Key Points
Frontline FCA increases progression-free survival in CLL and, in a post hoc analysis, also survival in younger patients.
With the low-dose approach, no increase in treatment related mortality is seen.
Abstract
The randomized Haemato Oncology Foundation for Adults in The Netherlands 68 phase 3 trial compared front-line chemotherapy with chemotherapy plus the CD52 monoclonal antibody alemtuzumab for high-risk chronic lymphocytic leukemia, defined as at least 1 of the following: unmutated immunoglobulin heavy chain genes, deletion 17p or 11q, or trisomy 12. Fit patients were randomized to receive either 6 28-day cycles of oral FC chemotherapy (days 1 through 3: fludarabine 40 mg/m2 per day and cyclophosphamide 250 mg/m2 per day: n = 139) or FC plus subcutaneous alemtuzumab 30 mg day 1 (FCA, n = 133). FCA prolonged the primary end point, progression-free survival (3-year progression-free survival 53 vs 37%, P = .01), but not the secondary end point, overall survival (OS). However, a post hoc analysis showed that FCA increased OS in patients younger than 65 years (3-year OS 85% vs 76%, P = .035). FCA also increased the overall response rate (88 vs 78%, P = .036), and the bone marrow minimal residual disease–negative complete remission rate (64% vs 43%, P = .016). Opportunistic infections were more frequent following FCA, but without an increase in treatment related mortality (FCA: 3.8%, FC: 4.3%). FCA improves progression-free survival in high-risk chronic lymphocytic leukemia. As anticipated, FCA is more immunosuppressive than FC, but with due vigilance, does not lead to a higher treatment-related mortality. This study was registered at www.trialregister.nl as trial no. NTR529.
Introduction
The first-line treatment of chronic lymphocytic leukemia (CLL) is continuously in transition. During the past decade, randomized trials have moved the standard treatment from single alkylating agents to immunochemotherapy with fludarabine (F) plus cyclophosphamide (C) combined with a CD20 antibody for patients without comorbidity.1-5 However, even with such optimal immunochemotherapy, the outcome remains heterogeneous. The prognostic value of clinical stage is limited because the majority of patients, including those who progress later on, present with a low stage. Genomic aberrations6 and immunoglobulin heavy chain (IGHV) gene mutational status7,8 enable a more reliable biological risk stratification across clinical stage.9 Unmutated IGHV genes and deletions of 17p or 11q are high-risk factors and trisomy 12 an intermediate-risk factor. Although patients with 11q deletion, trisomy 12, or unmutated IGHV genes do benefit from optimal (immuno)-chemotherapy, patients with 17p deletion do not.4,5 TP53 deletion and/or mutation remains the worst prognostic factor in CLL.6,10,11
The CD52 antigen is uniformly and strongly expressed on CLL cells, and the CD52 monoclonal antibody alemtuzumab is more effective than rituximab when used as single agent in first-line therapy of CLL,12,13 particularly in CLL with 17p deletion.14,15 Because T lymphocytes and natural killer (NK) cells also express CD52, alemtuzumab leads also to a profound and long lasting T- and NK-cell depletion.16
In 2005, we initiated this trial in patients with high-intermediate risk CLL, exploring the efficacy and safety of the addition of alemtuzumab to F plus C combination chemotherapy, at that time considered to be the most effective treatment of CLL,2 whereas immunochemotherapy was just emerging as promising in phase 2 trials.17,18 The only known alemtuzumab-based immunochemotherapy regimen at that time was fludarabine + alemtuzumab (FluCam)18 in which both agents were given IV on days 1 through 3 every 28 days, alemtuzumab as 30 mg daily. This regimen was highly effective in relapsed or refractory CLL with an overall response rate (ORR) of 83%, but led to severe infections and long-lasting T lymphocytopenia. Furthermore, 2 trials testing alemtuzumab maintenance in CLL patients responding to initial F or FC induction therapy had to be stopped prematurely or amended because of severe opportunistic infections.19,20 Hence, the addition to FluCam of yet another immunosuppressive agent, cyclophosphamide, was a concern. Based on these data, we modified the FluCam regimen to FC oral standard doses days 1 through 3 and alemtuzumab 30 mg day 1 only, except in cycle 1, where we started with an alemtuzumab loading dose, 30 mg days −1 to +1.
Methods
This prospective, randomized, open-label phase 3 trial was performed in 60 centers in 9 countries (see supplemental data on the Blood Web site). Patients with previously untreated CLL diagnosed and in need of treatment according to National Cancer Institute guidelines,21 18 to 75 years of age, with World Health Organization performance status <3 and no severe comorbidities, were eligible if they had high-risk CLL as defined by the presence of either unmutated IGHV (>98% homology to IGHV germline-rearranged genes), 17p deletion, 11q deletion, or trisomy 12 by fluorescent in situ hybridization (FISH) according to the hierarchical method.6 By an amendment in 2008, mutated cases with IGHV 3-21 usage were also considered to have high-risk CLL and were eligible.22 Because of the well-known difficulties in standardization,23,24 ZAP70 expression was not studied in this trial. The main exclusion criteria were: severe organ dysfunction including a creatinine clearance of <30 mL/min, suspected or documented central nervous system involvement by CLL, known seropositivity for HIV, hepatitis B or C (amendment 2008), and other active, uncontrolled infections. Eligible patients were randomly assigned at the Haemato Oncology Foundation for Adults in The Netherlands (HOVON) Data Centre in Rotterdam stratified by center, 17p deletion, and time from diagnosis (< or >2 years), to either standard chemotherapy or immunochemotherapy. All patients provided written informed consent. The trial was performed in accordance with the Declaration of Helsinki and was approved by all relevant ethics committees in the participating countries (Denmark, Finland, Norway, Sweden, The Netherlands, Belgium, Poland, Czech Republic, and Israel). FC chemotherapy consisted of 6 28-day courses of oral fludarabine (40 mg/m2 on days 1 through 3) and oral cyclophosphamide (250 mg/m2 on days 1 through 3). The FCA immunochemotherapy consisted of FC + alemtuzumab 30 mg subcutaneously, in the first cycle at days −1, 0, and +1, in cycles 2 through 6 at day 1 only. Maximum cumulative alemtuzumab dose would be 240 mg. For patients with creatinine clearance between 60 and 30 mL/min, the fludarabine dose should be reduced to 50%: 20 mg/m2 per day. The dose-modification protocol due to myelosuppression can be downloaded at www.hovon.nl.
Prophylaxis was mandatory against Pneumocystis jiroveci pneumonia (PCP) with cotrimoxazole 400/80 mg daily until 3 months after the end of treatment (amended in 2008 to 6 months after the end of treatment) and against herpes infections with acyclovir 400 mg 3 times daily or valacyclovir 500 mg twice daily during and until 3 months after the end of treatment.
Viral monitoring included quantitative polymerase chain reaction (PCR) for cytomegalovirus (CMV) and Epstein-Barr virus every 2 weeks in the FCA patients until 6 months after the end of treatment. For asymptomatic CMV reactivation, valganciclovir 900 mg by mouth twice daily until PCR negativity was recommended without discontinuation of alemtuzumab; symptomatic CMV reactivation was treated with ganciclovir 5 mg/kg IV twice daily until asymptomatic and PCR negative with interruption of alemtuzumab in that period.
Response evaluation
Response assessments were done after cycles 3 and 6, and subsequently every 6 months for 5 years according to National Cancer Institute guidelines, and, following an amendment in 2008, according to the International Workshop on Chronic Lymphocytic Leukemia 2008 guidelines.21,25 Assessments included physical examination; computed tomography scan of the neck, chest, and abdomen; and complete blood counts and biochemistry. Bone marrow examination with histomorphological and flow cytometric assessment was performed at diagnosis and repeated upon reaching clinical complete remission (CR). A CR status could not be attained without bone marrow examination and computed tomography scan.
Flow cytometry
Although the diagnosis could be established by standard 3-color flow cytometry, flow cytometry for minimal residual disease (MRD) (in patients otherwise in CR) should be done according to Böttcher et al.26 Flow cytometric CR was defined as <0.01% CD5/19/23–positive cells in the bone marrow.
Statistical methods
The primary study end point was progression-free survival (PFS) from randomization, defined as progression or relapse after previous response, or death from any cause. In accordance with other trials of CLL treatment,2,5 patients with no response or progressive disease after 3 cycles of induction treatment were included as events in the analysis of PFS. Secondary end points included overall survival (OS); clinical, flow cytometric, and molecular response rates; and toxicity. All outcome durations were measured from the date of randomization unless otherwise indicated. There was no independent review committee for response assessment.
Study assumptions
We assumed that the addition of alemtuzumab to FC would increase the median PFS from 28 to 42 months, corresponding to a relative hazard rate of 0.67. To detect these differences with a power of 80% and a 2-sided significance level of 0.05 would require 196 events to be observed, with inclusion of 300 patients in 3 years and additional follow-up of 3 years. All analyses were performed according to intention to treat. Probabilities of PFS and OS were estimated by the method of Kaplan-Meier and compared using the log-rank test. Cox regression analyses with and without adjustment for risk stratification and prognostic factors were applied to test for differences in PFS and OS between the treatment arms. Hazard ratios with 95% confidence intervals were determined. Differences in response rates including CR, partial response, MRD-negative CR, and ORR between the treatment arms, cytogenetic, and IGHV mutational subgroups were calculated using χ-square tests. The treatment effects in subgroups were explored in post hoc analyses by comparing the subgroup PFS and OS probabilities at 3 years, estimating the hazard ratios with 95% confidence intervals (CI) and performing tests, including Cox regression analyses, for interactions with treatment arm. The subgroups included genomic aberrations, IGHV mutational status, β2-microglobulin, clinical stage, sex, and age. In view of recent important reports of substantial differences in response to immunotherapy and PFS between younger and older patients,27 the unplanned post hoc analysis included the outcomes of patients <65 and ≥65 years of age, respectively. Differences in the number of occurrences of grade 3 or higher serious adverse events (SAE) were compared using a Kruskal-Wallis test. In all analyses, P values < .05, 2-sided, were considered statistically significant.
Results
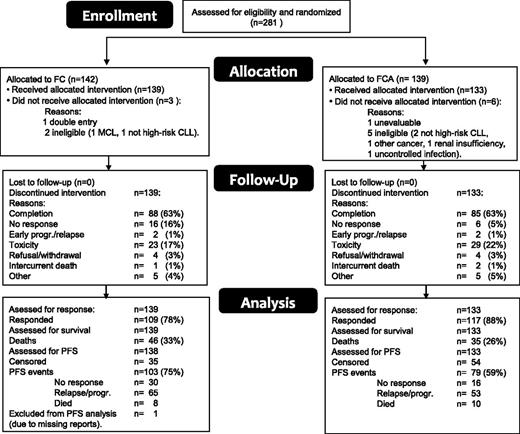
Figure 1 is a CONSORT diagram of the patient flow. A total of 281 patients were included between 2006 and 2010 and were randomly assigned to receive either FC or FCA. Nine patients (6 FCA, 3 FC) were subsequently excluded because of noneligibility (7), double entry (1), or nonevaluability (1); as a result, 272 patients were eligible and evaluated. At closure of the database (February 3, 2013), 191 were still alive with a median follow-up of 42.8 months (range 4-81.6). The reasons for going off the study included completion of treatment (173), unresponsive or early progression/relapse of CLL (26), toxicity (52), refusal/withdrawal (8), intercurrent death (3), and other reasons (10). One patient in the FC arm was not evaluable for PFS because of missing reports and was excluded from the PFS analysis only. Table 1 shows the patient characteristics according to treatment arm. Both treatment groups were well balanced regarding sex, age, performance status, stage, genomic aberrations, IGHV mutational status, and β2-microglobulin. Data regarding IGHV 3-21 usage were available in 126 patients and were found in 28. There was no difference in PFS between patients with and without IGHV 3-21 usage (P = .73).
Patient characteristics (total 281 included, 9 patients excluded later)
| . | n (%) . | n (%) . | Total . |
|---|---|---|---|
| . | FC (139) . | FCA (133) . | 272 . |
| Male sex | 103 (74) | 100 (75) | 203 |
| Age <65 y | 105 (76) | 98 (74) | 203 |
| Age >65 y | 34 (24) | 35 (26) | 69 |
| WHO perf. status 0 | 89 (64) | 78 (59) | 167 |
| WHO perf. status 1 | 46 (33) | 49 (37) | 95 |
| WHO perf. status 2 | 3 (2) | 4 (3) | 7 |
| Stage A | 17 (12) | 13 (10) | 30 |
| Stage B | 74 (53) | 75 (56) | 149 |
| Stage C | 48 (35) | 45 (34) | 93 |
| 17p deletion | 18 (13) | 14 (11) | 32 |
| 11q deletion | 38 (27) | 30 (23) | 68 |
| Trisomy 12 | 23 (17) | 34 (26) | 57 |
| 13q deletion | 24 (9) | 24 (18) | 48 |
| No FISH aberrations | 29 (21) | 18 (14) | 47 |
| Missing FISH results regarding | |||
| Del17p | 1 (1) | 1 (1) | 2 |
| Del11q | — | 1 (1) | 1 |
| +12 | 14 (10) | 18 (14) | 32 |
| Unmutated IGHV | 113 (81) | 104 (78) | 217 |
| IGHV status not done | 17 (13) | 6 (5) | 23 |
| Β2-microglobulin >3.5 mg/L | 62 (45) | 62 (47) | 124 |
| Β2-microglobulin not done | 29 (21) | 22 (17) | 51 |
| . | n (%) . | n (%) . | Total . |
|---|---|---|---|
| . | FC (139) . | FCA (133) . | 272 . |
| Male sex | 103 (74) | 100 (75) | 203 |
| Age <65 y | 105 (76) | 98 (74) | 203 |
| Age >65 y | 34 (24) | 35 (26) | 69 |
| WHO perf. status 0 | 89 (64) | 78 (59) | 167 |
| WHO perf. status 1 | 46 (33) | 49 (37) | 95 |
| WHO perf. status 2 | 3 (2) | 4 (3) | 7 |
| Stage A | 17 (12) | 13 (10) | 30 |
| Stage B | 74 (53) | 75 (56) | 149 |
| Stage C | 48 (35) | 45 (34) | 93 |
| 17p deletion | 18 (13) | 14 (11) | 32 |
| 11q deletion | 38 (27) | 30 (23) | 68 |
| Trisomy 12 | 23 (17) | 34 (26) | 57 |
| 13q deletion | 24 (9) | 24 (18) | 48 |
| No FISH aberrations | 29 (21) | 18 (14) | 47 |
| Missing FISH results regarding | |||
| Del17p | 1 (1) | 1 (1) | 2 |
| Del11q | — | 1 (1) | 1 |
| +12 | 14 (10) | 18 (14) | 32 |
| Unmutated IGHV | 113 (81) | 104 (78) | 217 |
| IGHV status not done | 17 (13) | 6 (5) | 23 |
| Β2-microglobulin >3.5 mg/L | 62 (45) | 62 (47) | 124 |
| Β2-microglobulin not done | 29 (21) | 22 (17) | 51 |
FC, fludarabine and cyclophosphamide; PR, Partial remission; WHO perf., World Health Organization performance.
Dose reductions and delays
In the FC arm, F was reduced in 20.1% and C in 15.6% of the cycles (supplemental Table 5A-B). In the FCA arm, F was reduced in 13.1%, C in 12.4%, and alemtuzumab in 5.3% of the cycles. Although F reductions occurred significantly more often in the FC arm (P = .0007), the median cumulative dose of F in the FC arm (1182 mg) was only 6% lower than in the FCA arm (1260 mg). Dose reductions occurred evenly over all 6 cycles, so higher adherence to protocol treatment in the FCA arm was apparently not due to the higher response rate in that arm. Delays occurred in about 10% of cycles with no difference between arms (supplemental Table 5B).
Efficacy
Table 2 shows the responses to treatment. The ORR (PR + CR) was significantly higher after FCA than FC (88% vs 78%, P = .036), whereas only a trend of increased CR rate was found (53% vs 42%, P = .071). Of the 130 patients in CR, flow cytometric assessment of the bone marrow was done in 126 (97%). Forty-five of 70 FCA (64%) and 24/56 FC (43%) patients achieved flow cytometric CR (P = .016). In the subgroups with 17p deletion, 11q deletion, trisomy 12, and mutated/unmutated IGHV, respectively, no significant differences in ORR or CR rates were found.
Response to treatment
| . | n (%) . | n (%) . | . |
|---|---|---|---|
| . | FC 139 (100) . | FCA 133 (100) . | P . |
| Overall response (CR + PR) | 109 (78) | 117 (88) | .036 |
| CR | 59 (42) | 71 (53) | .071 |
| CR by flow cytometry | 24/56 (43) | 45/70 (64) | .016 |
| PR | 50 (36) | 46 (35) | NS |
| Stable disease | 20 (14) | 9 (7) | NS |
| Progressive disease | 3 (2) | 1 (1) | NS |
| Unknown | 7 (5) | 6 (5) | NS |
| Response (CRR/ORR%) in subgroups | |||
| 17p deletion | 22/44 | 21/71 | .165 |
| 11q deletion | 21/79 | 43/90 | .323 |
| Trisomy 12 | 57/87 | 71/94 | .384 |
| IGHV unmutated | 44/81 | 56/88 | .263 |
| . | n (%) . | n (%) . | . |
|---|---|---|---|
| . | FC 139 (100) . | FCA 133 (100) . | P . |
| Overall response (CR + PR) | 109 (78) | 117 (88) | .036 |
| CR | 59 (42) | 71 (53) | .071 |
| CR by flow cytometry | 24/56 (43) | 45/70 (64) | .016 |
| PR | 50 (36) | 46 (35) | NS |
| Stable disease | 20 (14) | 9 (7) | NS |
| Progressive disease | 3 (2) | 1 (1) | NS |
| Unknown | 7 (5) | 6 (5) | NS |
| Response (CRR/ORR%) in subgroups | |||
| 17p deletion | 22/44 | 21/71 | .165 |
| 11q deletion | 21/79 | 43/90 | .323 |
| Trisomy 12 | 57/87 | 71/94 | .384 |
| IGHV unmutated | 44/81 | 56/88 | .263 |
CRR, complete response rate; NS, not significant.
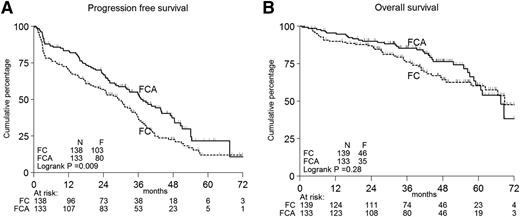
Figure 2A-B shows the PFS and OS according to treatment in all patients. Per protocol analysis, the primary end point PFS was significantly longer in FCA-treated patients: 3-year PFS 53% vs 37%, median PFS 37 months vs 29 months, P < .01 (Figure 2A). The secondary end point, survival, did not differ (Figure 2B). PFS based on true progression only gave identical results (supplemental Figure 4).
Kaplan-Meier curves. Progression-free (A) and OS (B) according to treatment in all patients. F, events found; N, numbers.
Kaplan-Meier curves. Progression-free (A) and OS (B) according to treatment in all patients. F, events found; N, numbers.
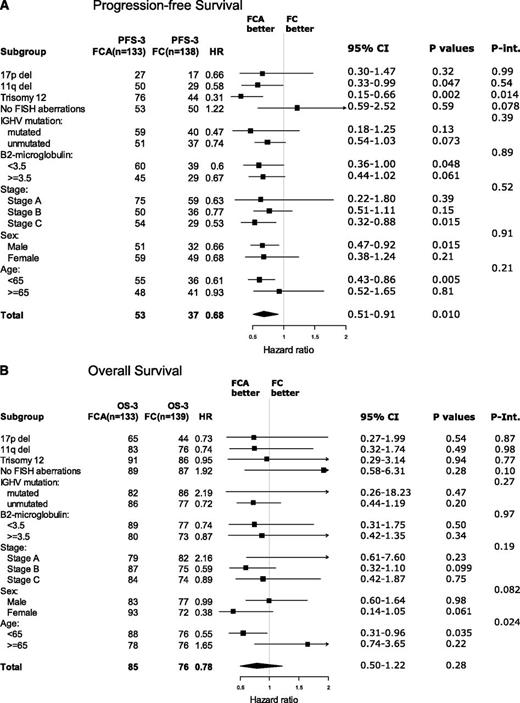
Figure 3A-B shows forest plots of post hoc analyses of the outcome in subgroups according to treatment. Regarding PFS, FCA was associated with a significantly longer PFS than FC in patients with trisomy 12, stage C, males, and age younger than 65 years. However, in interaction analysis, only the interaction between treatment arm and trisomy 12 remained significant, in which FCA prolonged the 3-year PFS from 44% to 76% (P = .014) (supplemental Figure 5C).
Forest plots. Three-year PFS (A) and OS (B) according to treatment in prognostic subgroups. Int., interaction.
Forest plots. Three-year PFS (A) and OS (B) according to treatment in prognostic subgroups. Int., interaction.
Regarding OS, the only significant interaction with treatment arm was age. In patients younger than 65 years, FCA prolonged the 3-year survival from 76% to 88% (P = .035). In contrast, in patients 65 years or older, FCA did not prolong OS or PFS (supplemental Figure 6A-C). When searching for an explanation to this finding, no other significant subgroup results arose. The patient characteristics (Table 1) were also evenly distributed in the elderly. A trend of shorter survival in elderly patients with opportunistic infections may be part of the explanation for this finding.
The outcome between patients with mutated and unmutated IGHV genes did not differ because patients with mutated IGHV could only be included if they had other unfavorable characteristics (unfavorable genomic aberrations or IGHV 3-21).
The results of post hoc Cox regression analyses of the impact of the various factors including treatment arm on PFS and OS are shown in Table 3 (multivariate) and supplemental Table 6 (univariate). In univariate PFS analysis, FCA treatment (P = .01), 17p deletion (P < .001), trisomy 12 (P = .007), 11q deletion (P < .041), and increased β2 microglobulin levels (P = .028) were significant, whereas in multivariate PFS analysis only treatment with FCA (P = .016) and 17p deletion (P = .004) retained independent significance. In univariate OS analysis, 17p deletion, increased β2-microglobulin, and younger age were significant, but in multivariate OS analysis only 17p deletion retained independent significance.
Cox regression multivariate analysis of the effects of some prognostic variables on PFS and OS in 272 patients
| . | PFS . | OS . | ||
|---|---|---|---|---|
| Variable . | Hazard ratio (95% CI) . | P value . | Hazard ratio (95% CI) . | P value . |
| Treatment arm | 0.65 (0.46-0.92) | .016 | 0.75 (0.44-1.27) | .284 |
| Age ≥65 y | 1.09 (0.73-1.61) | .681 | 1.42 (0.82-2.45) | .213 |
| 17p deletion | 2.39 (1.32-4.33) | .004 | 3.25 (1.45-7.31) | .004 |
| 11q deletion | 1.58 (0.95-2.62) | .077 | 1.38 (0.64-2.98) | .406 |
| Trisomy 12 | 0.78 (0.44-1.38) | .399 | 0.65 (0.27-1.61) | .336 |
| β2 microglobulin ≥3.5 | 1.21 (0.83-1.76) | .333 | 1.71 (0.94-3.11) | .078 |
| . | PFS . | OS . | ||
|---|---|---|---|---|
| Variable . | Hazard ratio (95% CI) . | P value . | Hazard ratio (95% CI) . | P value . |
| Treatment arm | 0.65 (0.46-0.92) | .016 | 0.75 (0.44-1.27) | .284 |
| Age ≥65 y | 1.09 (0.73-1.61) | .681 | 1.42 (0.82-2.45) | .213 |
| 17p deletion | 2.39 (1.32-4.33) | .004 | 3.25 (1.45-7.31) | .004 |
| 11q deletion | 1.58 (0.95-2.62) | .077 | 1.38 (0.64-2.98) | .406 |
| Trisomy 12 | 0.78 (0.44-1.38) | .399 | 0.65 (0.27-1.61) | .336 |
| β2 microglobulin ≥3.5 | 1.21 (0.83-1.76) | .333 | 1.71 (0.94-3.11) | .078 |
Safety
Table 4 shows the number of SAEs (grade 3 or higher) according to treatment arm. In the FC arm, 48/139 patients experienced 80 treatment-related SAEs. In comparison, in the FCA arm, 68/133 patients experienced 118 treatment-related SAEs (P = .0057). In addition, 8 FC patients went off study because of hematological or unspecified toxicity not recorded as SAEs. Although the total number of serious infections (neutropenic, non-neutropenic, and opportunistic together) did not differ significantly, the number of opportunistic infections was 2.5-fold higher among patients receiving FCA (P = .002). Among the 133 FCA patients, CMV reactivation occurred in 66 (50%). Except in 4 patients, these CMV reactivations were asymptomatic. Symptomatic CMV disease also occurred in 1 FC patient. In all cases, CMV reactivation resolved upon antiviral treatment. Other opportunistic infections mainly observed in FCA patients included PCP, mycobacterial infection, hepatitis B virus reactivation, Epstein-Barr virus reactivation, and 1 case of progressive multifocal leukoencephalopathy. A higher number of late, often infectious, adverse events occurred after FCA than after FC: for example, 18 vs 5 events >1 year after end of treatment including late PCP in 3 FCA- and 1 FC-treated patients. When this trend was noted, the PCP prophylaxis was extended from 3 to 6 months after the end of treatment (amendment 1 2008). Importantly, the number of deaths from infection did not differ: 5 after FC and 6 after FCA. The cumulative treatment-related mortality of the FC and FCA patients was 6/139 (4.3%) and 5/133 (3.8%), respectively (Table 4). However, among patients ≥65 years of age, a trend was noted of a shorter OS among patients with opportunistic infections (P = .07) (data not shown).
Incidence of severe adverse events (≥grade 3) per treatment arm
| Treatment arm . | FC (139) . | FCA (133) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade . | All . | 3 . | 4 . | 5 . | All . | 3 . | 4 . | 5 . | P . |
| Infections, all: | 70 | 63 | 2 | 5 | 81 | 67 | 8 | 6 | NS |
| Neutropenic | 28 | 24 | 2 | 2 | 26 | 25 | 1 | 0 | NS |
| Non-neutropenic | 31 | 31 | 0 | 0 | 27 | 21 | 3 | 3 | NS |
| Opportunistic | 11 | 8 | 0 | 3 | 28 | 21 | 4 | 3 | .002 |
| Organ-related | 13 | 10 | 2 | 1 | 36 | 33 | 1 | 2 | .0005 |
| Flulike syndrome | 3 | 3 | 0 | 0 | 27 | 27 | 0 | 0 | <.0001 |
| Allergy | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| Hemorrhage | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | NS |
| Other cancer | 2 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | NS |
| Total | 91 | 80 | 4 | 7 | 147 | 128 | 10 | 9 | <.0001 |
| Total related | 80 | 70 | 4 | 6 | 118 | 104 | 9 | 5 | <.0001 |
| Patients affected | 53 | 82 | .0001 | ||||||
| Patients affected by related SAEs | 48 | 68 | .0057 | ||||||
| Treatment arm . | FC (139) . | FCA (133) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade . | All . | 3 . | 4 . | 5 . | All . | 3 . | 4 . | 5 . | P . |
| Infections, all: | 70 | 63 | 2 | 5 | 81 | 67 | 8 | 6 | NS |
| Neutropenic | 28 | 24 | 2 | 2 | 26 | 25 | 1 | 0 | NS |
| Non-neutropenic | 31 | 31 | 0 | 0 | 27 | 21 | 3 | 3 | NS |
| Opportunistic | 11 | 8 | 0 | 3 | 28 | 21 | 4 | 3 | .002 |
| Organ-related | 13 | 10 | 2 | 1 | 36 | 33 | 1 | 2 | .0005 |
| Flulike syndrome | 3 | 3 | 0 | 0 | 27 | 27 | 0 | 0 | <.0001 |
| Allergy | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| Hemorrhage | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | NS |
| Other cancer | 2 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | NS |
| Total | 91 | 80 | 4 | 7 | 147 | 128 | 10 | 9 | <.0001 |
| Total related | 80 | 70 | 4 | 6 | 118 | 104 | 9 | 5 | <.0001 |
| Patients affected | 53 | 82 | .0001 | ||||||
| Patients affected by related SAEs | 48 | 68 | .0057 | ||||||
Also significantly, more organ-related events were found after FCA, notably vascular and neurological events (Table 4) without, however, any obvious relationship to occult infection. Bone marrow toxicity was comparable in the 2 treatment arms. Organ affection did not influence survival.
Discussion
We report here on the efficacy and safety of the addition of FCA as first-line treatment of fit patients with high-risk CLL. FCA resulted in a significant improvement of PFS, the primary trial end point, and—as shown in a post hoc analysis—an increase in OS in patients younger than 65 years of age. In patients with trisomy 12, FCA was beneficial independently of other factors. However, even with the low-dose alemtuzumab used in our study, FCA also led to an increased incidence of opportunistic infections, but not to an increased treatment-related mortality compared with FC alone and with that reported following FC with rituximab.5,27
When our study was launched, FC had just been established as the standard chemotherapy of CLL based on results of trials not even published at that time,2-4 whereas now the treatment of choice in fit CLL patients is immunochemotherapy with FC and rituximab (FCR).5 Our FCA results are in agreement with other first-line immunochemotherapy trials in CLL (supplemental Table 7) and with a trial of single-agent alemtuzumab used at much higher doses (90 mg/wk for up to 12 weeks).28 The FCR regimen was recently compared with bendamustine plus rituximab.27 Whereas in patients younger than 65 years, FCR continues to be the standard recommendation, in older patients the higher efficacy of FCR was outbalanced by a higher rate of severe infections; overall, the treatment-related mortality rates were 3.9% after FCR and 2.1% after bendamustine plus rituximab. There is no simple explanation to our observation in a post hoc analysis of a lack of benefit from FCA in elderly patients. Also among the elderly there were significantly more SAEs in the FCA arm, mainly from affected organs and opportunistic infection; the latter was associated with a trend of shorter OS, a finding that may have contributed to the lack of a survival benefit from FCA in elderly patients.
The trial was not powered for subgroup analysis, but the outcome according to genomic aberrations and treatment (supplemental Figure 5A-C) suggests that FCA is effective in most subgroups that constituted our high-risk category (11q deletion, trisomy 12, and unmutated CLL), but least in patients with 17p deletion. Thus, the poor prognosis associated with 17p deletion could not be overcome by low-dose alemtuzumab–based immunochemotherapy. Interestingly, though, in a recent analysis of a subset of HOVON68 patients in which TP53 mutations were also included, a trend was noted that FCA improved ORR in patients with TP53 mutations (14% vs 67%, P = .06).29
The clear benefit of FCA in our trisomy 12 patients is remarkable. Trisomy 12 has been considered both an intermediate- and a high-risk feature,6,30 but the mechanism behind its clinical impact is unknown. Recently, an association between trisomy 12 and NOTCH1 mutations has been observed,11,31 indicating that trisomy 12 patients with NOTCH1 mutations have a worse prognosis than those without. We have confirmed this association in an analysis of a subgroup of the HOVON68 patients.29 Recent data suggest that in patients harboring NOTCH1 mutations, alemtuzumab but not rituximab leads to longer PFS than in patients with wild-type NOTCH1.32,33 Identification of NOTCH1 and other novel somatic mutations may soon become standard as part of prognosis prediction in CLL,11 and FCA or alemtuzumab—depending on the presence of bulky disease or not—should be relevant to consider in the trisomy 12/Notch1 mutated subgroup.
Our CR rates after both FC (42%) and FCA (53%) markedly exceed not only the study assumption of 20% and 40%, respectively, but also the reported CR rates of other trials, including the CLL8 trial5,28,34,35 (supplemental Table 7). This is remarkable because we have selected for high-risk CLL patients. Possibly because of the high CR rate with FC alone, the increment in CR after FCA did not reach statistical significance. Notably, even after FC alone, almost half (43%) of the CRs were MRD-negative as assessed by flow cytometry. FC given orally is at least as effective as when given IV.36,37 Our high efficacy of FC was achieved despite dose reductions, but compared with the CLL8 study,5 the median cumulative doses of F and C (F corrected for the different route of administration) did not differ.
Two studies using a higher dose of alemtuzumab (90 mg/cycle) in combination with FC reached seemingly conflicting results. With FCA + rituximab, a CR rate of 70% was achieved, and, notably, in the 17p deletion subgroup, 57% CR (95% CI 44-70%).34 In the Multi-Center Phase II Efficacy and Pharmacokinetic Study Evaluating Fludarabine, Cyclophosphamide, and Subcutaneous Campath (FCCam) vs FCR study,35 only 19% CR was obtained in the FCCam arm (95% CI 11-30%), probably because of early study termination resulting from FCCam-induced toxicity. Other trials of F combined with alemtuzumab at a dose of 90 mg per cycle38 as well as of alemtuzumab maintenance 90 mg weekly in patients responding to F or FC19,20 have been amended or closed prematurely because of excessive infection-related mortality. In contrast, a trial combining low-dose alemtuzumab (30 mg weekly) with FC in relapsed or refractory CLL had no safety problems.39 Concerned by these early observations, we chose to reduce the alemtuzumab dose, well aware of potentially jeopardizing efficacy. On the whole, however, this proved to be the right balance, and the increased PFS and OS were not obtained at the cost of higher mortality. Our treatment-related mortalities of 4.3% and 3.8% after FC and FCA, respectively, are in line with the safety data of otherwise comparable non-alemtuzumab trials (supplemental Table 7).5,27,36 Still, even with low-dose alemtuzumab, FCA was clearly much more immunosuppressive than FC and resulted in a significant increase in opportunistic infections, in line with a second-line trial of alemtuzumab plus fludarabine without cyclophosphamide.40 Our rate of CMV reactivation is similar to that of alemtuzumab single-agent treatment at much higher doses.28 In principle, the pharmacokinetics of alemtuzumab, when given concomitantly with or just after effective chemotherapy, differs from that when given alone, where a clear dose-response effect can be seen.41 In combination with chemotherapy, however, the more effective this treatment, the fewer residual tumor target cells are left to bind the antibody, and the more antibody available for binding to and inhibiting normal immune cells.42 This also explains why infections occur particularly in responders19,20 and why some opportunistic infections occur remarkably late, in line with the reportedly long periods of reduced T- and NK-cell counts following alemtuzumab.16 Clearly, the combination even of low-dose alemtuzumab with chemotherapy demands a strong vigilance for infection, including regular viral monitoring and preemptive antiviral therapy, and—even more than a year after end of treatment—a high awareness of possibly persisting immunosuppression.
In conclusion, we confirm that immunochemotherapy with low-dose alemtuzumab and FC is superior to FC chemotherapy alone in younger, fit patients with high-risk CLL, where it prolongs both PFS and OS. With proper awareness as to infections, it is also safe. Our low-dose approach probably represents the most feasible balance in FC-based immunochemotherapy with alemtuzumab, and clearly illustrates the limitations of the use of this antibody because of immunosuppression. Despite these limitations, this and other trials32,43 confirm the value of this antibody in CLL and even though other compounds (eg, Bruton’s kinase inhibitors)44 are emerging with promising activity in high-risk CLL, alemtuzumab should keep its place in the CLL armamentarium and may even be combined with such emerging new drugs.
Presented in abstract form at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, December 10-13, 2011, and the XV International Workshop on CLL, Cologne, Germany, September 8-11, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Independent Safety Monitoring Committee, which was convened by the authors and included professors Peter Hillmen, Stefan Stilgenbauer, and Harald Anderson. The members of the committee have no financial interest in the trial. The authors also thank all patients and colleagues for their participation in the study, Fokje Spoelstra for excellent data management, and Wim van Putten for excellent statistical contribution during the planning of the trial.
This work was supported by the Schering and Genzyme corporations and by The Danish Cancer Society, the Novo Nordisk Foundation, and the Research Board of Rigshospitalet (C.H.G.).
Authorship
Contribution: C.H.G., M.H.J.v.O., M.B.v.t.V., J.J., J.W., G.T., M.I.R., E.K., T.K., and A.P. designed the trial, treated the patients, collected the data, and all except J.J. wrote the paper; K.L.W., S.W., and J.D. treated patients and wrote the paper; M.C.J.A.-T. managed the data and reviewed the paper; and W.G.A. was responsible for the statistical analyses and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian H. Geisler, Rigshospitalet, Department of Hematology 4042, 9 Blegdamsvej, DK2100 Copenhagen, Denmark; e-mail: christian.geisler@regionh.dk.