Key Points
Within MDS/MPN, the WHO 2008 criteria for aCML identify a subgroup of patients with aggressive clinical features distinct from MDS/MPN-U.
The MDS/MPN-U category is heterogeneous, and patient risk can be further stratified by a number of clinicopathological parameters.
Abstract
Atypical chronic myeloid leukemia (aCML) is a rare subtype of myelodysplastic/myeloproliferative neoplasm (MDS/MPN) largely defined morphologically. It is, unclear, however, whether aCML-associated features are distinctive enough to allow its separation from unclassifiable MDS/MPN (MDS/MPN-U). To study these 2 rare entities, 134 patient archives were collected from 7 large medical centers, of which 65 (49%) cases were further classified as aCML and the remaining 69 (51%) as MDS/MPN-U. Distinctively, aCML was associated with many adverse features and an inferior overall survival (12.4 vs 21.8 months, P = .004) and AML-free survival (11.2 vs 18.9 months, P = .003). The aCML defining features of leukocytosis and circulating myeloid precursors, but not dysgranulopoiesis, were independent negative predictors. Other factors, such as lactate dehydrogenase, circulating myeloblasts, platelets, and cytogenetics could further stratify MDS/MPN-U but not aCML patient risks. aCML appeared to have more mutated RAS (7/20 [35%] vs 4/29 [14%]) and less JAK2p.V617F (3/42 [7%] vs 10/52 [19%]), but was not statistically significant. Somatic CSF3R T618I (0/54) and CALR (0/30) mutations were not detected either in aCML or MDS/MPN-U. In conclusion, within MDS/MPN, the World Health Organization 2008 criteria for aCML identify a subgroup of patients with features clearly distinct from MDS/MPN-U. The MDS/MPN-U category is heterogeneous, and patient risk can be further stratified by a number of clinicopathological parameters.
Introduction
The World Health Organization (WHO) classification1,2 recognizes a group of myeloid neoplasms with overlapping features of both myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN) and places them under a separate category of myelodysplastic/myeloproliferative neoplasms (MDS/MPN). Within this category, the diagnostic criteria for chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia, and refractory anemia with ring sideroblasts with marked thrombocytosis (RARS-T), are well-defined and easy to follow. In contrast, the criteria for the remaining 2 MDS/MPN entities—atypical chronic myeloid leukemia, BCR-ABL1 negative (aCML), and MDS/MPN, unclassifiable (MDS/MPN-U) other than RARS-T—are less clearly defined. It remains to be determined if the existing WHO 2008 classification criteria for aCML reliably define a distinct disease or if aCML is similar to MDS/MPN-U in terms of its clinical and molecular-genetic features.
aCML was initially described as a subtype of myeloid neoplasm closely resembling chronic myelogenous leukemia, but lacking the pathognomonic Philadelphia chromosome.3 Detailed guidelines for the diagnosis of aCML were initially published by the French-American-British group in 19944 and later incorporated into the third edition of the WHO classification in 2001. Since then, only a few aCML patient cohorts5-7 have been reported, with the largest series consisting of 55 patients.5 These studies indicated a median overall survival (OS) of 14 to 30 months, and an acute myeloid leukemia (AML) progression rate of approximately 40%.
In the 2008 WHO classification, aCML is defined by “persistent leukocytosis (≥13 × 109/L) with immature circulating myeloid precursors (≥10% of leukocytes) and marked dysgranulopoiesis, with absent/minimal monocytosis (<1 × 109/L and <10% of leukocytes) or basophilia (often <2%).”8 The presence of BCR-ABL1 or rearrangements of PDGFRA, PDGFRB, or FGFR1 precludes a diagnosis of aCML and any MDS/MPN subtype. Myeloid neoplasms with mixed proliferative and dysplastic features that do not meet the criteria for CMML, juvenile myelomonocytic leukemia, or aCML are classified as MDS/MPN-U. RARS-T, a provisional entity within the MDS/MPN-U subgroup, is clearly defined by the presence of marked thrombocytosis, ring sideroblasts, and a high frequency of JAK2 V617F and SF3B1 mutations.6,9 Compared with RARS-T, the remaining cases in the MDS/MPN-U category appear to have an inferior prognosis.7,10,11
Recently, oncogenic mutations in the granulocyte-colony stimulating factor receptor (CSF3R) have been reported to be frequent in chronic neutrophilic leukemia (CNL),12,13 a rare MPN subtype sharing overlapping features with aCML. Further study showed that the JAK1/2 inhibitor ruxolitinib was capable of lowering the white blood count (WBC) and spleen size in a murine model of CSF3R mutation–associated CNL,14 which raises the potential applicability of Janus kinase (JAK) or SRC kinase inhibitors in CNL patients with CSF3R mutations. In contrast, the CSF3R mutation status is not as clear in aCML. Maxson et al reported frequent CSF3R mutations in aCML, similar to CNL,12 whereas Pardanani et al showed that CSF3R mutations were essentially absent in aCML.13 The current lack of a precise phenotypic-genotypic association prompted us to undertake this study based on a large database of patients in the hope of achieving a more adequate disease definition. In addition, somatic mutations in calreticulin (CALR) have recently been identified in BCR-ABL1–negative and JAK2/MPL-unmutated MPNs.15,16 CALR mutations are very infrequent in MDS, RARS-T, CMML,15,16 or CNL.17 To test CALR mutations in our precisely defined aCML and MDS/MPN-U terms may provide useful information in understanding these hematological neoplasms sharing overlapping features with MPN.
We conducted our multicenter study focusing on 2 infrequent and relatively poorly defined MDS/MPNs: aCML and MDS/MPN-U. We sought to determine if the 2008 WHO criteria can reliably identify aCML and whether this myeloid neoplasm, as currently defined, is clinicopathologically distinct from MDS/MPN-U. We also examined the risk factors associated with the outcome of patients with aCML and MDS/MPN-U as well as the CSF3R and CALR mutation status in these 2 groups of patients.
Materials and methods
Cases
We searched the pathology archives at 7 institutions in the United States: M.D. Anderson Cancer Center (MDACC), Cleveland Clinic Foundation (CCF), Massachusetts General Hospital, Stanford University Medical Center, Weill Cornell Medical College, the University of Pennsylvania Hospital, and the University of New Mexico (UNM) for cases of MDS/MPN between 2004 and 2012. All included cases had to meet the minimal 2008 WHO classification requirements for MDS/MPN, defined as sustained (≥3 months) leukocytosis (WBC ≥13 × 109/L) or thrombocytosis (platelets ≥450 × 109/L) with at least 1 sustained cytopenia (hemoglobin <12 g/dL in women and <13 g/dL in men; platelets <140 × 109/L; and ANC <1.8 × 109/L)18 ; and significant (>10%) dysplasia in at least 1 hematopoietic lineage. Cases fulfilling the 2008 WHO classification criteria for CMML, juvenile myelomonocytic leukemia, and RARS-T were excluded. Cases with a preceding history of MDS or MPN were also excluded. All included cases had <20% blasts in peripheral blood (PB) and bone marrow (BM) at presentation. Clinical information was retrieved from the electronic medical records. This study was approved by the Institutional Review Boards of all participating institutions. This study was conducted in accordance with the Declaration of Helsinki.
Pathology review
PB smears, BM aspirate smears, and core biopsy specimens were reviewed by at least 1 observer at respective institution to confirm the diagnosis. PB myeloid precursors included promyelocytes, myelocytes, and metamyelocytes, but not band forms or myeloblasts. BM cellularity and the myeloid:erythroid ratio were estimated on the core biopsy. Myelofibrosis was assessed by reticulin and trichrome stains and graded according to the European BM Fibrosis Network criteria.19 The numbers of megakaryocytes were recorded as increased, decreased, or normal, and the megakaryocyte morphology was recorded as predominantly MDS-like (small with hypolobated or abnormally lobated nuclei), predominantly MPN-like (large and hypersegmented nuclei with clustering), mixed MDS and MPN-like, or normal. A differential count based on 500 cells or all available cells was performed, and ring sideroblasts were assessed on Prussian blue/Perls-stained BM aspirate smears. For dyspoiesis, dysplastic changes had to be present in at least 10% of the cells in that lineage. Data from all cases were reevaluated by S.A.W. and a subset by A.O. Cases with uncertain diagnoses/classification was rereviewed at a meeting attended by participants from all 7 institutions and a consensus diagnosis was reached.
Cytogenetics
Conventional cytogenetic analysis was performed on G-banded metaphase cells prepared from unstimulated BM aspirate cultures using standard techniques. Twenty metaphases were analyzed and the results reported using the International System for Human Cytogenetic Nomenclature. Fluorescence in situ hybridization for BCR/ABL1, PDGFRA, PDGFRB, or FGFR1 was performed as necessary at some institutes.
Mutation studies
Polymerase chain reaction and Sanger sequencing for CSF3R were performed at 3 laboratories (MDACC, n = 45; CCF, n = 6; and UNM, n = 3); and CALR mutations were performed at MDACC (n = 30) on genomic DNA isolated from PB/BM mononuclear cells or from DNA extracted from fresh frozen tissue (MDACC) and/or paraffin sections (BM clot without decalcification, subset cases at CCF and UNM). The detailed methods used at 3 institutions are provided in the supplemental material on the Blood Web site. Tests on other mutations, including JAK2 p.V617F, KRAS, NRAS, CEBPA, KIT, FLT3, and MPL, were performed as part of the routine clinical workup in various subsets of the cases, and no additional test was performed specifically for this study.
Statistical analyses
For continuous variables, data are reported as median and range. For nominal variables, data are reported as the number of patients if not specified. OS was calculated from the day of diagnosis to the last follow-up. AML-free survival (AMLFS) was calculated from the time of diagnosis either to death or AML transformation. Patients who received hematopoietic stem cell transplant were censored at the time of the procedure. Distributions of OS and AMLFS were estimated by Kaplan-Meier curves. Univariate and multivariate Cox proportional hazards regression were performed to assess the impact of clinicopathologically relevant variables. Fisher’s exact and χ-square tests were used for categorical comparisons. All P values are 2-tailed and considered significant when <.05. No adjustments for multiplicity were made. Statistical analysis was performed using SAS 9.3 for Windows.
Results
Patients
From an initial cohort of 174 cases, 40 were excluded, either because of a prior history of MDS or MPN (n = 16); missing pathology slides or complete blood count data at diagnosis (n = 6); reclassified as other entities (MPN-unclassifiable; n = 2), CMML (n = 1), RARS-T (n = 2), CNL (n = 1), chronic eosinophilic leukemia (n = 2), primary myelofibrosis (n = 2), MDS with fibrosis (n = 4), and MDS with del(5)(q31-33) with JAK2 p.V617F mutation (n = 1), or transient rather than sustained leukocytosis or thrombocytosis (n = 3).
A final cohort of 134 cases that fulfilled the criteria for MDS/MPN formed the study group (MDACC = 62; CCF = 18, Massachusetts General Hospital = 17, Stanford = 13, University of Pennsylvania = 9, Weill Cornell Medical College = 9, and UNM = 6). Of these cases, absence of t(9;22) or BCR-ABL1 was confirmed. Fluorescence in situ hybridization for PDGFA, FIP1L1, CHIC2, or FGFR1 was performed and was negative in 10 cases with diagnostic difficulty. Ten (8%) patients had a prior history of cytotoxic exposure, including both chemotherapy and radiation (n = 4), chemotherapy only (n = 1), and radiation only (n = 5), for breast cancer (n = 4), lung cancer (n = 1), colon cancer (n = 1), or prostate cancer (n = 4), respectively.
All patients were adults, 72 years (range 42-88), and male predominant (2:1) (Table 1). Complete blood count showed hybrid features with significant and sustained cytosis and cytopenia(s) in all patients. Leukocytosis was present in 90% of patients and thrombocytosis in 19%. Cytopenias were mainly manifested as anemia (121/134, 90%), followed by thrombocytopenia (79/134, 60%), and rarely neutropenia (4/134, 3%). Increased lactate dehydrogenase (LDH) (beyond the normal upper limit of each institution) was seen in 74% of patients, organomegaly (spleen and/or liver) in 34%, and significant BM fibrosis (≥myelofibrosis 2 [MF2]) in 29%.
Comparison of aCML with MDS/MPN-U
| . | aCML (n = 65) . | MDS/MPN-U (n = 69) . | P value . |
|---|---|---|---|
| Age (y) (median, range) | 72 (42-86) | 71 (55-88) | .6071 |
| ≥70 | 43 (66.2%) | 42 (59.2%) | .5256 |
| Sex (male:female) | 45/20 | 44/25 | .5034 |
| Increased LDH | 48/57 (84.2%) | 41/63 (65.1%) | .0168 |
| Organomegaly | 29/65 (44.6%) | 15/66 (22.7%) | .0080 |
| WBC (×109/dL) (median, range) | 40.8 (13.8-227.1) | 19.4 (1.5-98.7) | <.0001 |
| ≥40 | 34/65 (52.3%) | 12/69 (17.4%) | .0000 |
| Hemoglobin (g/dL) (median, range) | 9.4 (5.7-13.6) | 10.1 (5.2-13.6) | .0042 |
| <10 | 43/65 (66.5%) | 27/69 (39.1%) | .0017 |
| Platelets (×109/dL) (median, range) | 87 (7-974) | 190 (9-1040) | .0020 |
| ≥450 | 3 (4.6%) | 22 (31.9%) | .0002 |
| 100-450 | 26 (40.0) | 23 (33.3%) | |
| <100 | 36 (55.4%) | 24 (34.8%) | |
| Blood myeloid precursors (%) | 17 (10-65) | 4 (0-45) | <.0001 |
| ≥10% | 65/65 (100%) | 13/68 (19.1%) | <.0001 |
| BM blasts (median, range) (%) | 3 (0-17) | 2 (0-17) | .2550 |
| <5% | 43 (66.2%) | 49 (72.1%) | .5335 |
| 5-9% | 11 (16.9%) | 12 (17.6%) | |
| ≥10% | 11 (16.9%) | 7 (10.3%) | |
| Blood blasts (median, range) (%) | 2 (0-17) | 0 (0-13) | .0009 |
| ≥5% | 17/65 (25.2%) | 8/68 (11.8%) | .0337 |
| Basophils (%) (median, range) | 0 (0-5) | 0 (0-10) | .0159 |
| Eosinophils (%) (median, range) | 0.4 (0-11) | 1.0 (0-11) | .5837 |
| Monocytes (%) (median, range) | 2.3 (0-9) | 2.0 (0-9) | .0879 |
| Cytogenetics | NS | ||
| Normal or −Y | 35/63 (56%) | 42/65 (65%) | .3670 |
| Single or double abnormalities without −7/7q | 20/63 (32%) | 19/65 (29%) | NS |
| +8 | 11 (17.5%) | 12 (18.5%) | NS |
| i17(q) | 5 (7.9%) | 1 (1.5%) | .1120 |
| −7/−7q | 5 (7.9%) | 4 (6.2%) | NS |
| Complex | 5 (7.9%) | 2 (3.1%) | .2963 |
| Mutations | |||
| JAK2 V617F | 3/42 (7.3%) | 10/52 (18.9%) | .1336 |
| RAS (KRAS/NRAS) | 7/20 (35.0%) | 4/29 (13.7%) | .0965 |
| CSF3R T618I | 0/27 | 0/27 | NS |
| CALR | 0/17 | 0/13 | NS |
| AML progression | 24/65 (36.9%) | 16/69 (23.2%) | .0922 |
| . | aCML (n = 65) . | MDS/MPN-U (n = 69) . | P value . |
|---|---|---|---|
| Age (y) (median, range) | 72 (42-86) | 71 (55-88) | .6071 |
| ≥70 | 43 (66.2%) | 42 (59.2%) | .5256 |
| Sex (male:female) | 45/20 | 44/25 | .5034 |
| Increased LDH | 48/57 (84.2%) | 41/63 (65.1%) | .0168 |
| Organomegaly | 29/65 (44.6%) | 15/66 (22.7%) | .0080 |
| WBC (×109/dL) (median, range) | 40.8 (13.8-227.1) | 19.4 (1.5-98.7) | <.0001 |
| ≥40 | 34/65 (52.3%) | 12/69 (17.4%) | .0000 |
| Hemoglobin (g/dL) (median, range) | 9.4 (5.7-13.6) | 10.1 (5.2-13.6) | .0042 |
| <10 | 43/65 (66.5%) | 27/69 (39.1%) | .0017 |
| Platelets (×109/dL) (median, range) | 87 (7-974) | 190 (9-1040) | .0020 |
| ≥450 | 3 (4.6%) | 22 (31.9%) | .0002 |
| 100-450 | 26 (40.0) | 23 (33.3%) | |
| <100 | 36 (55.4%) | 24 (34.8%) | |
| Blood myeloid precursors (%) | 17 (10-65) | 4 (0-45) | <.0001 |
| ≥10% | 65/65 (100%) | 13/68 (19.1%) | <.0001 |
| BM blasts (median, range) (%) | 3 (0-17) | 2 (0-17) | .2550 |
| <5% | 43 (66.2%) | 49 (72.1%) | .5335 |
| 5-9% | 11 (16.9%) | 12 (17.6%) | |
| ≥10% | 11 (16.9%) | 7 (10.3%) | |
| Blood blasts (median, range) (%) | 2 (0-17) | 0 (0-13) | .0009 |
| ≥5% | 17/65 (25.2%) | 8/68 (11.8%) | .0337 |
| Basophils (%) (median, range) | 0 (0-5) | 0 (0-10) | .0159 |
| Eosinophils (%) (median, range) | 0.4 (0-11) | 1.0 (0-11) | .5837 |
| Monocytes (%) (median, range) | 2.3 (0-9) | 2.0 (0-9) | .0879 |
| Cytogenetics | NS | ||
| Normal or −Y | 35/63 (56%) | 42/65 (65%) | .3670 |
| Single or double abnormalities without −7/7q | 20/63 (32%) | 19/65 (29%) | NS |
| +8 | 11 (17.5%) | 12 (18.5%) | NS |
| i17(q) | 5 (7.9%) | 1 (1.5%) | .1120 |
| −7/−7q | 5 (7.9%) | 4 (6.2%) | NS |
| Complex | 5 (7.9%) | 2 (3.1%) | .2963 |
| Mutations | |||
| JAK2 V617F | 3/42 (7.3%) | 10/52 (18.9%) | .1336 |
| RAS (KRAS/NRAS) | 7/20 (35.0%) | 4/29 (13.7%) | .0965 |
| CSF3R T618I | 0/27 | 0/27 | NS |
| CALR | 0/17 | 0/13 | NS |
| AML progression | 24/65 (36.9%) | 16/69 (23.2%) | .0922 |
NS, not significant.
Karyotype information was available in 128 patients (95.5%). An abnormal karyotype other than –Y (5 patients) was detected in 51 (40.5%) cases (Table 1). Of the patients with a prior history of cytotoxic exposure, 1 had a complex karyotype and no case showed −7/−7q; overall the karyotypic features were not different from the rest of patients.
After a diagnosis of MDS/MPN, patients received various treatment modalities (not for AML transformation) including hydroxyurea (n = 66), hypomethylating agents (n = 64), histone deacetylase inhibitors(n = 10); low-intensity chemotherapy (n = 19); induction chemotherapy (n = 13); tyrosine kinase inhibitors (n = 17) or JAK2, RAS, FLT3, MAPK, MYC, or AKT inhibitors (n = 10); immunomodulatory agents (thalidomide, lenalidomide, or interferon, n = 13); and supportive care only (n = 18). Six (6) patients received hematopoietic stem cell transplant.
The median follow-up period was 13.8 months (range 0.1-110 months, including living and deceased patients and patients censored upon receiving hematopoietic stem cell transplant), the median OS for the whole group was 17.7 months (95% confidence interval [CI]: 13.8-21.0), and the median AMLFS was 13.5 months (95% CI: 11.9-17.8).
Subclassification of aCML and MDS/MPN-U based on hematologic and BM features
Of all the cases, a WBC ≥13 × 109/L was seen in majority of patients (121/134, 90%); ≥10% PB myeloid precursors were seen in more than half of patients (78/133, 58%). Dysgranulopoiesis (≥10% granulocytes) was present in 101/134 (75%) of patients, 3 of which were only observed on PB smears. In the WHO classification, dysgranulopoiesis in aCML is indicated as “marked” but without detailed quantification. In this study, we used a 10% cutoff. Overall, 65 cases fulfilled all 3 criteria and were assigned a diagnosis of aCML. The remaining 69 cases were assigned to MDS/MPN-U. Of these cases, 36 cases from MDACC were included in a previously study.11 Notably, substantial overlapping features between MDS/NMPN-U and aCML were observed (detail is illustrated in Figure 1). The other criteria “no basophilia (often ≤2% basophils)” was not specifically used for exclusion. The cases that fulfilled the first 3 criteria for aCML had a median basophil percentage of 0% (0-5%), with only 2 patients having >2% basophils (3% and 5%, respectively). In a subset of aCML cases, dysgranulopoiesis was in the form of abnormal nuclear segmentation with multiple lobes (Figure 2).
Subclassification of aCML and MDS/MPN by the presence of leukocytosis (≥13 × 109/L), PB myeloid precursors (≥10%), and dysgranulopoiesis (≥10%). Of all 134 patients, 65 (49%) patients fulfilled all 3 criteria for aCML, and the remaining 69 patients were placed under MDS/MPN-U. Of the latter group, 1 patient (arrow) had leukocytosis, no dysgranulopoiesis, and no information on blood myeloid precursors; 5 patients (arrowhead) had neither leukocytosis nor dysgranulopoiesis but did have thrombocytosis.
Subclassification of aCML and MDS/MPN by the presence of leukocytosis (≥13 × 109/L), PB myeloid precursors (≥10%), and dysgranulopoiesis (≥10%). Of all 134 patients, 65 (49%) patients fulfilled all 3 criteria for aCML, and the remaining 69 patients were placed under MDS/MPN-U. Of the latter group, 1 patient (arrow) had leukocytosis, no dysgranulopoiesis, and no information on blood myeloid precursors; 5 patients (arrowhead) had neither leukocytosis nor dysgranulopoiesis but did have thrombocytosis.
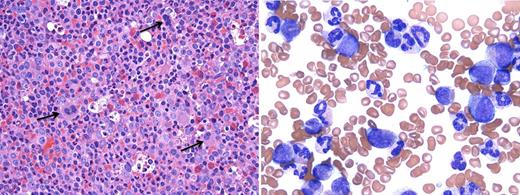
A case of aCML. (Left) BM biopsy (hematoxylin and eosin, ×500) reveals a hypercellularity (100%) with markedly increased myeloid:erythroid ratio (26:1). Dysplastic megakaryocytes are indicated by arrows; (right) PB smear (Wright Giemsa, ×1000) shows marked leukocytosis with many myeloid precursors (promyelocytes, myelocytes, and metamyelocytes, 23%). Neutrophils show peculiar abnormal nuclear segmentation. (Microscope: Olympus, Tokyo, Japan; camera and software: Q-Capture, Surrey, Canada.)
A case of aCML. (Left) BM biopsy (hematoxylin and eosin, ×500) reveals a hypercellularity (100%) with markedly increased myeloid:erythroid ratio (26:1). Dysplastic megakaryocytes are indicated by arrows; (right) PB smear (Wright Giemsa, ×1000) shows marked leukocytosis with many myeloid precursors (promyelocytes, myelocytes, and metamyelocytes, 23%). Neutrophils show peculiar abnormal nuclear segmentation. (Microscope: Olympus, Tokyo, Japan; camera and software: Q-Capture, Surrey, Canada.)
Clinical and histological comparison between aCML and MDS/MPN-U
In comparison with MDS/MPN-U (Table 1), aCML patients had more frequently increased LDH, organomegaly, worse anemia, thrombocytopenia, higher circulating blasts, and a lower basophil percentage. The median WBC was 40.8 × 109/L for aCML patients and 19.4 × 109/L for MDS/MPN-U patients (P < .0001). In terms of BM features, aCML had a higher cellularity (95% [10-100] vs 92.5% [10-100], P = .0097) and a higher myeloid:erythroid ratio than MDS/MPN-U (9.4 [0.8-100] vs 6 [0.5-63], P = .0110). By definition, dysgranulopoiesis was seen in all aCML (100%), whereas it was observed in 36/69 (52.2%) of MDS/MPN-U patients (P < .0001). Dyserythropoiesis was observed in a similar frequency in aCML (24/60, 40.0%) and MDS/MPN-U (33/67, 49.3%) (P = .3719). Megakaryocyte numbers as “increased,” “decreased,” and “unremarkable” were seen in 31 (48.5%), 18 (28.1%), 15 (23.4%) aCML, and 42 (60.8%), 14 (20.3%), and 13 (18.9%) in MDS/MPN-U, not different (P = .3473). Megakaryocyte morphology, as categorized as MDS-like, MPN-like, mixed MDS, and MPN-like and unremarkable, showed no differences between aCML (33 [54.1%], 16 [26.2], 5 [8.2%], and 7 [11.5%], respectively), and MDS/MPN-U (39 [56.5%], 18 [26.1%], 6 [8.7%], and 6 [8.7%], respectively) (P = .9611). Significant myelofibrosis (MF 2 or 3) was observed in 20/65 (30.8%) aCML and 18/68 (26.5%) MDS/MPN-U, but the difference was not significant (P = .5833).
The treatment modalities given to aCML and MDS/MPN-U were comparable (data not shown). Patients with aCML had a median OS of 12.4 and an AMLFS of 11.2 months, which was significantly inferior to patients with MDS/MPN-U who had a median OS of 21.8 and AMLFS of 18.9 months (P = .004 and .003, respectively) (Figure 3).
Survival rates. Compared with MDS/MPN-U, patients with aCML showed a significant inferior OS (12.4 months, 95% CI [9.0-16.1] vs 21.8 months, 95% CI [17.6-28.8]) and ACL-free survival (11.2 months, 95% CI [7.0-13.5] vs 18.9 months, 95% CI [12.3-26.3]).
Survival rates. Compared with MDS/MPN-U, patients with aCML showed a significant inferior OS (12.4 months, 95% CI [9.0-16.1] vs 21.8 months, 95% CI [17.6-28.8]) and ACL-free survival (11.2 months, 95% CI [7.0-13.5] vs 18.9 months, 95% CI [12.3-26.3]).
Molecular genetic comparison between aCML and MDS/MPN-U
Karyotype findings showed no statistical significance between aCML and MDS/MPN-U, either assessed as normal vs abnormal or by individual alterations (Table 1). JAK2 V617F mutations were evaluated in 94 cases, and were positive in 3/42 (7%) aCML and 10/52 MDS/MPN-U (18%) (P = .134). RAS mutation was detected in 7/20 (35%) aCML and 4/29 (14%) MDS/MPN (P = .096). Other mutations, including FLT3 (2/28 vs 1/30), CEBPA (2/17 vs 0/13), MPL (0/10 and 0/7), KIT (0/17, 1/17), IDH1/IDH2 (0/8 and 0/11), and NPM1 (0/20 and 0/17) were either very infrequent or not present in both groups of diseases. The single case with mutated KIT p.D816V was a MDS/MPN-U with associated systemic mastocytosis.
CSF3R mutation studies were performed in 27 aCML and 27 MDS/MPN-U patients. One patient with aCML showed a p.G683R variant in exon 17, which is likely a germline polymorphism based on the reported minor allele frequency of 0.042 in the Single Nucleotide polymorphism database (Reference SNP ID: rs3918001); however, germline DNA was not available for confirmatory testing. The remaining cases were negative for any other mutations in CSF3R gene. CALR exon 9 mutations were not detected in 17 (0/17) aCML or MDS/MPN-U (0/13).
Factors related to patient prognosis
Because of substantial overlapping features between aCML and MDS/MPN-U, we first performed statistical analysis in all patients as a group. This analysis was also to examine if the defining parameters for aCML were prognostically relevant.
Table 2 shows that of the 3 aCML defining parameters, a higher WBC as a continuous variable was an adverse prognostic factor for both OS and AMLFS in univariate and multivariate analysis, and a count of 40 × 109/L was the most significant divider by the optimal cutpoint approach by Contal and O’Quigley.20 The percentage of PB myeloid precursors as a continuous variable was a strong discriminator for both OS and AMLFS in univariate as well as multivariate analysis. Dysgranulopoiesis showed a trend for an inferior OS (P = .0839) and AMLFS (P = .0544) in univariate analysis, but became insignificant in multivariate analysis. Other relevant factors independently predicted an inferior prognosis included higher BM blast counts (≥5%), elevated LDH, and lower platelet counts (<450 × 109/L).
Parameters in relationship with patient outcomes by univariate and multivariate Cox regression analysis of 134 patients as a cohort
| Univariate analysis . | OS . | AMLFS . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
| Age (y) | 1.02 (0.99-1.05) | .3163 | 1.00 (0.97-1.03) | .8394 |
| Sex (female) | 1.23 (0.81-1.85) | .3295 | 1.20 (0.80-1.79) | .3756 |
| Increased LDH | 2.53 (1.44-4.44) | .0012 | 2.58 (1.50-4.44) | .0006 |
| Organomegaly | 1.27 (0.84-1.93) | .2511 | 1.32 (0.87-2.00) | .1711 |
| WBC ×109/dL | 1.02 (1.01-1.02) | .0000 | 1.025 (1.010-1.020) | .0000 |
| Hemoglobin (g/dL) | 0.97 (0.88-1.07) | .5381 | 0.96 (0.87-1.06) | .4211 |
| Platelets ×109/dL | 0.99 (0.998-1.00) | .0021 | 0.99 (0.99-1.000) | .0112 |
| Blood myeloid precursors (%) | 1.05 (1.03-1.06) | .0000 | 1.040 (1.025-1.055) | .0000 |
| PB blasts | 1.04 (0.99-1.09) | .1015 | 1.049 (1.005-1.095) | .0284 |
| BM blasts | 1.08 (1.03-1.13) | .0008 | 1.11 (1.07-1.17) | .0000 |
| Myelofibrosis (MF 2 or 3) | 1.03 (0.67-1.59) | .8860 | 0.99 (0.65-1.52) | .9657 |
| Dysgranulopoiesis | 1.52 (0.94-2.45) | .0839 | 1.59 (0.99-2.55) | .0544 |
| Cytogenetics | ||||
| Category 1, reference | — | — | — | — |
| Category 2 | 1.12 (0.72-1.75) | .6155 | 1.11 (0.72-1.72) | .6265 |
| Category 3 | 2.31 (1.13-4.72) | .0216 | 3.90 (1.94-7.85) | .0001 |
| Category 3 vs 2 | 2.06 (0.97-4.40) | .0616 | 3.5 (1.67-7.33) | .0009 |
| aCML diagnosis | 1.80 (1.20-2.69) | .0042 | 1.79 (1.21-1.64) | .0035 |
| Multivariate analysis* | ||||
| Increased LDH | 1.55 (0.83-2.88) | .1668 | 1.71 (0.96-3.08) | .0707 |
| WBC ×109/dL | 1.01 (1.00-1.02) | .0283 | 1.01 (1.00-1.02) | .0098 |
| Platelets ×109/dL | 0.99 (0.99-1.00) | .0574 | 0.99 (0.99-1.00) | .0886 |
| Blood myeloid precursors (%) | 1.03 (1.01-1.05) | .0040 | 1.02 (1.00-1.04) | .0711 |
| BM blasts (%) | 1.06 (0.98-1.15) | .1461 | 1.11 (1.03-1.19) | .0069 |
| Peripheral blasts (%) | 1.01 (0.91-1.11) | .8894 | 1.02 (0.93-1.12) | .7316 |
| Cytogenetics (category 3)† | 1.14 (0.51-2.52) | .7489 | 2.69 (1.32-5.49) | .0065 |
| Dysgranulopoiesis | 0.88 (0.51-1.51) | .6455 | 0.95 (0.55-1.63) | .8500 |
| Univariate analysis . | OS . | AMLFS . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
| Age (y) | 1.02 (0.99-1.05) | .3163 | 1.00 (0.97-1.03) | .8394 |
| Sex (female) | 1.23 (0.81-1.85) | .3295 | 1.20 (0.80-1.79) | .3756 |
| Increased LDH | 2.53 (1.44-4.44) | .0012 | 2.58 (1.50-4.44) | .0006 |
| Organomegaly | 1.27 (0.84-1.93) | .2511 | 1.32 (0.87-2.00) | .1711 |
| WBC ×109/dL | 1.02 (1.01-1.02) | .0000 | 1.025 (1.010-1.020) | .0000 |
| Hemoglobin (g/dL) | 0.97 (0.88-1.07) | .5381 | 0.96 (0.87-1.06) | .4211 |
| Platelets ×109/dL | 0.99 (0.998-1.00) | .0021 | 0.99 (0.99-1.000) | .0112 |
| Blood myeloid precursors (%) | 1.05 (1.03-1.06) | .0000 | 1.040 (1.025-1.055) | .0000 |
| PB blasts | 1.04 (0.99-1.09) | .1015 | 1.049 (1.005-1.095) | .0284 |
| BM blasts | 1.08 (1.03-1.13) | .0008 | 1.11 (1.07-1.17) | .0000 |
| Myelofibrosis (MF 2 or 3) | 1.03 (0.67-1.59) | .8860 | 0.99 (0.65-1.52) | .9657 |
| Dysgranulopoiesis | 1.52 (0.94-2.45) | .0839 | 1.59 (0.99-2.55) | .0544 |
| Cytogenetics | ||||
| Category 1, reference | — | — | — | — |
| Category 2 | 1.12 (0.72-1.75) | .6155 | 1.11 (0.72-1.72) | .6265 |
| Category 3 | 2.31 (1.13-4.72) | .0216 | 3.90 (1.94-7.85) | .0001 |
| Category 3 vs 2 | 2.06 (0.97-4.40) | .0616 | 3.5 (1.67-7.33) | .0009 |
| aCML diagnosis | 1.80 (1.20-2.69) | .0042 | 1.79 (1.21-1.64) | .0035 |
| Multivariate analysis* | ||||
| Increased LDH | 1.55 (0.83-2.88) | .1668 | 1.71 (0.96-3.08) | .0707 |
| WBC ×109/dL | 1.01 (1.00-1.02) | .0283 | 1.01 (1.00-1.02) | .0098 |
| Platelets ×109/dL | 0.99 (0.99-1.00) | .0574 | 0.99 (0.99-1.00) | .0886 |
| Blood myeloid precursors (%) | 1.03 (1.01-1.05) | .0040 | 1.02 (1.00-1.04) | .0711 |
| BM blasts (%) | 1.06 (0.98-1.15) | .1461 | 1.11 (1.03-1.19) | .0069 |
| Peripheral blasts (%) | 1.01 (0.91-1.11) | .8894 | 1.02 (0.93-1.12) | .7316 |
| Cytogenetics (category 3)† | 1.14 (0.51-2.52) | .7489 | 2.69 (1.32-5.49) | .0065 |
| Dysgranulopoiesis | 0.88 (0.51-1.51) | .6455 | 0.95 (0.55-1.63) | .8500 |
HR, hazard ratio.
WBC, hemoglobin, platelets, blood myeloid precursors, blood, and BM blasts are all analyzed as continuous variables.
In multivariate analysis, cytogenetic category 3 (complex and −7/−7q) is compared with category 1 (normal or –Y) combined with category 2 (all other abnormalities).
Cytogenetic categorization was first referred to the recently published cytogenetic categorization for CMML.21 However, unlike what was reported in CMML patients,21 +8, as an isolated abnormality, was not associated with a poor prognosis in our patients. The risk profile was more similar to that used in the International Prognostic Scoring System model for MDS.22 The karyotypes were grouped into category 1, a normal karyotype and –Y; category 3, a complex karyotype (3 or more abnormalities) or −7/−7q, and category 2: all other abnormalities according to the International Prognostic Scoring System. Patients with category 3 cytogenetics had a significantly inferior OS and AMLFS both in univariate and multivariate analyses.
Factors related to patient prognosis within the aCML and MDS/MPN-U groups
Within the aCML group, in the univariate Cox regression analysis, a higher WBC, either as a continuous variable or a cutoff of 50 × 109/L by the optimal cutpoint approach or a higher number of PB myeloid precursors, were significant risks for an inferior OS and AMLFS. A higher number of BM blasts was a significant hazard for AMLFS but not for OS. Other factors, including LDH, platelet count, cytogenetic categories, and PB blasts became not significant for survivals (supplemental Table 1). Because of too few factors being significant in the univariate analysis, multivariate Cox regression analysis was not performed for aCML patients. In contrast, the group of MDS/MPN-U patients showed significant clinical and pathological heterogeneity, and the same factors that showed prognostic significance for the entire group of 134 patients remained significant in predicting the prognosis for this group of patients in univariate (supplemental Table 1) and multivariate analysis (Table 3).
Multivariate Cox regression analysis of patients with MDS/MPN-U
| Variables . | OS . | AMLFS . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
| LDH (increased) | 2.00 (0.90-4.44) | .0873 | 1.81 (0.86-3.79) | .1163 |
| WBC ≥40 × 109/dL | 1.91 (0.64-5.69) | .2446 | 2.62 (0.96-7.13) | .0583 |
| Platelets ≥ 450 × 109/dL | – | – | – | – |
| 100-450 | 1.05 (0.43-2.56) | .9127 | 0.71 (0.30-1.66) | .4328 |
| <100 | 1.82 (0.67-4.91) | .2330 | 1.10 (0.42-2.86) | .8372 |
| <100 vs 100-450 | 1.73 (0.71-4.22) | .2234 | 1.55 (0.66-3.64) | .3128 |
| Blood myeloid precursors ≥10% | 1.57 (0.44-5.56) | .4815 | 3.19 (0.91-11.10) | .0677 |
| BM blasts ≥5% | 2.02 (0.81-5.01) | .1264 | 2.60 (1.13-5.98) | .0242 |
| PB blasts ≥5% | 0.35 (0.10-1.25) | .1076 | 0.17 (0.04-0.68) | .0125 |
| Cytogenetics 3* | 7.24 (1.60-32.68) | .0100 | 76.62 (10.21-574.66) | <.0001 |
| Dysgranulopoiesis | 1.18 (0.49-2.83) | .7048 | 1.48 (0.62-3.53) | .3704 |
| Variables . | OS . | AMLFS . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
| LDH (increased) | 2.00 (0.90-4.44) | .0873 | 1.81 (0.86-3.79) | .1163 |
| WBC ≥40 × 109/dL | 1.91 (0.64-5.69) | .2446 | 2.62 (0.96-7.13) | .0583 |
| Platelets ≥ 450 × 109/dL | – | – | – | – |
| 100-450 | 1.05 (0.43-2.56) | .9127 | 0.71 (0.30-1.66) | .4328 |
| <100 | 1.82 (0.67-4.91) | .2330 | 1.10 (0.42-2.86) | .8372 |
| <100 vs 100-450 | 1.73 (0.71-4.22) | .2234 | 1.55 (0.66-3.64) | .3128 |
| Blood myeloid precursors ≥10% | 1.57 (0.44-5.56) | .4815 | 3.19 (0.91-11.10) | .0677 |
| BM blasts ≥5% | 2.02 (0.81-5.01) | .1264 | 2.60 (1.13-5.98) | .0242 |
| PB blasts ≥5% | 0.35 (0.10-1.25) | .1076 | 0.17 (0.04-0.68) | .0125 |
| Cytogenetics 3* | 7.24 (1.60-32.68) | .0100 | 76.62 (10.21-574.66) | <.0001 |
| Dysgranulopoiesis | 1.18 (0.49-2.83) | .7048 | 1.48 (0.62-3.53) | .3704 |
Cytogenetic category 3 (complex and −7/−7q) is compared with category 1 (normal or –Y) combined with category 2 (all other abnormalities).
Discussion
We studied a large series of MDS/MPN patients to gain a better understanding of aCML and MDS/MPN-U, the 2 less well-characterized entities within this disease category. Our results show that the 2008 WHO classification diagnostic criteria for aCML do indeed identify a group of patients with clinical features distinct from those with a diagnosis of MDS/MPN-U.
This conclusion is based on the identification of a cohort of cases that strictly fulfilled the WHO classification definitions of aCML and MDS/MPN-U. To increase diagnostic specificity, from an initial cohort of 174 cases, 40 cases were excluded. A significant subset (n = 22) of those cases was excluded either because of a history of MDS and MPN before the diagnosis of MDS/MPN or because the initial diagnostic material was unavailable for review. This is relevant because the development of hybrid MDS/MPN features can be considered a sign of disease progression in MDS or MPN patients,23 and the natural history of these secondary diseases may not be the same as de novo MDS/MPN. In reviewing the cases, we also become aware that some disease entities may share overlapping features with MDS/MPN. In MDS with fibrosis, MPN-like megakaryocytes are commonly seen in association with myelofibrosis and increased CD34+ myeloblasts.24,25 However, MDS with fibrosis patients often present with severe cytopenia(s) with no leukocytosis or thrombocytosis “proliferative” hematological features. The morphologic features seen in chronic eosinophilic leukemia, CNL, primary myelofibrosis, and MPN-U may share some features with MDS/MPN, but significant dysplasia or significant cytopenia(s) are absent.26 An iron stain is required in all cases to correctly diagnose RARS-T. MDS with isolated del(5q) with concurrent JAK2 P.V617F mutations often show overlapping features of MDS and MPN27,28 ; however, the disease phenotype, efficacy of lenalidomide, and patient prognosis are not different from other 5q deletion syndrome.
We compared the main diagnostic parameters of these 2 MDS/MPN subtypes. Leukocytosis, as defined by ≥13 × 109/L, was common in both groups of patients and did not differentiate aCML from MDS/MPN-U; however, the median WBC was significantly higher in aCML patients. The percentage of PB myeloid precursors may fluctuate and would be more reliably evaluated on smears from at least 2 separate time points. The most debatable criterion was dysgranulopoiesis, which is defined as “severe/prominent” in the current WHO classification. In the prior series of aCML by Breccia et al, “marked” dysgranulopoiesis was only seen in 32% of cases.5 In our series, based on the cases assessed at MDACC, marked dysgranulopoiesis (≥50% of granulocytes) was seen in 52% aCML patients, and the remaining 48% had 10% to 49% dysgranulopoiesis. In a few cases, we did observe abnormal chromatin clumping in neutrophils, as reported previously.29 Of note, in some cases, dysgranulopoiesis was manifested as abnormal nuclear hypersegmentation, which is not commonly seen in MDS dysgranulopoiesis. Prognostically, of the aCML defining parameters, a higher WBC as a continuous variable and the presence of ≥10% PB myeloid precursors were both significantly correlated with an inferior OS and AMLFS in both univariate and multivariate analysis. Dysgranulopoiesis, however, only showed borderline significance for OS and AMLFS in univariate analysis but insignificant in multivariate analysis. Notably, we had 12 (9%) patients who presented with leukocytosis and ≥10% PB immature myeloid cells but no significant dysgranulopoiesis. They had clinicopathological features similar to aCML rather than their assigned MDS/MPN-U category (data not shown). Our study findings, in conjunction with the inherent subjectivity of evaluating dysgranulopoiesis, raise the question as to whether dysgranulopoiesis is mandatory for a diagnosis of aCML, provided that there is leukocytosis with a substantial fraction of circulating myeloid precursors and dysplasia in at least 1 hematopoietic lineage.
We further questioned if any cytogenetic or molecular genetic alterations were unique for aCML. An abnormal karyotype, a complex karyotype, and i(17q) appeared to be slightly more frequent in aCML than in MDS/MPN-U; however, the differences were not statistically significant. Similar to that reported previously, RAS mutations were relatively frequent, whereas JAK2 p.V617F mutations were infrequent in aCML.30 Other tested mutations were infrequent in both groups. CSF3R T618I mutations were not detected in any of the 27 aCML and 27 MDS/MPN-U patients tested. This result is similar to that reported by Pardanani et al13 who detected CSF3R T618I mutations in 10/12 CNL but in 0/19 aCML patients. Recently, Kosmider et al31 reported variant CSF3R somatic mutations other than T618I in 6/196 (4%) CMML patients. Notably, G683R mutations were found in germline DNA. One of our aCML cases showed mutation in CSF3R exon17 G683R, likely representing a constitutional variant. Our findings that somatic CSF3R mutations are essentially absent in aCML and MDS/MPN-U endorse the recent proposal by Tefferi et al for redefining CNL by its molecular characteristics.26 The presence of a membrane-proximal CSF3R mutation in a patient with predominantly neutrophilic MPN should be considered diagnostic for CNL and essentially excludes a diagnosis of aCML. CALR mutations in exon 9, reported in a very low frequency (1/30, 3%) in aCML by Nangalia et al,16 were not detected in our patients, either in aCML or in MDS/MPN-U.
Within the group of aCML patients, a higher WBC and percent of PB myeloid precursors could further stratify the risk of patients, both for OS and AMLFS. BM blasts were relevant for AMLFS but not OS. Surprisingly, other parameters became irrelevant to prognosis. In contrast, the cases assigned to the MDS/MPN-U were heterogeneous. Recently, Dinardo et al11 studied the natural history of cases diagnosed as MDS/MPN-U between 1987 and 2013 at a single tertiary center and observed a median OS of 12.4 months in their cohort of patients. However, unlike our study (a median OS of 21.4 months), survival in that study was measured from the date of referral rather than the date of diagnosis. Notably, slides were not available for most of the older cases (1987-2003) to confirm diagnosis, and a significant subset of these patients were diagnosed before the availability of hypomethylating agents and various kinase inhibitors. In our MDS/MPN-U patients, a high WBC and the presence of PB myeloid precursors were significant negative predictors for inferior OS and AMLFS. Unlike aCML, a complex karyotype or −7/−7q, BM and PB blast percentage, and high LDH level were also adverse risk factors for OS. Dysgranulopoiesis was not significant for OS or AMLFS, either in univariate or multivariate analysis, but patients with an increased platelet count appeared to have a better outcome.
In summary, we showed that the WHO definition for aCML identifies within the MDS/MPN category a distinct subgroup of patients with a number of adverse clinical features as well as inferior outcomes. MPN-related mutations are either absent or very infrequent in aCML, and the detection of CSF3R T618I, MPL CALR, or JAK2 V617F mutations should prompt a differential diagnosis of CNL, PMF, or MPN-U, which can share overlapping features with aCML. The MDS/MPN-U category is heterogeneous, but a number of clinical, morphologic, and cytogenetic factors were identified that should allow for a better patient risk stratification. Recently, frequent ASXL1, TET2, and SRSF mutations have been found in MDS/MPN, particularly in CMML.32-34 SETBP1 mutations are detected in 25% to 32% of aCML32,35 and 10% of MDS/MPN-U,32 which correlate with higher WBC, lower hemoglobin levels, and lower platelet counts. Incorporation of molecular characteristics may help in achieving a better understanding of these myeloid neoplasms and perhaps will allow a clinically meaningful subcategorization.36
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Roxanna Anaya at The University of Texas M.D. Anderson Cancer Center for her excellent work on proofreading and meticulous data verification.
This research is partially supported by a Cancer Center Support Grant (P30 CA016672) (F.C.S. and P.S.F.).
Authorship
Contribution: S.A.W. designed, performed, and analyzed data, and wrote the paper; A.O. designed, performed, and wrote the paper; R.P.H., H.J.R., J.T.G., DC., E.W., J.H., J.J., R.K., M.M., K.P.P., C.B., K.H.Y., C.D.D., S.V., R.V.T., A.B., E.D.H., D.A.A., K.F., and R.L. gathered data and conducted experiments; P.S.F. and F.C.S. analyzed data; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sa A. Wang, Department of Hematopathology, Unit 72, 1515 Holcombe Blvd, The University of Texas M.D. Anderson Cancer Center, Houston, TX 77030; e-mail: swang5@mdanderson.org.


![Figure 3. Survival rates. Compared with MDS/MPN-U, patients with aCML showed a significant inferior OS (12.4 months, 95% CI [9.0-16.1] vs 21.8 months, 95% CI [17.6-28.8]) and ACL-free survival (11.2 months, 95% CI [7.0-13.5] vs 18.9 months, 95% CI [12.3-26.3]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/17/10.1182_blood-2014-02-553800/4/m_2645f3.jpeg?Expires=1767700011&Signature=QOSfnxfvSgXhVPALbbbuwg80HfgLyjSMh-0c-CMxGYrEHShmMjr7E-5PbGw~nOmT6SkXxguXKG7mi81yas~5NuphPPyGWJzTwZ0~XDdBrkcmGfwEmPzKTYRGH9mvl30D3Z8kD0C7OYRvObmQRdJOI86--04xI0og~OJ3e5ATj9xf0eBj3ktHSYlhczDcukEBS~urS4l-IUmANzMccDUDXZLvq68NjpUBkZHnPRDT1wzapAQJ4IwhWShunhayB9Rf1P6HLJN52rjgFRYokshKsyj8j884xroJwUKOaf8kUOtUjCkZudaFZR8ePR~JyN1IiG4mseljhRyR4LsapU1cRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal