor in megakaryocytes. (A-B) RT-PCR and western blot analysis of GHR expression in M07e, Meg-01, HEL cell lines, and human primary megakaryocytes. IM-9 and Chinese hamster ovary cells were taken as positive and negative controls, respectively. (C) Human cord blood-derived CD34+ cells were cultured in serum-free medium with 20 ng/ml TPO for 13 days. The expressions of GHR (green fluorescence), CD34, and CD41 (red fluorescence) in the cells were detected by confocal microscopy. DAPI was used for nuclear staining (blue fluorescence). All data are from 3 independent experiments.
hGH promotes the differentiation of megakaryocytes
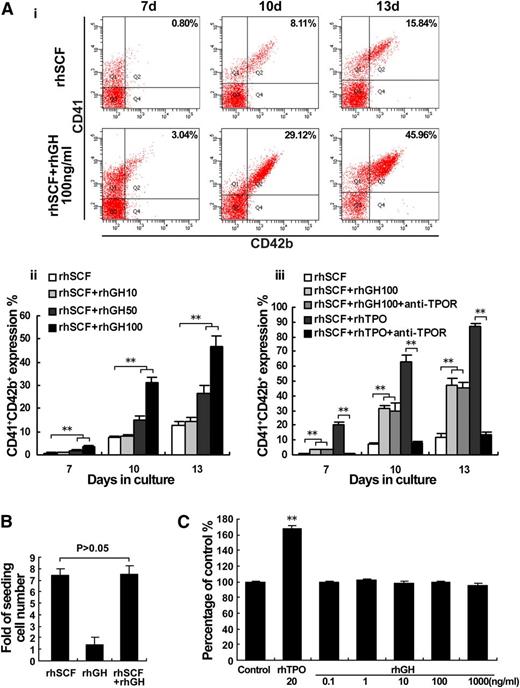
To detect whether hGH has a direct effect on megakaryocyte proliferation and/or differentiation, cord blood-derived CD34+ cells were cultured with a different dose of rhGH, in the presence of rhSCF (20 ng/mL). After culturing for 7 days with rhGH, the expression of CD41/CD42b began to increase, and reached a relatively higher level at day 10 and an even higher level at day 13 (Figure 2Ai). The effect of rhGH on megakaryocyte differentiation was prominent at the concentration of 100 ng/mL (Figure 2Aii), and it was not significantly influenced in the presence of anti-c-Mpl (TPOR) antibody (20 μg/mL), which has the ability to neutralize the action of TPO (10 ng/mL) (Figure 2Aiii). However, rhGH alone could not support the survival and expansion of cord blood-derived CD34+ cells (Figure 2B), consistent with the finding that rhGH treatment could not induce the proliferation of M07e megakaryoblastic cells, in contrast to rhTPO (Figure 2C).
hGH promotes the differentiation of megakaryocytes. (A) Human cord blood-derived CD34+ cells were cultured in the presence of rhSCF (20 ng/ml) with or without different concentrations of rhGH (10, 50, and 100 ng/ml) or rhTPO (10 ng/ml) for 13 days. (i) The expressions of CD41 and CD42b in the cells treated with 100 ng/ml rhGH for 7, 10, and 13 days were analyzed by flow cytometry. (ii) Histogram showing the proportions of CD41+CD42b+ cells for each group. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (iii) The influence of anti-TPOR treatment on the expression of CD41/42b induced by rhGH and rhTPO. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (B) The increased numbers of cells in the culture at day 7 were analyzed by a cytometer and compared with seeding numbers of CD34+ cells at day 0. Data are from 3 independent experiments. Error bars denote SD. (C) Viability of M07e cells cultured with the indicated concentrations of rhGH for 72 hours as analyzed by CCK-8 assay. The data are from 6 independent assays with a single batch of cells. Error bars denote SD. **P < .01.
hGH promotes the differentiation of megakaryocytes. (A) Human cord blood-derived CD34+ cells were cultured in the presence of rhSCF (20 ng/ml) with or without different concentrations of rhGH (10, 50, and 100 ng/ml) or rhTPO (10 ng/ml) for 13 days. (i) The expressions of CD41 and CD42b in the cells treated with 100 ng/ml rhGH for 7, 10, and 13 days were analyzed by flow cytometry. (ii) Histogram showing the proportions of CD41+CD42b+ cells for each group. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (iii) The influence of anti-TPOR treatment on the expression of CD41/42b induced by rhGH and rhTPO. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (B) The increased numbers of cells in the culture at day 7 were analyzed by a cytometer and compared with seeding numbers of CD34+ cells at day 0. Data are from 3 independent experiments. Error bars denote SD. (C) Viability of M07e cells cultured with the indicated concentrations of rhGH for 72 hours as analyzed by CCK-8 assay. The data are from 6 independent assays with a single batch of cells. Error bars denote SD. **P < .01.
hGH stimulates proplatelet formation and platelet production
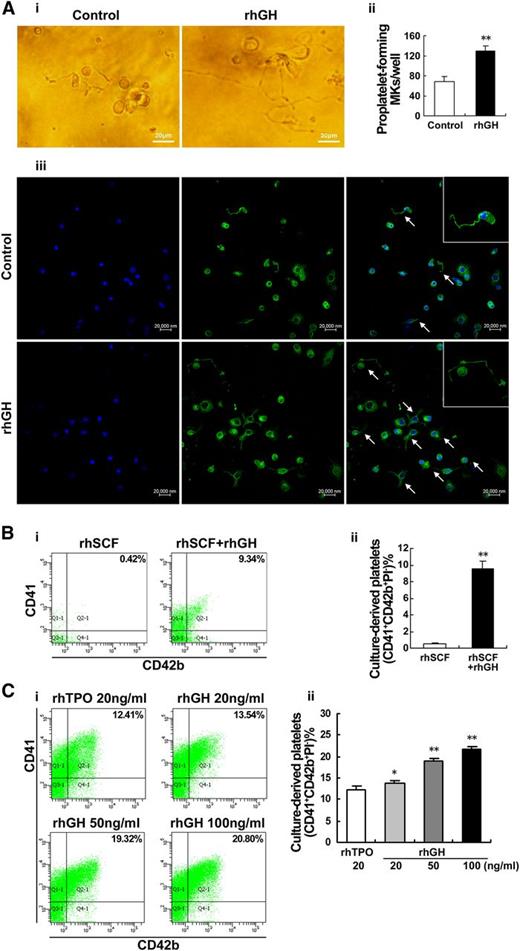
The above results indicate that hGH may exert its effects primarily on the late stage of megakaryocyte differentiation. We, therefore, chose mature megakaryocytes to detect the effect of rhGH on proplatelet formation. As observed under an inverted microscope, typical proplatelet formations characterized by threadlike cytoplasmic extensions with bead-like nubs were observed in rhGH-treated human primary megakaryocytes derived from cord blood CD34+ cells (Figure 3Ai). Immunofluorescent anti-β1-tubulin staining further revealed that the frequency of proplatelet-forming megakaryocytes was markedly increased after rhGH treatment (Figure 3Aii-iii).
hGH promotes proplatelet formation and platelet production by cultured megakaryocytes. (A) Human cord blood-derived CD34+ cells were cultured with rhTPO (20 ng/ml) for 7 days and then treated with rhGH (100 ng/ml) for 3 days. Proplatelet formations in representative megakaryocytes were detected by phase contrast imaging (i), and visualized by confocal microscopy (iii) after being stained with anti-β1-tubulin (green) and DAPI (blue). Arrows indicate typical proplatelet formations. (ii) Histogram showing the number of proplatelet-forming megakaryocytes for each group. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (B) Human cord blood-derived CD34+ cells were treated with rhSCF (20 ng/ml) in the presence or absence of rhGH (100 ng/ml) for 13 days. (i) Culture-derived platelets were stained with APC-CD41, FITC-CD42b, and PI, and analyzed by flow cytometry. (ii) Mean percentages of CD41+CD42b+PI− culture-derived platelets are shown. Data are representative of 3 independent experiments. Error bars denote SD. **P < .01. (C) Human cord blood-derived CD34+ cells were treated with rhTPO (20 ng/ml) for 7 days and then treated with rhGH (20, 50, or 100 ng/ml) or rhTPO (20 ng/ml) for 6 days. (i) Culture-derived platelets stained with APC-CD41, FITC-CD42b, and PI was analyzed by flow cytometry. (ii) Histogram showing the percentage of CD41+CD42b+PI− culture-derived platelets in 3 independent experiments. Error bars denote SD. *P < .05; **P < .01.
hGH promotes proplatelet formation and platelet production by cultured megakaryocytes. (A) Human cord blood-derived CD34+ cells were cultured with rhTPO (20 ng/ml) for 7 days and then treated with rhGH (100 ng/ml) for 3 days. Proplatelet formations in representative megakaryocytes were detected by phase contrast imaging (i), and visualized by confocal microscopy (iii) after being stained with anti-β1-tubulin (green) and DAPI (blue). Arrows indicate typical proplatelet formations. (ii) Histogram showing the number of proplatelet-forming megakaryocytes for each group. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (B) Human cord blood-derived CD34+ cells were treated with rhSCF (20 ng/ml) in the presence or absence of rhGH (100 ng/ml) for 13 days. (i) Culture-derived platelets were stained with APC-CD41, FITC-CD42b, and PI, and analyzed by flow cytometry. (ii) Mean percentages of CD41+CD42b+PI− culture-derived platelets are shown. Data are representative of 3 independent experiments. Error bars denote SD. **P < .01. (C) Human cord blood-derived CD34+ cells were treated with rhTPO (20 ng/ml) for 7 days and then treated with rhGH (20, 50, or 100 ng/ml) or rhTPO (20 ng/ml) for 6 days. (i) Culture-derived platelets stained with APC-CD41, FITC-CD42b, and PI was analyzed by flow cytometry. (ii) Histogram showing the percentage of CD41+CD42b+PI− culture-derived platelets in 3 independent experiments. Error bars denote SD. *P < .05; **P < .01.
To further observe whether hGH has a direct effect on platelet production, the activated platelets (CD41+CD42b+PI–) produced by cord blood-derived CD34+ cells were measured by flow cytometry. After 13 days of treatment with rhGH (100 ng/mL) in the presence of rhSCF (20 ng/mL), an obvious production of platelets was detected in the culture (Figure 3B). In another experiment, the cord blood-derived CD34+ cells were cultured with rhTPO (20 ng/mL) for 7 days to obtain enriched megakaryocytes, and then the cells were cultured with rhGH (100 ng/mL) for 6 days. As shown in Figure 3C, rhGH treatment increased the production of platelets in a dose-dependent manner. The above data demonstrate that GH possesses a distinct ability to promote proplatelet formation and platelet production from cultured megakaryocytes.
The stage- and time-specific activation of ERK1/2 and Akt is involved in hGH-promoted megakaryocyte differentiation and platelet production
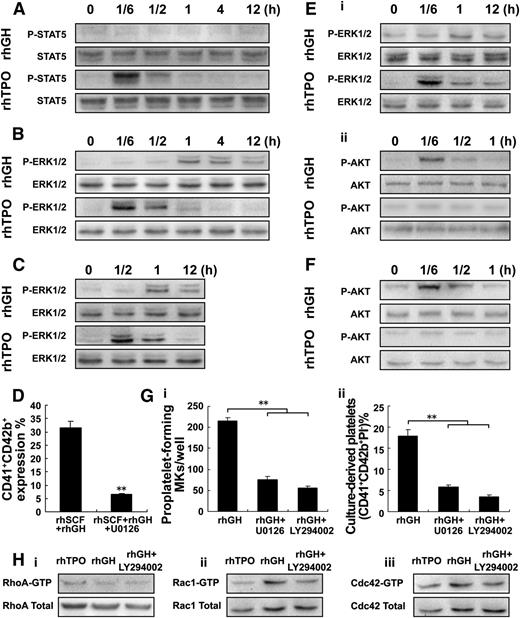
To uncover the underlying mechanism of hGH action, the known cellular signal pathways that are involved in megakaryocyte proliferation and differentiation32,33 were investigated. In M07e megakaryoblastic cells, rhGH treatment displayed an obscure effect on the activation of STAT5, which could be markedly activated by rhTPO (Figure 4A). In contrast to a rapid and transient phosphorylation of ERK1/2 induced by rhTPO, a delayed and prolonged activation of ERK1/2 occurred after rhGH treatment (Figure 4A-B). Unfortunately, there was no observable activation of Akt following the treatment with rhGH or rhTPO in M07e cells (data not shown). Similarly, the lagged and prolonged activation of ERK1/2 induced by rhGH was also found in human primary megakaryocytes at an early differentiation stage (Figure 4C). On the other hand, treatment with U0126 (10 μM), a specific blocker of ERK1/2, significantly inhibited the increased expression of CD41/CD42b in human primary megakaryocytes induced by rhGH (Figure 4D), suggesting that the delayed and prolonged activation of ERK1/2 most likely contributes to the rhGH-induced megakaryocyte differentiation.
hGH enhances dTMP-induced platelet production. (A-B) Western blot analysis of p-STAT5 and p-ERK1/2 in whole-cell lysates from M07e cells after exposure to rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times. (C) Western blot analysis of p-ERK1/2 in whole-cell lysates from human cord blood-derived CD34+ cells cultured with rhTPO (20 ng/ml) for 6 days, and treated with rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times after starvation. (D) Human cord blood-derived CD34+ cells were cultured with rhGH (100 ng/ml) in the presence of rhSCF (20 ng/ml) for 7 days; then the cells were further treated with rhGH (100 ng/ml) for another 3 days with or without U0126 (10 μM) in the culture. Histogram showing the percentage of CD41+CD42b+ megakaryocytes from 3 independent experiments analyzed by flow cytometry. Error bars denote SD. (E) Western blot analysis of p-ERK1/2 (i) and p-Akt (ii) in whole-cell lysates from human cord blood-derived CD34+ cells cultured with rhTPO (20 ng/ml) for 11 days, and then treated with rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times after starvation. (F) Western blot analysis of p-Akt in whole-cell lysates from Meg-01 cells after exposure to rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times. (G) Human cord blood-derived CD34+ cells were cultured with rhTPO (20 ng/ml) for 7 days and then treated with rhGH (100 ng/ml) with or without U0126 (10 μM) or LY294002 (20 μM) for another 3 or 6 days. (i) Proplatelet-forming megakaryocytes were counted in each well 3 days later. (ii) Culture-derived platelets were analyzed 6 days later. Mean ± SD of 3 experiments. **P < .01. (H) Western blot analysis of the activation of ρ GTPases, RhoA (i), Rac1 (ii), and Cdc42 (iii) in whole-cell lysates from human cord blood-derived CD34+ cells cultured with rhTPO (20 ng/ml) for 7 days, and then treated with rhGH (100 ng/ml) for 3 days with or without LY294002 (20 μM).
hGH enhances dTMP-induced platelet production. (A-B) Western blot analysis of p-STAT5 and p-ERK1/2 in whole-cell lysates from M07e cells after exposure to rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times. (C) Western blot analysis of p-ERK1/2 in whole-cell lysates from human cord blood-derived CD34+ cells cultured with rhTPO (20 ng/ml) for 6 days, and treated with rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times after starvation. (D) Human cord blood-derived CD34+ cells were cultured with rhGH (100 ng/ml) in the presence of rhSCF (20 ng/ml) for 7 days; then the cells were further treated with rhGH (100 ng/ml) for another 3 days with or without U0126 (10 μM) in the culture. Histogram showing the percentage of CD41+CD42b+ megakaryocytes from 3 independent experiments analyzed by flow cytometry. Error bars denote SD. (E) Western blot analysis of p-ERK1/2 (i) and p-Akt (ii) in whole-cell lysates from human cord blood-derived CD34+ cells cultured with rhTPO (20 ng/ml) for 11 days, and then treated with rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times after starvation. (F) Western blot analysis of p-Akt in whole-cell lysates from Meg-01 cells after exposure to rhTPO (20 ng/ml) or rhGH (100 ng/ml) for the indicated times. (G) Human cord blood-derived CD34+ cells were cultured with rhTPO (20 ng/ml) for 7 days and then treated with rhGH (100 ng/ml) with or without U0126 (10 μM) or LY294002 (20 μM) for another 3 or 6 days. (i) Proplatelet-forming megakaryocytes were counted in each well 3 days later. (ii) Culture-derived platelets were analyzed 6 days later. Mean ± SD of 3 experiments. **P < .01. (H) Western blot analysis of the activation of ρ GTPases, RhoA (i), Rac1 (ii), and Cdc42 (iii) in whole-cell lysates from human cord blood-derived CD34+ cells cultured with rhTPO (20 ng/ml) for 7 days, and then treated with rhGH (100 ng/ml) for 3 days with or without LY294002 (20 μM).
In human primary megakaryocytes at a late differentiation stage, in addition to a slow activation of ERK1/2, a rapid activation of Akt was observed after rhGH treatment (Figure 4E). The rhGH-induced activation of Akt was also found in Meg-01 mature megakaryocytes (Figure 4F). Moreover, pretreatment with inhibitors of ERK1/2 (U0126) and Akt (LY294002), especially Akt inhibitor, significantly blocked the rhGH-induced proplatelet formation and platelet production by cultured megakaryocytes (Figure 4G). We then found that GH treatment led to an evident increase in the activation of ρ GTPases, Rac1, and Cdc42 in mature megakaryocytes, whereas pretreatment with Akt inhibitor (LY294002) significantly restrained the activation of Rac1/Cdc42 induced by GH (Figure 4H).
hGH has a complementary effect on dTMP-induced thrombopoiesis
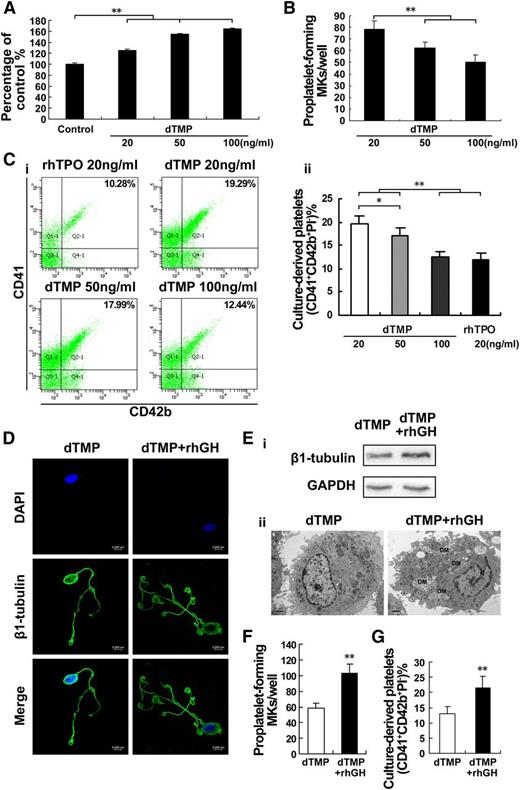
Our preliminary work revealed that dTMP, a linear peptide containing two tandem 14-mer TMPs joined by a four-amino-acid linker (PSGP), displayed a similar property to TPO in binding with c-Mpl. As shown in Figure 5A, dTMP dose-dependently promoted the proliferation of M07e cells. Similarly, this peptide alone could support and stimulate the differentiation of human cord blood-derived CD34+ cells into megakaryocytes. However, the proplatelet formation and platelet production were decreased with increasing concentrations of dTMP (Figure 5B-C), indicating that dTMP might have an inhibitory effect on the terminal differentiation of megakaryocytes. Noticeably, compared with dTMP alone, cotreatment with rhGH and dTMP resulted in more proplatelet formations by megakaryocytes (Figure 5D). In addition, an increased expression of β1-tubulin, typical demarcation membrane formations, and more pseudopod formations were obeserved in the cells treated with dTMP plus rhGH (Figure 5E). Accordingly, cotreatment with dTMP and rhGH significantly increased the proportion of proplatelet-forming megakaryocytes at day 10 (Figure 5F and supplemental Figure 2) and the platelet production at day 13 (Figure 5G), in comparison with dTMP treatment alone. These results indicate that GH may have a complementary effect to dTMP on the terminal differentiation of megakaryocytes and platelet production.
hGH enhances dTMP-induced platelet production. (A) Viability of M07e cells cultured with dTMP (20 ng/ml, 50 ng/ml, or 100 ng/ml) for 72 hours was analyzed by CCK-8 assay. The data are from 6 independent assays with a single batch of cells. Error bars denote SD. **P < .01. (B) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml, 50 ng/ml, or 100 ng/ml) for 10 days and then the proplatelet formations were observed under a phase-contrast microscope. Histogram shows the number of proplatelet-forming megakaryocytes. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (C) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml, 50 ng/ml, or 100 ng/ml) or rhTPO (20 ng/ml) for 13 days. (i) Culture-derived platelets stained with APC-CD41, FITC-CD42b, and PI was analyzed by flow cytometry. (ii) Histogram showing the percentage of CD41+CD42b+PI− culture-derived platelets from 3 independent experiments. Error bars denote SD. *P < .05; **P < .01. (D) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml) in the absence or presence of rhGH (100 ng/ml) for 10 days. Proplatelet formations in representative megakaryocytes were stained with β1-tubulin (green) and DAPI (blue), and visualized by confocal microscope. (E) The expression level of β1-tubulin in human cord blood-derived CD34+ cells cultured with dTMP (20 ng/ml) in the absence or presence of rhGH (100 ng/ml) for 10 days was analyzed by western blot (i) and the cells were visualized by TEM (ii). DM, demarcation membrane; N, nucleus. (F) Histogram showing the number of proplatelet-forming megakaryocytes for each group. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (G) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml) in the absence or presence of rhGH (100 ng/ml) for 13 days. Histogram shows the percentage of CD41+CD42b+PI− culture-derived platelets in 3 independent experiments. Error bars denote SD. **P < .01.
hGH enhances dTMP-induced platelet production. (A) Viability of M07e cells cultured with dTMP (20 ng/ml, 50 ng/ml, or 100 ng/ml) for 72 hours was analyzed by CCK-8 assay. The data are from 6 independent assays with a single batch of cells. Error bars denote SD. **P < .01. (B) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml, 50 ng/ml, or 100 ng/ml) for 10 days and then the proplatelet formations were observed under a phase-contrast microscope. Histogram shows the number of proplatelet-forming megakaryocytes. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (C) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml, 50 ng/ml, or 100 ng/ml) or rhTPO (20 ng/ml) for 13 days. (i) Culture-derived platelets stained with APC-CD41, FITC-CD42b, and PI was analyzed by flow cytometry. (ii) Histogram showing the percentage of CD41+CD42b+PI− culture-derived platelets from 3 independent experiments. Error bars denote SD. *P < .05; **P < .01. (D) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml) in the absence or presence of rhGH (100 ng/ml) for 10 days. Proplatelet formations in representative megakaryocytes were stained with β1-tubulin (green) and DAPI (blue), and visualized by confocal microscope. (E) The expression level of β1-tubulin in human cord blood-derived CD34+ cells cultured with dTMP (20 ng/ml) in the absence or presence of rhGH (100 ng/ml) for 10 days was analyzed by western blot (i) and the cells were visualized by TEM (ii). DM, demarcation membrane; N, nucleus. (F) Histogram showing the number of proplatelet-forming megakaryocytes for each group. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (G) Human cord blood-derived CD34+ cells were cultured with dTMP (20 ng/ml) in the absence or presence of rhGH (100 ng/ml) for 13 days. Histogram shows the percentage of CD41+CD42b+PI− culture-derived platelets in 3 independent experiments. Error bars denote SD. **P < .01.
The dTMP-GH fusion protein strongly promotes thrombopoiesis
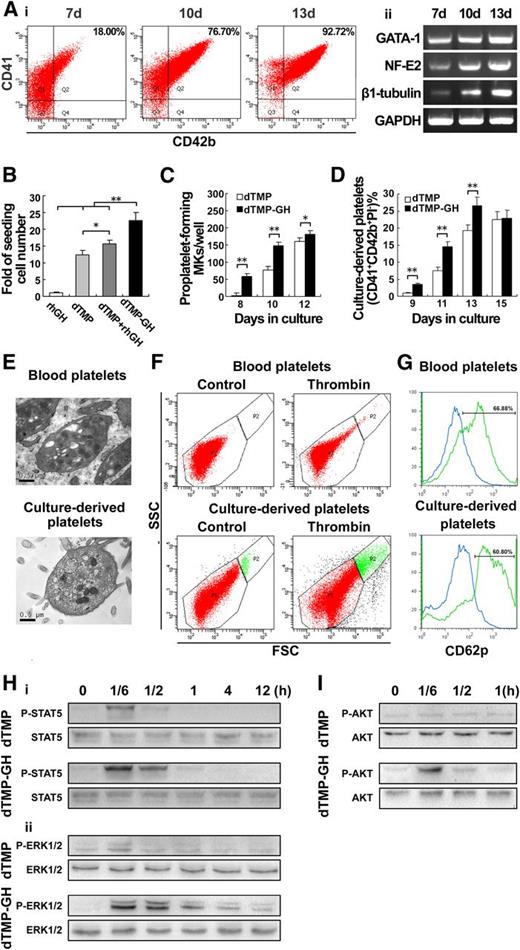
We next found that dTMP-GH, the dTMP, and GH fusion protein generated by gene-engineering approach,34 exhibited a distinct effect on the differentiation and maturation of megakaryocytes, manifested by increased expressions of CD41/CD42b (Figure 6Ai), GATA-1, NF-E2, as well as β1-tubulin (Figure 6Aii). Noticeably, dTMP-GH also possessed a prominent activity in promoting the survival of human cord blood-derived CD34+ cells (Figure 6B). More importantly, compared with equimolar dTMP, treatment with 120 ng/mL dTMP-GH resulted in an earlier and increased proportion of proplatelet formation and platelet production (Figure 6C-D). In addition, the platelets produced by dTMP-GH–treated megakaryocytes displayed the typical ultrastructure of normal platelets, as viewed under transmission electron microscopy (TEM) (Figure 6E), and the functionality of these platelets was validated by their shape change and increased expression of CD62p in response to thrombin (Figure 6F-G).
The dTMP-GH fusion protein strongly promotes thrombopoiesis. (A) Human cord blood-derived CD34+ cells were cultured with dTMP-GH (120 ng/ml) for 7, 10, and 13 days. (i) The proportion of CD41+CD42b+ cells was analyzed by flow cytometry. (ii) The expression levels of GATA-1, NF-E2, and β1-tubulin were analyzed by RT-PCR. (B) Histogram showing the proliferation of human cord blood-derived CD34+ cells cultured with dTMP-GH (120 ng/ml), dTMP (20 ng/ml), or rhGH (100 ng/ml) at day 7. The cells were counted by cytometer. Data are from 3 independent experiments. Error bars denote SD. *P < .05; **P < .01. (C) Histogram showing the number of proplatelet-forming megakaryocytes derived from human cord blood-derived CD34+ cells cultured with dTMP (20 ng/ml) or dTMP-GH (120 ng/ml) at days 8, 10, and 12. Data are from 3 independent experiments. Error bars denote SD. *P < .05; **P < .01. (D) Culture-derived platelets were stained with CD41-PE, CD42b-APC, and PI. Histogram shows the percentage of CD41+CD42b+PI− culture-derived platelets detected by flow cytometry. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (E) TEM analysis of blood- and culture-derived platelets (×30 000). (F) Aggregation of platelets. Platelets were stimulated with 2 U/ml thrombin for 15 minutes and analyzed by flow cytometry. Unstimulated platelets were used as control. Top and bottom panels represent blood-derived platelets and culture-derived platelets, respectively. (G) Platelets were stimulated with or without 2 U/ml thrombin for 15 minutes. Expression of P-selection was analyzed by CD62p-PE staining (blue line indicates unstimulated platelets; green line indicates thrombin-stimulated platelets). (H) Western blot analysis of p-STAT5 (i), and p-ERK1/2 (ii), in whole-cell lysates from M07e cells after exposure to dTMP (20 ng/ml) or dTMP-GH (120 ng/ml) for the indicated times. (I) Western blot analysis of p-Akt in whole-cell lysates from Meg-01 cells after exposure to dTMP (20 ng/ml) or dTMP-GH (120 ng/ml) for the indicated times.
The dTMP-GH fusion protein strongly promotes thrombopoiesis. (A) Human cord blood-derived CD34+ cells were cultured with dTMP-GH (120 ng/ml) for 7, 10, and 13 days. (i) The proportion of CD41+CD42b+ cells was analyzed by flow cytometry. (ii) The expression levels of GATA-1, NF-E2, and β1-tubulin were analyzed by RT-PCR. (B) Histogram showing the proliferation of human cord blood-derived CD34+ cells cultured with dTMP-GH (120 ng/ml), dTMP (20 ng/ml), or rhGH (100 ng/ml) at day 7. The cells were counted by cytometer. Data are from 3 independent experiments. Error bars denote SD. *P < .05; **P < .01. (C) Histogram showing the number of proplatelet-forming megakaryocytes derived from human cord blood-derived CD34+ cells cultured with dTMP (20 ng/ml) or dTMP-GH (120 ng/ml) at days 8, 10, and 12. Data are from 3 independent experiments. Error bars denote SD. *P < .05; **P < .01. (D) Culture-derived platelets were stained with CD41-PE, CD42b-APC, and PI. Histogram shows the percentage of CD41+CD42b+PI− culture-derived platelets detected by flow cytometry. Data are from 3 independent experiments. Error bars denote SD. **P < .01. (E) TEM analysis of blood- and culture-derived platelets (×30 000). (F) Aggregation of platelets. Platelets were stimulated with 2 U/ml thrombin for 15 minutes and analyzed by flow cytometry. Unstimulated platelets were used as control. Top and bottom panels represent blood-derived platelets and culture-derived platelets, respectively. (G) Platelets were stimulated with or without 2 U/ml thrombin for 15 minutes. Expression of P-selection was analyzed by CD62p-PE staining (blue line indicates unstimulated platelets; green line indicates thrombin-stimulated platelets). (H) Western blot analysis of p-STAT5 (i), and p-ERK1/2 (ii), in whole-cell lysates from M07e cells after exposure to dTMP (20 ng/ml) or dTMP-GH (120 ng/ml) for the indicated times. (I) Western blot analysis of p-Akt in whole-cell lysates from Meg-01 cells after exposure to dTMP (20 ng/ml) or dTMP-GH (120 ng/ml) for the indicated times.
Western blot analysis revealed that in M07e megakaryoblastic cells, dTMP-GH treatment (120 ng/mL) caused a rapid activation of STAT5 and a strong and sustained activation of ERK1/2, whereas in human Meg-01 mature megakaryocytes, dTMP-GH treatment resulted in a rapid and strong activation of Akt (Figure 6H-I). All the data indicated that dTMP-GH has the ability to promote the whole progression of thrombopoiesis.
The dTMP-GH fusion protein accelerates platelet recovery in mice with severe thrombopenia induced by TBI
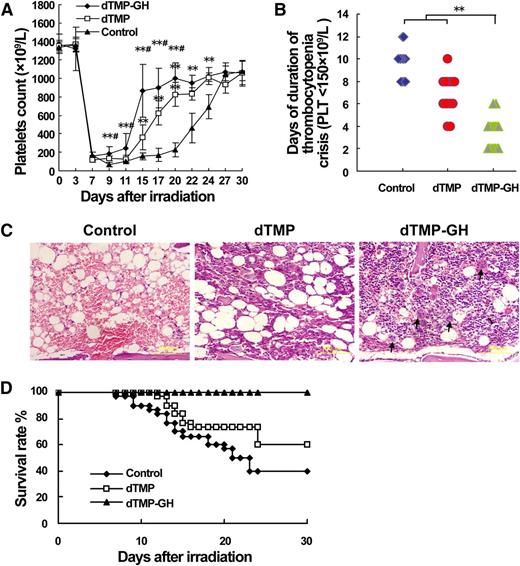
The in vivo study revealed that subcutaneous administration with this fusion protein could dose-dependently increase the platelet level in the peripheral blood in normal mice (supplemental Figure 3). Finally, 120 μg/kg dTMP-GH was used to treat BALB/c mice with severe thrombocytopenia induced by 6.5 Gy TBI, by subcutaneous injection, in parallel with a dTMP treatment group (100 μg/kg). Compared with vehicle control, both dTMP-GH and dTMP treatments for 10 days were effective in promoting platelet recovery after irradiation (Figure 7A). However, the starting time of platelet recovery in dTMP-GH–treated mice was at least 3 days earlier than that of dTMP-treated mice (Figure 7A). Correspondingly, dTMP-GH treatment resulted in a significantly shorter duration of thrombocytopenia crisis (platelets <150 × 109/L) (Figure 7B). As observed by H&E staining, more hematopoietic cells, especially the mature megakaryocytes near the sinusoid, were found in the BM of dTMP-GH–treated mice at day 14 after TBI (Figure 7C). Moreover, dTMP-GH treatment significantly increased the 30-day survival rate of irradiated mice (Figure 7D). This result demonstrates that dTMP-GH has a capacity to quickly promote platelet recovery, which is conducive to the therapy of severe thrombocytopenia.
dTMP-GH fusion protein accelerates platelet recovery after ionizing irradiation. BALB/c mice were irradiated with 6.5 Gy TBI and then treated with dTMP-GH (120 μg/kg), dTMP (100 μg/kg), or vehicle once a day for 10 days. Each group contained 20 animals. Three mice from each group were euthanized on day 14, and their tibias were H&E-stained. (A) Peripheral platelet counts. Error bars denote SD. **P < .01 vs control group; #P < .05 vs dTMP group. (B) Duration of thrombocytopenia crisis (platelets <150 × 109/L). **P < .01. (C) Histologic analysis. Blank arrows indicate the mature megakaryocytes near the sinusoid. (D) Survival rates.
dTMP-GH fusion protein accelerates platelet recovery after ionizing irradiation. BALB/c mice were irradiated with 6.5 Gy TBI and then treated with dTMP-GH (120 μg/kg), dTMP (100 μg/kg), or vehicle once a day for 10 days. Each group contained 20 animals. Three mice from each group were euthanized on day 14, and their tibias were H&E-stained. (A) Peripheral platelet counts. Error bars denote SD. **P < .01 vs control group; #P < .05 vs dTMP group. (B) Duration of thrombocytopenia crisis (platelets <150 × 109/L). **P < .01. (C) Histologic analysis. Blank arrows indicate the mature megakaryocytes near the sinusoid. (D) Survival rates.
Discussion
It’s known that TPO is the primary but not the only regulator of thrombopoiesis.35 To our knowledge, this is the first report on the direct effect of GH on thrombopoiesis. As a universal growth factor, GH has the ability to promote cell proliferation and/or differentiation either directly by binding to its receptor or indirectly by insulin-like growth factor-1 (IGF-1)–mediated signaling.36 In view of the different cell types or the same cell type at different status, GH may function as a regulator for cell proliferation and/or differentiation.37,38 In this study, we found that rhGH is incapable of supporting the survival or proliferation of HSCs or megakaryoblastic cells but has the ability to promote megakaryocyte differentiation. These results are consistent with the report that GH is inefficient in maintaining the survival/proliferation machinery of CD34+ cells and Sca-1+ cells in BM cultures, although it does promote B-lymphocyte differentiation.39 The action of GH was further illustrated by the finding that rhGH did not induce a significant activation of STAT5 (Figure 4A), the transcription factor that accounts for most of the proliferation-promoting action of GH.40 Furthermore, rhGH also could not induce significant phosphorylation of janus kinase 2, the main mediator of STAT5, in megakaryoblastic cells (data not shown). It was reported that the growth-promoting action of GH appears to require IGF-1R signaling,36 whereas our previous study demonstrated that IGF-1 has no direct effect on the proliferation of M07e megakaryoblastic cells.41 Therefore, the finding that GH is insufficient to promote the proliferation of HSCs and megakaryocyte progenitor cells might be attributed to the lack of IGF-1R signaling in these cells.
ERK1/2 is another important cellular signal transduction pathway involved in the proliferation and differentiation of megakaryocytes.33 Interestingly, we found that rhGH induced a slow but prolonged activation of ERK1/2 not only in M07e megakaryoblastic cells, but also in primary megakaryocytes at an early stage, in contrast to the quick and short activation of ERK1/2 induced by rhTPO. This result may explain why rhGH can promote the differentiation but not the proliferation of megakaryocytes to some extent, because it has been reported that, in some kind of cells, short activation of ERK1/2 led to cell proliferation, whereas sustained activation resulted in cell differentiation.42,43 In fact, the sustained activation of ERK1/2 was required for megakaryocyte differentiation induced by 12-O-tetradecanoylphorbol-13-acetate and phorbol esters, and for the terminal differentiation of megakaryocytes induced by fibronectin.44-46 However, the intrinsic reason for the different patterns of ERK1/2 activation induced by GH and TPO is unknown.
In addition, we also found that a marked activation of Akt was induced by rhGH in mature megakaryocytes but not in megakaryoblastic cells, suggesting that activation of the Akt pathway may contribute to the terminal differentiation of megakaryocytes induced by rhGH. Consistent with these data, Guerriero et al reported that PI3K-Akt signaling is crucial for the late stage of megakaryocyte differentiation because they observed that the level and activity of p-Akt gradually increased during the progression of megakaryocyte differentiation, while the level of p-ERK1/2 gradually decreased.47 Similarly, a study by Limb et al revealed that PI3K-Akt signaling plays a predominant role in promoting megakaryocyte differentiation and platelet production induced by a lysophosphatidylcholine derivative.48 In this study, we also observed that rhGH treatment promoted the activation of ρ GTPases, Rac1, and Cdc42, which are crucial for microtubule sliding and proplatelet elongation49 ; whereas both the Rac1/Cdc42 activation and proplatelet formation were significantly suppressed by the Akt inhibitor (Figure 4G-H), which is consistent with previous reports that Rac1 and Cdc42 could be stimulated by PI3K/Akt signaling,50,51 indicating that Akt-stimulated ρ GTPases signaling might contribute to GH-induced megakaryocyte terminal differentiation.
The failure to develop TPO-derived drugs has led to the search for new reagents to treat thrombocytopenia. To exert the advantages of both dTMP and hGH, we recently generated a chimeric protein by fusing hGH with dTMP. Here, we found that the dTMP-GH fusion protein displayed a comprehensive effect on the full spectrum of thrombopoiesis. Compared with dTMP, dTMP-GH treatment led to an earlier formation of proplatelets and platelet production. Besides, the proliferation-promoting activity of dTMP-GH was also dramatically enhanced in comparison with equimolecular dTMP (Figure 6B). Because it has been reported that GHR signal activation has the ability to transactivate other growth factor receptor signaling,52,53 we speculate that there may exist a synergy between the activations of signal pathways mediated by GHR and c-Mpl, respectively. In addition, the enhanced activity of dTMP-GH is not completely reversed by pretreatment with the GH antagonist, pegvisomant (data not shown). Thus, there is also a possibility that fusion with GH might alter the topology of dTMP, thereby increasing its bioactivity.
We also found that subcutaneous injection of 120 μg/kg of dTMP-GH led to a much quicker platelet recovery in mice with severe thrombocytopenia induced by TBI, in comparison with a fivefold molar excess of dTMP (100 μg/kg). The superiority of dTMP-GH is probably ascribed to the property of GH in promoting megakaryocyte terminal differentiation, which results in earlier and more platelet production. In fact, the in vitro study has demonstrated that dTMP-GH is efficient in promoting the whole progression of thrombopoiesis (Figure 6), wherein dTMP mainly acts on megakaryocytopoiesis, and GH is possibly responsible for megakaryocyte terminal differentiation; while treatment with equimolar rhGH (100 μg/kg) alone had no obvious effects on platelet level recovery (data not shown). Previous studies have demonstrated that a high dose of rhGH (500 μg/kg or 1 mg/kg) has the ability to promote hematopoietic reconstruction, including platelet recovery, in mice after severe irradiation.4,7 Therefore, we presume that fusion with dTMP also increases the bioavailability of GH because c-Mpl is primarily expressed in megakaryocytes, and GH can be specifically delivered to and act on megakaryocytes when it is fused with dTMP. As a consequence, the lower usage of GH, in the form of the dTMP-GH protein, might result in fewer and/or weaker side-effects to the body, especially in cancer patients.
Collectively, the findings of this study sheds new light on the functional role of GH in promoting thrombopoiesis and provides a clue for the treatment of thrombocytopenia, although the specific properties of the dTMP-GH fusion protein need to be further defined.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Natural Science Fund of China (31071025), the National “863” High-tech Development Plan (2007AA02Z152), the People’s Liberation Army (BSW11J009), and the State Key Laboratory of Trauma, Burns and Combined Injury (SKLZZ201115, SKLZZ201016, SKLKF200911) (J.W.).
Authorship
Contribution: Y.X. and S.W. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; M.S., Z.Z., F.C., and A.W. participated in the in vitro experiments; S.C. and M.C. participated in the animal experiments; D.Z., J.Z., and T.C. contributed to designing the study and discussed the results; Y.S. designed and supervised the research; J.W. designed and supervised the research, analyzed data, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junping Wang, Institute of Combined Injury, State Key Laboratory of Trauma, Burns and Combined Injury, College of Preventive Medicine, Third Military Medical University, Gaotanyan St 30 Chongqing 400038, China; e-mail: wangjunping@tmmu.edu.cn or wangjunp@yahoo.com; and Yongping Su, Institute of Combined Injury, State Key Laboratory of Trauma, Burns and Combined Injury, College of Preventive Medicine, Third Military Medical University, Gaotanyan St 30 Chongqing 400038, China; e-mail: pingsw2005@126.com.
References
Author notes
Y.X. and S.W. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal