Key Points
JAK/STAT signaling is constitutively increased in AML stem and progenitor cells.
JAK2 inhibition significantly inhibits AML but not normal stem cell growth.
Abstract
Acute myeloid leukemia (AML) is sustained by small populations of leukemia stem cells (LSCs) that can resist available treatments and represent important barriers to cure. Although previous studies have shown increased signal transducer and activator of transcription (STAT)3 and STAT5 phosphorylation in AML leukemic blasts, the role of Janus kinase (JAK) signaling in primary AML compared with normal stem cells has not been directly evaluated. We show here that JAK/STAT signaling is increased in LSCs, particularly from high-risk AML. JAK2 inhibition using small molecule inhibitors or interference RNA reduced growth of AML LSCs while sparing normal stem cells both in vitro and in vivo. Increased JAK/STAT activity was associated with increased expression and altered signaling through growth factor receptors in AML LSCs, including receptor tyrosine kinase c-KIT and FMS-related tyrosine kinase 3 (FLT3). Inhibition of c-KIT and FLT3 expression significantly inhibited JAK/STAT signaling in AML LSCs, and JAK inhibitors effectively inhibited FLT3-mutated AML LSCs. Our results indicate that JAK/STAT signaling represents an important signaling mechanism supporting AML LSC growth and survival. These studies support continued evaluation of strategies for JAK/STAT inhibition for therapeutic targeting of AML LSCs.
Introduction
Acute myeloid leukemia (AML) is driven by a subpopulation of leukemia stem cells (LSCs) with self-renewal properties that generate the bulk of leukemic cells.1 Human AML LSCs are functionally defined by capacity to regenerate leukemia in immunodeficient mice.1,2 Whereas normal hematopoietic stem cells (HSCs) are restricted to the lineage− (Lin−)CD34+CD38− population, AML LSCs may express markers associated with normal committed progenitors including Lin+, CD38+, and CD45RA+.3-6 Current treatments for AML are limited by failure to induce remission and high relapse rates that may be related to resistance of LSCs to elimination.7,8 High numbers of LSCs9 or expression of a LSC gene signature3 is independently associated with poor prognosis in AML, supporting a role for LSCs as important targets for therapeutic development.
Improved targeting of LSCs requires better understanding of mechanisms supporting their maintenance and expansion. AML results from collaboration of different classes of mutations including those affect transcription factors and in growth factor (GF) receptor tyrosine kinases such as FMS-related tyrosine kinase 3 (FLT3) and receptor tyrosine kinase c-KIT and downstream signaling pathways such as neuroblastoma RAS viral (v-ras) oncogene homolog (N-RAS).10 The Janus kinase (JAK) family of nonreceptor tyrosine kinases are important mediators of cytokines and GF signaling, activating signal transducer and activator of transcription (STAT) proteins and other downstream signaling pathways that modulate cell cycling and apoptosis.11 STATs are constitutively activated in several solid tumors and hematological malignancies.12-14 Gain-of-function JAK2 V617F mutations are common in myeloproliferative disorders15 but are rare in AML.16 Conversely, increased JAK2, STAT3, and STAT5 phosphorylation is reported in AML blasts.17-19 Treatment with combined JAK2 and FLT3 inhibitors significantly reduces proliferation of AML cells,20 and a multikinase inhibitor targeting FLT3, JAK2, and several cyclin dependent kinases inhibited leukemia growth in animal models.21 STAT signaling was critical for LSC self-renewal in a meningioma (disrupted in balanced translocation) 1– and homeobox protein Hox-A9–expressing leukemia model.22 However the role of JAK signaling in primary human AML LSCs has not been evaluated, and prior studies have not included mechanistic investigation of altered JAK2 signaling in AML.
Several JAK2 inhibitors are in clinical development, of which INC424 (Ruxolitinib) is approved for treatment of primary myelofibrosis.23 A phase 2 study of INC424 in patients with relapsed/refractory AML showed good tolerance and modest antileukemic activity.24 Fifteen of 38 patients studied showed decreased or stabilized blasts in blood and marrow with complete remission achieved in 3 patients with prior myeloproliferative neoplasms. It is possible that additional benefit could be seen if the drug was used up front or in higher doses, or more potent and selective JAK2 inhibitors were used. To better understand the potential value of JAK/STAT inhibition in AML, there is a critical need to carefully evaluate the role of altered JAK signaling in growth and survival of human AML LSCs. Here we evaluated JAK/STAT activity in primary AML CD34+ cells, and the effects of potent JAK1/2 inhibitors and small interfering RNA (siRNA)-mediated knockdown of JAK and STAT expression on growth and maintenance of AML and normal stem/progenitor cells. We also studied the role of altered GF receptor expression and signaling in enhanced JAK/STAT activity in AML CD34+ cells.
Experimental procedures
Patient samples and cells
Peripheral blood (PB) or bone marrow (BM) samples were obtained from AML patients at diagnosis or at relapse. Patient characteristics are shown in supplemental Table 1. Risk groups were based on National Cancer Center Network criteria (supplemental Table 2 available on the Blood Web site).25 Normal peripheral blood stem cells (PBSCs) were obtained from transplant donors. Cord blood (CB) samples were obtained from StemCyte (Arcadia, CA). All subjects signed informed consent forms approved by the City of Hope (COH) Institutional Review Board in accordance with the Declaration of Helsinki. Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque (Sigma Diagnostics) separation. CD34+ cells were selected using immunomagnetic columns (Miltenyi Biotech).
Reagents
AZD1480 was synthesized by AstraZeneca. INC424 and AC220 were from Selleck Chemicals (Houston, TX). Imatinib (Novartis Pharmaceuticals) was purchased from the COH pharmacy. Antibodies to phosphorylated STAT3 (pSTAT3) (Y705), STAT3, pSTAT5 (Tyr694), STAT5, pJAK2 (Y1007/1008), JAK2, p-c-Kit (Tyr719), c-Kit, pFLT3 (Y591), FLT3 (8F2), and horseradish peroxidase (HRP)-conjugated anti-mouse and HRP-conjugated anti-rabbit antibodies were from Cell Signaling Technology (Danvers, MA). Anti-β-actin was from Sigma-Aldrich (St Louis, MO).
Cell culture
CD34+ cells were cultured in Iscove's modified Dulbecco's media supplemented with GF at concentrations similar to that found in conditioned medium from long-term BM cultures (granulocyte-macrophage colony-stimulating factor, 200 pg/mL; granulocyte colony-stimulating factor, 1 ng/mL; stem cell factor [SCF], 200 pg/mL; leukemia inhibitory factor, 50 pg/mL; macrophage inflammatory protein α, 200 pg/mL; and interleukin 6 [IL-6], 1 ng/mL).
Colony-forming cell assays
Cells were plated in methylcellulose progenitor culture with 30% fetal bovine serum; erythropoietin, 3 U/mL; IL-3, 5 ng/mL; SCF, 5 ng/mL; granulocyte colony-stimulating factor, 20 ng/mL; and granulocyte-macrophage colony-stimulating factor, 20 ng/mL), and hematopoietic colonies were counted after 14 days.
Apoptosis assay
Cells were labeled with Annexin V-phycoerythrin (PE; BDPharMingen, San Diego, CA) or fixed with 4% paraformaldehyde, permeabilized with 0.1% Saponin, and labeled with PE-conjugated active caspase 3 antibody (BDPharMingen) and analyzed by flow cytometry.
Cell cycle analysis
Cells were labeled with anti-Ki67-fluorescein isothiocyanate (BDPharMingen) and 7-aminoactinomycin D (7-AAD; BDPharMingen)26 and analyzed by flow cytometry. Ki67−7-AAD− cells were considered to be in G0, Ki67+7-AAD− cells in G1, and Ki67+7-AAD+ cells in S/G2/M.
Western blotting
Cells were lysed with radioimmunoprecipitation assay buffer buffer with a phosphatase inhibitor cocktail (Pierce Biotechnologies) and Calyculin A (Cell Signaling Technology). Proteins were resolved using 4% to 12% ν-polyacrylamide gel electrophoresis Bis-Tris gels (Invitrogen) and transferred to polyvinylidene fluoride membranes (Millipore). Membranes were blocked, incubated with indicated primary antibodies and HRP-conjugated secondary antibodies, and detected with an enhanced chemiluminescence system (Pierce Biotechnology).
Flow cytometry for pSTAT3 and pSTAT5
CD34+ cells were fixed, permeabilized, labeled with pSTAT3, pSTAT5, or isotype control antibodies, and analyzed by flow cytometry. Results were expressed as ratio of mean channel fluorescence (MCF) of pSTAT3/5 to MCF for isotype controls. For GF stimulation experiments, cells serum starved for 1 hour were exposed to 200 pg/mL SCF or 1 ng/mL FLT3 ligand.
RNA interference
Separate sets of siRNAs to JAK2, JAK1, STAT3, STAT5a, STAT5b, c-kit, FLT3, and control nonspecific siRNAs (ON-TARGETplus SMARTpools siRNAs; Thermo Scientific, Waltham, MA) and siRNAs from Applied BioSystems/Ambion (Foster City, CA) were used to transfect human AML CD34+ cells using the Amaxa Human CD34 Cell Nucleofector Kit. Cells were collected 48 and 72 hours later for analysis. Lentivirus vectors expressing short hairpin RNA (shRNA) to JAK2 and control shRNA (MISSION shRNA lentiviral transduction particles; Sigma-Aldrich) were used to transduce KG1a cells in the presence of polybrene (4 µg/mL), and cells were analyzed after 72 hours.
Engraftment of human cells in immunodeficient mice
Primary human AML or CB cells were CD3+ cell depleted using immunomagnetic columns (Miltenyi). For ex vivo treatment, 5 × 106 cells per mouse were cultured with dimethylsulfoxide (DMSO), AZD1480, or INC424 for 72 hours and transplanted intravenously into irradiated (300 cGy) 8-week-old female NOD/SCID γ-chain− (NSG) mice. Eight weeks posttransplantation, mice were euthanized, and human CD45+ cells and CD45+ subsets (CD34, CD33, CD14, CD15, CD19, and CD3) in BM were analyzed by flow cytometry. For in vivo treatment, AML cells were transplanted into NSG mice, engraftment was confirmed after 6 to 8 weeks, and mice were treated with AZD1480 (50 mg/kg/day) or vehicle by gavage for 2 weeks. To evaluate the effect of treatment on LSC capacity, BM cells from primary recipients were transplanted into secondary recipient NSG mice. Mouse care and procedures were performed using protocols approved by the COH Institutional Animal Care and Use Committee.
Measurement of cytokine receptor expression by quantitative polymerase chain reaction
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA), first-strand cDNA was synthesized using Superscript III First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA), and quantitative polymerase chain reaction analysis using SYBR Green master mix was performed using an HT7900 system (Applied Biosystems, Foster City, CA). Primer sequences are shown in supplemental Table 3.
Statistical analysis
Data obtained from independent experiments were reported as the mean ± standard error of the mean (SEM). Student t test, Mann-Whitney test, 2-way analysis of variance with multiple testing, or regression analysis was performed to determine statistical significance as appropriate.
Results
Increased JAK/STAT activity in CD34+ cells from AML patients
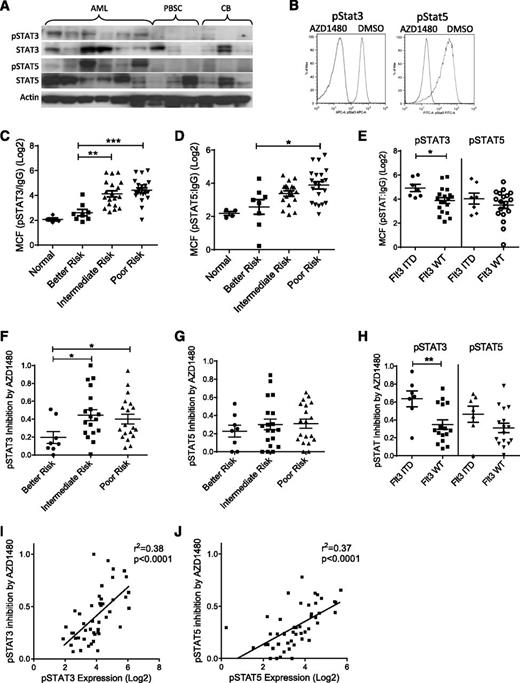
AML CD34+ cells demonstrated increased pSTAT3 and pSTAT5 levels compared with normal CB and PBSC CD34+ cells on western blotting (Figure 1A). AZD1480, a potent ATP competitive inhibitor of JAK1/2 kinases, resulted in rapid (30 minutes) and sustained (72 hours) inhibition of pJAK2 and pSTAT3/5 levels in AML CD34+ cells on western blotting (supplemental Figure 1A) and flow cytometry (Figure 1B; supplemental Figure 1B). Assessment of a larger group of samples using flow cytometry showed that the majority of AML CD34+ cells (n = 49) had increased pSTAT3 (41/46 with a ratio of MCF for pSTAT3:IgG > 5) and pSTAT5 (40/46 with pSTAT5:IgG ratio > 5) compared with CB samples (n = 4, pSTAT:IgG ratio ≤ 5). pSTAT3 and pSTAT5 levels were higher in CD34+ cells from poor and intermediate compared with better cytogenetic risk group patients (Figure 1C-D).25 Within the poor risk category, pSTAT3/5 expression was significantly higher in FLT3-ITD+ compared with FLT3-ITD− samples, consistent with reports of increased JAK/STAT signaling in FLT3-ITD+ cells27 (Figure 1E). AZD1480 resulted in greater fractional inhibition of pSTAT3 in the poor and intermediate risk compared with the better risk group (Figure 1F), whereas pSTAT5 reduction was similar in poor, intermediate, and better risk groups (Figure 1G). Significantly greater inhibition of pSTAT3 was seen in FLT3-ITD+ compared with FLT3-ITD− samples (Figure 1H). We observed a positive correlation between fractional inhibition of pSTAT3/5 by AZD1480 and basal pSTAT3/5 levels (Figure 1I-J), further suggesting that increased pSTAT3/5 expression in AML CD34+ cells was related to increased JAK2 activity. Finally, expression of pSTAT3/5 was increased overall in CD34+CD38− and inhibited by AZD1480 in both CD34+CD38− and CD34+CD38+ AML cells (supplemental Figure 1C).
Increased STAT3 and STAT5 activity in human AML stem/progenitor cells and inhibition by AZD1480 treatment. (A) AML, PBSCs, and CB CD34+ cells were evaluated for phosphorylated and total STAT3/5 and actin by western blotting. (B) AML CD34+ cells treated with 0.5 µM AZD1480 or DMSO for 30 minutes were evaluated for pSTAT3/5 by flow cytometry. Representative plots are shown. (C-D) Expression of pSTAT3 and pSTAT5 measured by flow cytometry in 4 cord blood and 48 primary AML CD34+ samples organized by cytogenetic risk category, represented as log2(MCF pSTAT3/5/MCF isotype control). (E) Expression of pSTAT3/5 in AML CD34+ cells by FLT3-internal tandem duplication (ITD) mutation status. (F-G) Fractional inhibition of pSTAT3/5 in 46 AML CD34+ cells organized by cytogenetic risk category exposed to 0.1 µM AZD1480 for 30 minutes. Inhibition was calculated as 1 − (MCF pSTAT3/5-treated cells/MCF pSTAT3/5-untreated cells). (H) Fractional inhibition of pSTAT3/5 inhibition in AML CD34+ cells by FLT3-ITD mutation status. (I-J) Correlation of pSTAT3/5 expression (log2) in AML CD34+ cells with pSTAT3/5 inhibition by AZD1480. Results represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
Increased STAT3 and STAT5 activity in human AML stem/progenitor cells and inhibition by AZD1480 treatment. (A) AML, PBSCs, and CB CD34+ cells were evaluated for phosphorylated and total STAT3/5 and actin by western blotting. (B) AML CD34+ cells treated with 0.5 µM AZD1480 or DMSO for 30 minutes were evaluated for pSTAT3/5 by flow cytometry. Representative plots are shown. (C-D) Expression of pSTAT3 and pSTAT5 measured by flow cytometry in 4 cord blood and 48 primary AML CD34+ samples organized by cytogenetic risk category, represented as log2(MCF pSTAT3/5/MCF isotype control). (E) Expression of pSTAT3/5 in AML CD34+ cells by FLT3-internal tandem duplication (ITD) mutation status. (F-G) Fractional inhibition of pSTAT3/5 in 46 AML CD34+ cells organized by cytogenetic risk category exposed to 0.1 µM AZD1480 for 30 minutes. Inhibition was calculated as 1 − (MCF pSTAT3/5-treated cells/MCF pSTAT3/5-untreated cells). (H) Fractional inhibition of pSTAT3/5 inhibition in AML CD34+ cells by FLT3-ITD mutation status. (I-J) Correlation of pSTAT3/5 expression (log2) in AML CD34+ cells with pSTAT3/5 inhibition by AZD1480. Results represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
JAK inhibitors reduce growth and survival of AML CD34+ cells in vitro
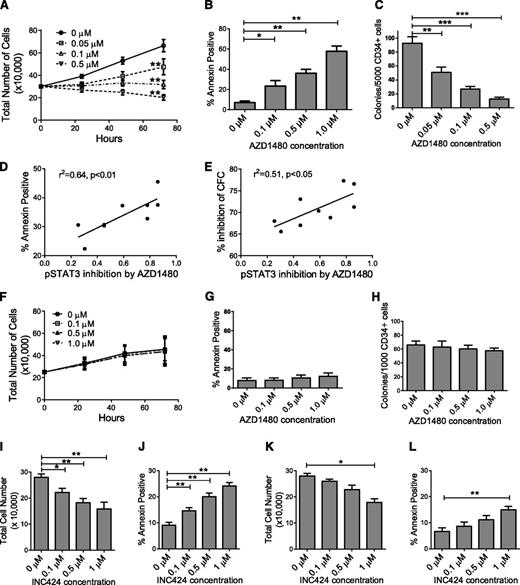
Treatment with AZD1480 resulted in a significant dose-dependent reduction in AML CD34+ cell numbers (Figure 2A), increased apoptosis based on Annexin-V (Figure 2B), increased active caspase 3 labeling (data not shown), increased G0 cells by Ki67/7AAD staining (supplemental Figure 2A-B), and reduced colony-forming cell (CFC) numbers (Figure 2C). AZD1480-mediated apoptosis (Figure 2C) and CFC inhibition (Figure 2D) correlated with AZD1480-mediated pSTAT3 inhibition in AML CD34+ cells. AZD1480-induced apoptosis (supplemental Figure 2C) and CFC inhibition (supplemental Figure 2D) also correlated with baseline pSTAT3 expression. AZD1480 induced apoptosis (supplemental Figure 2E) and inhibited CFC formation (supplemental Figure 2F) in both AML CD34+38− and CD34+38+ cells. In contrast, AZD1480 did not significantly reduce CB CD34+ cell numbers (Figure 2F), survival (Figure 2G), and colony-forming ability (Figure 2H), or significantly alter cell cycle (data not shown). Similarly, AZD1480 did not significantly reduce cell numbers, survival, proliferation, or colony-forming capacity of normal PBSC CD34+ cells (data not shown). These observations suggest that AZD1480 selectively targets AML but spares normal progenitors. A second JAK1/JAK2 inhibitor, INCB18424 (INC424), also resulted in dose-dependent (0.05-1.0 μM) inhibition of pJAK2, pSTAT3, and pSTAT5 (supplemental Figure 2G-I), reduced numbers (Figure 2I), and increased apoptosis of AML CD34+ cells (Figure 2J) but to a lesser extent than AZD1480. In contrast, INC424 did not significantly inhibit normal CD34+ cells except at high concentration (1.0 μM) (Figure 2K-L). INC424 resulted in significantly higher inhibition of cell numbers normalized to controls (0.1 and 1 µM, P < .05; 0.5 µM, P < .01) and an increase in apoptosis over controls (0.1, 0.5, and 1 µM, P < .01) in AML compared with CB samples (data not shown).
JAK inhibitor treatment reduces in vitro growth and survival of primary human AML but not normal CD34+ cells. (A) Enumeration of AML CD34+ cells (n = 8, AML 008, 056, 090, 111, 179, 216, 282, and 404) cultured with AZD1480 for 72 hours. (B) AML CD34+ cells (n = 13, AML 008, 028, 056, 090, 111, 179, 216, 282, 294, 335, 404, 422, and 493) treated with AZD1480 for 72 hours were evaluated for apoptosis by Annexin-V labeling. (C) CFC growth from AML CD34+ cells (n = 9, AML 090, 179, 282, 335, 404, 419, 422, 493, and 526) treated with AZD1480 for 72 hours. (D-E) Correlation between (D) fractional pSTAT3 inhibition in AML CD34+ cells and AZD1480-mediated apoptosis and (E) percent inhibition of CFC with AZD1480 (0.1 µM; n = 9). (F-H) Cord blood CD34+ cells (n = 3) treated with AZD1480 for 72 hours were (F) enumerated and evaluated for (G) apoptosis and (H) CFC growth. (I-J) AML CD34+ samples (n = 6, AML 373, 413, 519, 532, 663, and 704) treated with INC424 for 72 hours were (I) enumerated and (J) evaluated for apoptosis. (K-L) CB CD34+ samples (n = 4) were treated with INC424 for 72 hours and (K) enumerated and (L) evaluated for apoptosis. Results represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
JAK inhibitor treatment reduces in vitro growth and survival of primary human AML but not normal CD34+ cells. (A) Enumeration of AML CD34+ cells (n = 8, AML 008, 056, 090, 111, 179, 216, 282, and 404) cultured with AZD1480 for 72 hours. (B) AML CD34+ cells (n = 13, AML 008, 028, 056, 090, 111, 179, 216, 282, 294, 335, 404, 422, and 493) treated with AZD1480 for 72 hours were evaluated for apoptosis by Annexin-V labeling. (C) CFC growth from AML CD34+ cells (n = 9, AML 090, 179, 282, 335, 404, 419, 422, 493, and 526) treated with AZD1480 for 72 hours. (D-E) Correlation between (D) fractional pSTAT3 inhibition in AML CD34+ cells and AZD1480-mediated apoptosis and (E) percent inhibition of CFC with AZD1480 (0.1 µM; n = 9). (F-H) Cord blood CD34+ cells (n = 3) treated with AZD1480 for 72 hours were (F) enumerated and evaluated for (G) apoptosis and (H) CFC growth. (I-J) AML CD34+ samples (n = 6, AML 373, 413, 519, 532, 663, and 704) treated with INC424 for 72 hours were (I) enumerated and (J) evaluated for apoptosis. (K-L) CB CD34+ samples (n = 4) were treated with INC424 for 72 hours and (K) enumerated and (L) evaluated for apoptosis. Results represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
RNA interference-mediated inhibition of JAK2, STAT3, and STAT5 reduces growth and survival of AML CD34+ cells
We further assessed the role of the JAK signaling in AML CD34+ cells by siRNA-mediated knockdown of JAK1, JAK2, STAT3, and STAT5a and STAT5b (in combination). We verified knockdown of targeted proteins in siRNA-treated cells (Figure 3A). JAK2, STAT3, and STAT5a/b knockdown significantly decreased cell numbers (Figure 3B), increased apoptosis (Figure 3C), and inhibited CFC (Figure 3D) compared with control siRNA, with less inhibition seen with JAK1 knockdown. We used a second set of siRNAs targeting different sequences in JAK1, JAK2, STAT3, and STAT5 to validate these results. We confirmed inhibition of target gene expression in AML cells (Figure 3E). Inhibition of JAK1 resulted in reduced pSTAT3 but not STAT5 levels, whereas inhibition of JAK2 resulted in inhibition of both pSTAT3 and pSTAT5 levels (Figure 3F). We observed significant reduction of cell numbers (Figure 3G), survival (Figure 3H), and CFC (Figure 3I) following JAK2, STAT3, and STAT5 knockdown. In contrast, the second JAK1 siRNA did not inhibit AML CD34+ cell survival or growth. JAK2 inhibition by lentivirus-mediated JAK2 shRNA expression also resulted in marked inhibition of pSTAT3 and pSTAT5 expression in AML KG1a cells (supplemental Figure 3A), with significant inhibition of cell growth (supplemental Figure 3B) and apoptosis induction (supplemental Figure 3C). JAK1, JAK2, STAT3, and STAT5 knockdown (Figure 3J) did not significantly induce apoptosis in CB CD34+ cells (Figure 3K) or inhibit CFC growth, other than modest but significant inhibition with STAT3 knockdown (Figure 3L). Although target gene inhibition in AML and normal cells could vary over time and influence results of colony assays, these results indicate that JAK inhibition selectively targets AML cells. Our results indicate that the JAK inhibitor effects on AML CD34+ cells are related to JAK2 inhibition to a greater extent than JAK1 inhibition and that JAK2, STAT3, and STAT5 play important roles in AML CD34+ cell growth and survival.
RNA interference-mediated inhibition of JAK/STAT expression reduces growth and survival of human AML CD34+ cells. (A) AML CD34+ cells (AML 404, 571, and 636) were exposed to siRNA to JAK1, JAK2, STAT3, and STAT5a/b and negative control siRNA by nucleofection. Knockdown of target proteins was evaluated by western blotting. (B-D) Nucleofected cells were cultured for 72 hours and (B) enumerated (n = 3) and assessed for (C) apoptosis (n = 4) and (D) CFC growth (n = 3). (E) AML CD34+ cells (AML 545, 614, 704, and 755) were exposed to a second set of siRNA to JAK1, JAK2, STAT3, and STAT5a/b and control siRNA (negative control), and (F) the effect of JAK1/2 knockdown on pSTAT3/5 expression was evaluated by western blotting. Nucleofected cells were cultured for 72 hours and (G) enumerated (n = 3) and assessed for (H) apoptosis (n = 4) and (I) CFC growth (n = 3). (J-L) CB CD34+ cells (n = 3) were exposed to siRNA to JAK1, JAK2, STAT3, and STAT5a/b and control siRNA (negative control) and assessed for (J) knockdown of target proteins by western blotting and for (K) apoptosis and (L) CFC growth. Results represent the mean ± SEM. Significance values: *P < .05, **P < .01.
RNA interference-mediated inhibition of JAK/STAT expression reduces growth and survival of human AML CD34+ cells. (A) AML CD34+ cells (AML 404, 571, and 636) were exposed to siRNA to JAK1, JAK2, STAT3, and STAT5a/b and negative control siRNA by nucleofection. Knockdown of target proteins was evaluated by western blotting. (B-D) Nucleofected cells were cultured for 72 hours and (B) enumerated (n = 3) and assessed for (C) apoptosis (n = 4) and (D) CFC growth (n = 3). (E) AML CD34+ cells (AML 545, 614, 704, and 755) were exposed to a second set of siRNA to JAK1, JAK2, STAT3, and STAT5a/b and control siRNA (negative control), and (F) the effect of JAK1/2 knockdown on pSTAT3/5 expression was evaluated by western blotting. Nucleofected cells were cultured for 72 hours and (G) enumerated (n = 3) and assessed for (H) apoptosis (n = 4) and (I) CFC growth (n = 3). (J-L) CB CD34+ cells (n = 3) were exposed to siRNA to JAK1, JAK2, STAT3, and STAT5a/b and control siRNA (negative control) and assessed for (J) knockdown of target proteins by western blotting and for (K) apoptosis and (L) CFC growth. Results represent the mean ± SEM. Significance values: *P < .05, **P < .01.
AZD1480 treatment inhibits human AML LSC capable of engrafting NSG mice
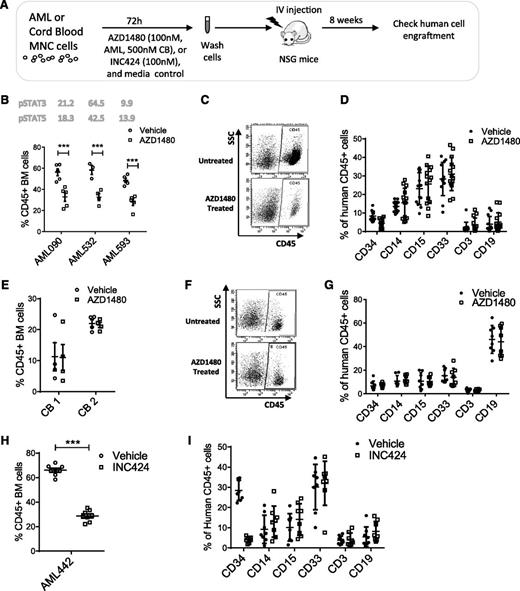
We evaluated the effect of AZD1480 on AML LSCs with in vivo repopulating capacity. AML MNCs were depleted of T cells, treated with AZD1480 or DMSO for 72 hours, and injected into sublethally irradiated NSG mice (supplemental Table 4). Human CD45+ cell engraftment in BM was assessed after 8 weeks (Figure 4A). Analysis of 3 samples showed that AZD1480 (0.1 µM) treatment significantly reduced human AML engraftment (Figure 4B-D). Conversely, AZD1480 (0.5 µM) treatment did not significantly reduce CB CD45+ cell engraftment (Figure 4E-G). Treatment with INC424 (0.1 μM) also significantly reduced AML cell engraftment in BM of NSG mice (Figure 4H-I).
JAK inhibitors reduce engraftment of primary human AML but not normal stem cells in NSG mice. (A) Human primary AML and cord blood MNCs were treated with AZD1480 or DMSO for 72 hours and transplanted into sublethally irradiated NSG mice. After 8 weeks, BM cells were analyzed for expression of human CD45+ cells. (B) Human CD45+ cell engraftment for AZD1480 (0.1 μM)-treated and control DMSO-treated cells from 3 different AML samples (AML090, n = 5 mice per group; AML532, n = 3-4 mice per group; AML 593, n = 4 mice per group). The baseline levels of pSTAT3/5 in each AML sample are shown in the figure. (C) Representative fluorescence-activated cell sorter plot of human AML CD45+ cell engraftment in BM. (D) Expression of differentiation markers on human AML CD45+ cells engrafted in BM. (E) Engraftment of human cord blood CD45+ cells exposed to AZD1480 (0.5 µM) or DMSO for 72 hours in NSG mice 8 weeks after transplant (2 different samples, n = 4 for each group per sample). (F) Representative fluorescence-activated cell sorter plot of human CB CD45+ cell engraftment in BM. (G) Expression of differentiation markers on human CB CD45+ cell engrafted in BM. (H) Human CD45+ cell engraftment for INC424 (0.1 μM)-treated and control DMSO-treated AML cells (AML442, n = 8 mice per group; MCF pSTAT3 = 16, MCF pSTAT5 = 22.8). (I) Expression of differentiation markers on human AML CD45+ cells engrafted in BM.
JAK inhibitors reduce engraftment of primary human AML but not normal stem cells in NSG mice. (A) Human primary AML and cord blood MNCs were treated with AZD1480 or DMSO for 72 hours and transplanted into sublethally irradiated NSG mice. After 8 weeks, BM cells were analyzed for expression of human CD45+ cells. (B) Human CD45+ cell engraftment for AZD1480 (0.1 μM)-treated and control DMSO-treated cells from 3 different AML samples (AML090, n = 5 mice per group; AML532, n = 3-4 mice per group; AML 593, n = 4 mice per group). The baseline levels of pSTAT3/5 in each AML sample are shown in the figure. (C) Representative fluorescence-activated cell sorter plot of human AML CD45+ cell engraftment in BM. (D) Expression of differentiation markers on human AML CD45+ cells engrafted in BM. (E) Engraftment of human cord blood CD45+ cells exposed to AZD1480 (0.5 µM) or DMSO for 72 hours in NSG mice 8 weeks after transplant (2 different samples, n = 4 for each group per sample). (F) Representative fluorescence-activated cell sorter plot of human CB CD45+ cell engraftment in BM. (G) Expression of differentiation markers on human CB CD45+ cell engrafted in BM. (H) Human CD45+ cell engraftment for INC424 (0.1 μM)-treated and control DMSO-treated AML cells (AML442, n = 8 mice per group; MCF pSTAT3 = 16, MCF pSTAT5 = 22.8). (I) Expression of differentiation markers on human AML CD45+ cells engrafted in BM.
AZD1480 inhibits human AML LSC growth in vivo
We next evaluated the effect of in vivo administration of AZD1480 on AML LSCs engrafted in NSG mice. AML cells were transplanted into NSG mice, engraftment was confirmed 6 weeks after transplant, and mice were treated with AZD1480 (50 mg/kg/day) or vehicle by gavage for 2 weeks (Figure 5A; supplemental Table 4). AZD1480 treatment markedly reduced spleen size and cellularity (data not shown) and reduced the percentage of human AML cells in murine BM compared with vehicle control (Figure 5B-C). In general, increased basal STAT3 expression was associated with increased inhibition of AML cells by AZD1480 treatment. Limiting dilution secondary transplantation of BM cells from AZD1480- or vehicle-treated mice were engrafted into NSG mice. Limiting dilutiion analysis indicated significant reduction in AML LSC frequency compared with vehicle-treated mice (Figure 5D-F). In contrast, AZD1480 treatment of mice engrafted with CB cells did not inhibit human cell engraftment (Figure 5G-H) and did not affect engraftment after secondary transplantation of BM from treated mice (Figure 5I), indicating a lack of significant effects on normal HSC growth and self-renewal.
In vivo administration of AZD1480 reduces primary human AML but not normal stem cells in NSG mice. (A) AML or CB mononuclear cells were transplanted into NSG mice. After 6 to 10 weeks, engraftment was confirmed, and mice were treated with AZD1480 (50 mg/kg) or vehicle daily for 2 weeks and evaluated for human cell engraftment. In 1 experiment, secondary transplant of BM cells to NSG mice was performed, and human cell engraftment was evaluated after 8 weeks. (B) Human CD45+ cells in BM of mice engrafted with AML cells (AML566, AML600, AML666, AML866, and AML1274) following 2 weeks of treatment with AZD1480 or DMSO. The pSTAT3 and pSTAT5 expression levels for each sample are indicated in the figure. (C-E) Pooled AML CD34+ cells from 2 patients (AML 404 and 755) were transplanted into NSG mice that were subsequently treated with AZD1480 or vehicle for 2 weeks, following which BM cells from primary recipient mice were transplanted in limiting dilutions (1.6 × 106, 0.8 × 106, 0.2 × 106 cells per mouse × 8 mice each) into secondary recipient mice, and human CD45+ cells in PB and BM (1.8 × 106 only) were evaluated after 8 weeks. (C) Human AML CD45+ cells and expression of differentiation markers on engrafted CD45+ cells in BM of primary recipients following AZD1480 or DMSO treatment. (D) Human CD45+ cells in PB cells of secondary recipients 8 weeks after limiting dilution transplant of BM cells from primary recipients. (E) Stem cell frequency in AZD1480- and DMSO-treated mice calculated using Poisson statistics. (F) Engraftment of human AML CD45+ cells and expression of differentiation markers on engrafted CD45+ cells in BM of secondary recipients at 8 weeks after transplant. (G) Human CD45+ cells in BM of mice engrafted with CB cells and treated for 2 weeks with AZD1480 or DMSO. (H) Expression of differentiation markers on human CB CD45+ cells in BM after AZD1480 or DMSO treatment. (I) Engraftment of human CD45+ cells and expression of differentiation markers on engrafted CD45+ cells in BM of secondary recipients at 8 weeks after transplantation of BM cells from AZD1480-treated and control mice. Results represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
In vivo administration of AZD1480 reduces primary human AML but not normal stem cells in NSG mice. (A) AML or CB mononuclear cells were transplanted into NSG mice. After 6 to 10 weeks, engraftment was confirmed, and mice were treated with AZD1480 (50 mg/kg) or vehicle daily for 2 weeks and evaluated for human cell engraftment. In 1 experiment, secondary transplant of BM cells to NSG mice was performed, and human cell engraftment was evaluated after 8 weeks. (B) Human CD45+ cells in BM of mice engrafted with AML cells (AML566, AML600, AML666, AML866, and AML1274) following 2 weeks of treatment with AZD1480 or DMSO. The pSTAT3 and pSTAT5 expression levels for each sample are indicated in the figure. (C-E) Pooled AML CD34+ cells from 2 patients (AML 404 and 755) were transplanted into NSG mice that were subsequently treated with AZD1480 or vehicle for 2 weeks, following which BM cells from primary recipient mice were transplanted in limiting dilutions (1.6 × 106, 0.8 × 106, 0.2 × 106 cells per mouse × 8 mice each) into secondary recipient mice, and human CD45+ cells in PB and BM (1.8 × 106 only) were evaluated after 8 weeks. (C) Human AML CD45+ cells and expression of differentiation markers on engrafted CD45+ cells in BM of primary recipients following AZD1480 or DMSO treatment. (D) Human CD45+ cells in PB cells of secondary recipients 8 weeks after limiting dilution transplant of BM cells from primary recipients. (E) Stem cell frequency in AZD1480- and DMSO-treated mice calculated using Poisson statistics. (F) Engraftment of human AML CD45+ cells and expression of differentiation markers on engrafted CD45+ cells in BM of secondary recipients at 8 weeks after transplant. (G) Human CD45+ cells in BM of mice engrafted with CB cells and treated for 2 weeks with AZD1480 or DMSO. (H) Expression of differentiation markers on human CB CD45+ cells in BM after AZD1480 or DMSO treatment. (I) Engraftment of human CD45+ cells and expression of differentiation markers on engrafted CD45+ cells in BM of secondary recipients at 8 weeks after transplantation of BM cells from AZD1480-treated and control mice. Results represent mean ± SEM. Significance values: *P < .05, **P < .01, ***P < .001.
Increased growth factor receptor expression and signaling in AML CD34+ cells
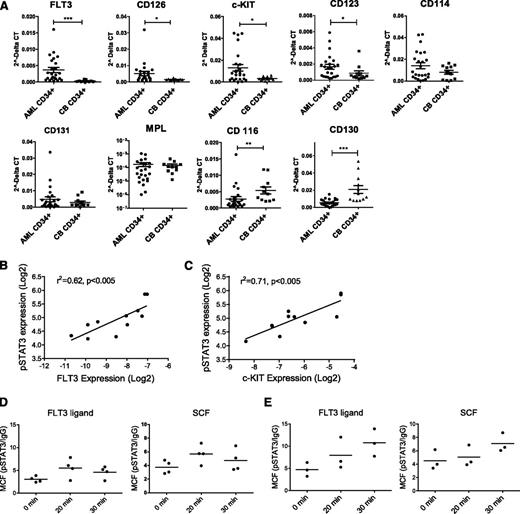
To explore the mechanisms underlying increased JAK/STAT activation, we compared expression of hematopoietic GF receptors in poor risk AML and normal CD34+ cells. FLT3, c-KIT, CD123, and CD126 mRNA expression was increased in AML CD34+ cells, with FLT3 up-regulation the most significant and consistent abnormality (Figure 6A). In contrast, expression of CD130 and CD116 was reduced in AML CD34+ cells. Seven of 24 samples had FLT3 ITD mutations and none had c-KIT mutations. FLT3 and c-KIT up-regulation occurred independently of receptor mutations. FLT3 and c-KIT receptor expression in AML CD34+ cells correlated with baseline pSTAT3 expression (Figure 6B-C) and AZD1480-mediated pSTAT3 inhibition (supplemental Figure 4A-B). In contrast, CD123 and CD114 expression did not significantly correlate with pSTAT3 expression (supplemental Figure 4C-D). Western blotting confirmed increased FLT3 and c-KIT expression and phosphorylation in AML CD34+ cells (supplemental Figure 4E). Flow cytometry demonstrated increased cell surface FLT3 receptor expression in FLT3 wild-type CD34+ cells but not FLT3-ITD AML CD34+cells compared with CB CD34+ cells (supplemental Figure 4F), consistent with reports of reduced cell surface expression of the FLT3-ITD mutant.28 AML CD34+ cells also showed increased cell surface c-KIT expression compared with CB CD34+ cells. To evaluate JAK/STAT signaling in response to GF stimulation, GF-starved AML and CB CD34+ cells were exposed to FLT3 ligand (FL) and SCF. In CB CD34+ cells, FL and SCF stimulation resulted in transient increase in pSTAT3/5 levels, which peaked at 20 minutes (Figure 6D; supplemental Figure 4G). In contrast, AML CD34+ cells demonstrated enhanced pSTAT3/5 response that continued to increase 30 minutes after stimulation (Figure 6E; supplemental Figure 4H) and was sustained even after 2 hours (data not shown). AZD1480 blocked increased pSTAT3/5 in AML CD34+ cells following FLT3 and c-KIT stimulation, indicating that STAT3/5 activation was JAK dependent (supplemental Figure 4I). The altered amplitude and kinetics of pSTAT response in AML CD34+ cells suggests both increased GF receptor-mediated activation and impaired down-regulation of JAK signaling.
Increased GF receptor expression and GF-mediated JAK/STAT signaling in AML CD34+ cells. (A) Expression of mRNA for growth factor receptors in AML (n = 24) and cord blood (n = 11) CD34+ cells, assessed by quantitative polymerase chain reaction. (B-C) Correlation between (B) FLT3 and (C) c-KIT mRNA expression (log2) and pSTAT3 expression (log2) in AML CD34+ cells. (D) Expression of pSTAT3 in CB CD34+ cells following exposure to FL (1 ng/mL) and SCF (200 pg/ml) (n = 3). (E) Expression of pSTAT3 in AML CD34+ cells (AML 600, 987, and 1050) following FL and SCF exposure (n = 3).
Increased GF receptor expression and GF-mediated JAK/STAT signaling in AML CD34+ cells. (A) Expression of mRNA for growth factor receptors in AML (n = 24) and cord blood (n = 11) CD34+ cells, assessed by quantitative polymerase chain reaction. (B-C) Correlation between (B) FLT3 and (C) c-KIT mRNA expression (log2) and pSTAT3 expression (log2) in AML CD34+ cells. (D) Expression of pSTAT3 in CB CD34+ cells following exposure to FL (1 ng/mL) and SCF (200 pg/ml) (n = 3). (E) Expression of pSTAT3 in AML CD34+ cells (AML 600, 987, and 1050) following FL and SCF exposure (n = 3).
Enhanced JAK/STAT signaling in AML CD34+ cells is dependent on GF receptor expression and signaling
We used siRNA-mediated knockdown to evaluate the contribution of GF receptors to enhanced JAK/STAT signaling in AML CD34+ cells (supplemental Figure 5A). FLT3 and c-KIT knockdown inhibited STAT3/5 phosphorylation in AML CD34+ cells (supplemental Figure 5B-E). A second set of siRNAs targeting different sequences in c-KIT and FLT3 (Figure 7A) also inhibited pSTAT3/5 expression (Figure 7B-C), cell number (Figure 7D), survival (Figure 7E), and colony formation (Figure 7F). These results support an important role for altered GF receptor expression and signaling in enhanced JAK signaling in AML CD34+ cells. Treatment with the FLT3 inhibitor AC220 (20 nM)29 (Figure 7G) and the c-KIT inhibitor Imatinib (5 µM) (Figure 7H) also inhibited FLT3 ligand and SCF-stimulated STAT3/5 activation in AML CD34+ cells, respectively. AC220 and Imatinib also inhibited STAT3/5 activity in AML CD34+ cells cultured with the low GF cocktail (Figure 7I). AZD1480 inhibited acute and sustained GF stimulation with FLT3-stimulated STAT3/5 activation to a similar extent as AC220 and inhibited c-KIT-stimulated STAT3/5 activation to a greater extent than Imatinib. Combined treatment with AZD1480 and AC220, or AZD1480 and Imatinib, did not further inhibit STAT3/5 activity compared with AZD1480 alone. AC220 moderately inhibited survival and colony growth of FLT3-ITD+ AML CD34+ cells (Figure 7J-K), consistent with reports of limited cytotoxicity toward primary FLT3-ITD+ AML cells,30 but did not significantly inhibit FLT3 WT samples (Figure 7L-M). The combination of AC220 and AZD1480 did not further inhibit AML CD34+ cell growth beyond AZD1480 alone. These results suggest that JAK kinases are a critical signaling hub linking GF receptor signaling to growth and survival pathways in AML CD34+ cells, including FLT3-ITD+ AML CD34+ cells.
Inhibition of growth factor receptor expression or signaling reduces JAK/STAT signaling in AML CD34+ cells. (A) AML CD34+ cells (AML 571, 656, and 704) were exposed to 2 different sets of siRNA targeting different sequences in FLT3 (FLT3 [T] and FLT3 [A]) and c-KIT (c-KIT [T] and c-KIT [A]) and control siRNA (n = 3). Expression of target proteins was evaluated by western blotting. (B-C) Expression of pSTAT3/5 in AML CD34+ cells treated with (B) FLT3 and (C) c-KIT siRNA. (D-F) Nucleofected cells were cultured for 72 hours and (D) enumerated and assessed for (E) apoptosis and (F) CFC growth. (G-I) Effect of inhibitor exposure on pSTAT3/5 expression in AML CD34+ cells (AML 028, 519, 545, 707, 755, and 813). (G) Effect of AZD1480 (0.1 µM) and the FLT3 inhibitor AC220 (20 nM) on pSTAT3/5 expression after FLT-3 stimulation (n = 6). (H) Effect of AZD1480 (0.1 µM) and the c-KIT inhibitor Imatinib (5 µM) on pSTAT3/5 expression after SCF stimulation (n = 6). (I) Inhibition of pSTAT3/5 after exposure to AC220, Imatinib, and AZD1480 alone or in combination (n = 6). (J-M) Apoptosis and CFC growth of (J-K) FLT3-ITD+ AML CD34+ cells (n = 4; AML 355, 653, 656, and 868) and (L-M) FLT3 wild-type AML CD34+ cells (n = 4; AML 373, 404, 413, and 666), exposed to AZD1480, AC220, or the combination.
Inhibition of growth factor receptor expression or signaling reduces JAK/STAT signaling in AML CD34+ cells. (A) AML CD34+ cells (AML 571, 656, and 704) were exposed to 2 different sets of siRNA targeting different sequences in FLT3 (FLT3 [T] and FLT3 [A]) and c-KIT (c-KIT [T] and c-KIT [A]) and control siRNA (n = 3). Expression of target proteins was evaluated by western blotting. (B-C) Expression of pSTAT3/5 in AML CD34+ cells treated with (B) FLT3 and (C) c-KIT siRNA. (D-F) Nucleofected cells were cultured for 72 hours and (D) enumerated and assessed for (E) apoptosis and (F) CFC growth. (G-I) Effect of inhibitor exposure on pSTAT3/5 expression in AML CD34+ cells (AML 028, 519, 545, 707, 755, and 813). (G) Effect of AZD1480 (0.1 µM) and the FLT3 inhibitor AC220 (20 nM) on pSTAT3/5 expression after FLT-3 stimulation (n = 6). (H) Effect of AZD1480 (0.1 µM) and the c-KIT inhibitor Imatinib (5 µM) on pSTAT3/5 expression after SCF stimulation (n = 6). (I) Inhibition of pSTAT3/5 after exposure to AC220, Imatinib, and AZD1480 alone or in combination (n = 6). (J-M) Apoptosis and CFC growth of (J-K) FLT3-ITD+ AML CD34+ cells (n = 4; AML 355, 653, 656, and 868) and (L-M) FLT3 wild-type AML CD34+ cells (n = 4; AML 373, 404, 413, and 666), exposed to AZD1480, AC220, or the combination.
Discussion
The current studies rigorously examine the role of JAK signaling in AML LSCs and demonstrate the ability of JAK inhibitors to selectively target AML LSCs while sparing normal stem cells. We show that JAK/STAT signaling is constitutively enhanced in human AML stem and progenitor cells. Small molecule JAK inhibitors significantly reduced AML CD34+ cell growth, survival, and CFC potential in vitro and engraftment in immunodeficient mice in vivo. Moreover, in vivo administration of a JAK inhibitor to immunodeficient mice engrafted with AML cells reduced levels of LSCs capable of regenerating AML cells after secondary transplantation. The effects of JAK inhibitors on AML CD34+ cells were recaptiulated by siRNAs targeting JAK2, STAT3, and STAT5, whereas JAK1 appeared to be less critically important in AML cells. Therefore, several lines of evidence support an important role for JAK2 signaling in maintenance of AML stem/progenitor cells.
Our studies also indicate that increased JAK/STAT activity in AML progenitors is related to increased GF receptor expression and altered GF signaling. Poor risk AML progenitors demonstrate increased expression of FLT3, c-KIT, and IL-3 receptors, which correlates with pSTAT levels and show enhanced and sustained JAK-dependent pSTAT activation following GF stimulation compared with normal progenitors. Inhibition of GF receptor expression using RNA interference or of signaling using small molecule inhibitors reduced pSTAT levels in AML CD34+ cells. JAK inhibitors were at least as effective in blocking GF stimulated pSTAT activation as small molecule GF receptor inhibitors. These observations identify JAK2 as a critical signaling hub linking GF receptors to downstream regulatory pathways in AML LSCs.
Enhanced and sustained pSTAT activation in response to GF stimulation in AML CD34+ cells may suggest impaired negative regulatory mechanisms. One possible explanation may be reduced activity of the serine-threonine protein phosphatase 2 (PP2A), a negative regulator of several kinase cascades in AML CD34+ cells.31,32 Although enhanced JAK signaling may itself contribute to reduced PP2A activity in AML CD34+ cells, additional mechanisms including alterations in PP2A regulatory subunits could also play a role.31 In addition to PP2A, alterations in other negative regulatory genes such as CD45,33 lymphocyte-specific adapter protein (LNK), suppressor of cytokine signaling 1 (SOCS-1), SOCS-6, and Casitas B-lineage lymphoma (CBL) could also contribute to enhanced GF receptor signaling and warrant further evaluation in future.27,34
JAK2 inhibitors and siRNA-mediated knockdown of JAK1/2 and STAT3/5 expression resulted in minimal inhibition of normal CD34+ cells and did not inhibit normal HSCs engrafted in immunodeficient mice, indicating selectively targeting of AML LSCs. Although studies using genetic mouse models show that JAK/STAT signaling is required for normal hematopoiesis, JAK inhibitors may not completely eliminate signaling as occurs in knockout mice. Low levels of JAK signaling may be sufficient to maintain normal cells, which also may be able to compensate through alternative signaling mechanisms. Our results suggest that AML and normal stem cells may require different thresholds of JAK/STAT activity to preserve viability. The increased activity of JAK inhibitors toward AML CD34+ cells may be a consequence of the higher and sustained levels of JAK activity, with remodeling of signaling networks and greater dependence on JAK signaling for survival and growth. Consistent with our results, JAK signaling was identified as a core enriched pathway in AML LSCs.3
Although INC424 demonstrated modest antileukemic activity as a single agent in patients with advanced AML, it is possible that use of JAK inhibitors up front or at higher doses or use of more potent and selective JAK inhibitors could result in greater benefit.24 Despite AZD1480 and INC424 both being putatively selective JAK1 and JAK2 inhibitors, higher concentrations of INC424 had increased inhibitory effects on normal stem/progenitor cells. Selectivity of individual inhibitors toward AML LSCs vs normal stem cells may depend on differences in effects on non-JAK kinases. Although AZD1480 has been withdrawn from clinical trials, our studies support continuing clinical evaluation of other more potent and selective JAK inhibitors in AML. Because AML is a complex disorder resulting from cooperation of multiple molecular abnormalities, a single agent is not expected to be sufficient to effectively treat this disease. Combinations of JAK inhibitors with other treatments directed against cooperating abnormalities in AML LSCs will likely be required. Patient selection will be an important consideration for future studies. Our results suggest that increased baseline STAT3/5 activation status could be a potential biomarker for response to JAK2 inhibition. Our results also support further investigation of JAK inhibitors for targeting of FLT3-mutated AML. The FLT3-ITD mutation is the most common mutation in AML and confers a poor prognosis. Several FLT3 inhibitors are in clinical trials, of which AC220 is the most potent. However, AC220 only modestly inhibits FLT3-ITD CD34+ cell survival and has only moderate clinical activity. FLT3-ITD+ AML CD34+ cells have increased pSTAT expression and increased sensitivity to JAK inhibition. JAK inhibitors may also overcome stroma-mediated drug resistance in FLT3-ITD–mutated AML cells.35 Our laboratory studies using stringent, clinically relevant models provide a strong scientific rationale and impetus for continued investigation of JAK2 inhibition as a therapeutic strategy in AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Dennis Huszar (Astra Zeneca Pharmaceuticals) for supplying AZD1480, StemCyte for CB samples, Dr Min Li for advice regarding statistical analysis, Jennifer Arceo and Linda Seymour for sample collection, and Tinisha McDonald for sample processing. The authors acknowledge the support of the Animal Resources Center and Analytical Cytometry Core.
This work was supported by a grant from the George Hoag Family Foundation, a translational research grant from the Leukemia and Lymphoma Society, and National Institutes of Health, National Cancer Institute grants P30CA033572 and CA172447.
Authorship
Contribution: A.M.C. designed and performed experiments, analyzed and interpreted data, and wrote manuscript; Liang Li designed experiments and interpreted data; Y.H. performed experiments; A.L. acquired samples and patient data; Ling Li performed experiments; A.S. acquired samples and interpreted data; S.F. and D.P. interpreted data; R.J. designed research and interpreted data; and R.B. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ravi Bhatia, Division of Hematopoietic Stem Cell and Leukemia Research, City of Hope National Medical Center, Duarte, CA 91010; e-mail: rbhatia@coh.org.



![Figure 7. Inhibition of growth factor receptor expression or signaling reduces JAK/STAT signaling in AML CD34+ cells. (A) AML CD34+ cells (AML 571, 656, and 704) were exposed to 2 different sets of siRNA targeting different sequences in FLT3 (FLT3 [T] and FLT3 [A]) and c-KIT (c-KIT [T] and c-KIT [A]) and control siRNA (n = 3). Expression of target proteins was evaluated by western blotting. (B-C) Expression of pSTAT3/5 in AML CD34+ cells treated with (B) FLT3 and (C) c-KIT siRNA. (D-F) Nucleofected cells were cultured for 72 hours and (D) enumerated and assessed for (E) apoptosis and (F) CFC growth. (G-I) Effect of inhibitor exposure on pSTAT3/5 expression in AML CD34+ cells (AML 028, 519, 545, 707, 755, and 813). (G) Effect of AZD1480 (0.1 µM) and the FLT3 inhibitor AC220 (20 nM) on pSTAT3/5 expression after FLT-3 stimulation (n = 6). (H) Effect of AZD1480 (0.1 µM) and the c-KIT inhibitor Imatinib (5 µM) on pSTAT3/5 expression after SCF stimulation (n = 6). (I) Inhibition of pSTAT3/5 after exposure to AC220, Imatinib, and AZD1480 alone or in combination (n = 6). (J-M) Apoptosis and CFC growth of (J-K) FLT3-ITD+ AML CD34+ cells (n = 4; AML 355, 653, 656, and 868) and (L-M) FLT3 wild-type AML CD34+ cells (n = 4; AML 373, 404, 413, and 666), exposed to AZD1480, AC220, or the combination.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/18/10.1182_blood-2013-05-505735/4/m_2826f7.jpeg?Expires=1765930373&Signature=p~rmeHnfZGi-6pjUr15TU4FcOdHedLwrjuwb7lBON1hLMiX5fTleCA1UFuUVvDRFTm033J3Wvk2efen8bSuyDo9ryhJUCtGm~26l5Ir9JJEpnMpV-6Kr1-yScqfr-gLCencd1kmExWiqvWS7NhxSBKjl5MBoBUcAX1PhFXmlPPuT8P9j-UgTxB7ZErRJyh-IwnPE3hWw9SMEJ1xRcYtNShsn51HCR8e3QUhajA7TNbc3TsnOeYWEtUnU76sX0qbMGiQ2WX0~HJMKWlhjXIChuQ6ef3jc4abjV7PXKC5Ztf9QVUIhimQzXOuAy0fPIne8rmJ3c-2PpKP9wBP8hTOTjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal