Key Points
When targeted to a single allele of the AAVS1 locus, the Gp1ba promoter drives a high level of expression specifically to megakaryocytes.
Transgene rescue in iPSCs provides a model for the return of surface αIIbβ3 expression to near-normal levels in patients with type I GT.
Abstract
Megakaryocyte-specific transgene expression in patient-derived induced pluripotent stem cells (iPSCs) offers a new approach to study and potentially treat disorders affecting megakaryocytes and platelets. By using a Gp1ba promoter, we developed a strategy for achieving a high level of protein expression in human megakaryocytes. The feasibility of this approach was demonstrated in iPSCs derived from two patients with Glanzmann thrombasthenia (GT), an inherited platelet disorder caused by mutations in integrin αIIbβ3. Hemizygous insertion of Gp1ba promoter-driven human αIIb complementary DNA into the AAVS1 locus of iPSCs led to high αIIb messenger RNA and protein expression and correction of surface αIIbβ3 in megakaryocytes. Agonist stimulation of these cells displayed recovery of integrin αIIbβ3 activation. Our findings demonstrate a novel approach to studying human megakaryocyte biology as well as functional correction of the GT defect, offering a potential therapeutic strategy for patients with diseases that affect platelet function.
Introduction
Inherited platelet disorders (IPDs) can be associated with a bleeding diathesis that may be life threatening. Individually rare, in aggregate, these disorders are estimated to affect 1 in 10 000 individuals.1 Glanzmann thrombasthenia (GT) is a rare, autosomal recessive disease resulting in the lack of functional αIIbβ3, leading to impaired platelet aggregation and bleeding.2 Therapy for patients with GT is limited, and patients suffer from lifelong, recurrent mucocutaneous bleeding that can be life threatening.3 Platelet transfusions are an effective therapy, but run the risk of alloimmunization.4 A few patients have undergone allogeneic bone marrow transplantation with its attendant complications.5 Gene therapy holds promise for a cure for GT and other IPDs. A recent study in an αIIb-deficient canine model for GT showed improved hemostasis with modest restoration of platelet surface αIIbβ3 by lentiviral transduction of mobilized hematopoietic stem cells.6 Here we describe the use of a targeted and specific transgenic construct that leads to a high level of expression of a gene of interest in megakaryocytes. We have used this system to restore the surface expression and function of integrin αIIbβ3 in induced pluripotent stem cell (iPSC)–derived megakaryocytes from patients with GT. Our results demonstrate an alternative corrective strategy for IPDs using GT as a model.
Study design
Peripheral blood mononuclear cells were collected from two patients with type 1 GT (GTP1 and GTP2), both having mutations in ITGA2B, and from a healthy control (control 1) and were reprogrammed as previously described.7 A second iPSC control line (control 2) was generated from healthy CD34+ bone marrow cells.8 All iPSC lines were created by using the doxycycline-regulated human stem cell cassette lentivirus expressing OCT4, SOX2, KLF4, and MYC9 and were analyzed for pluripotency by teratoma formation, flow cytometry, and quantitative gene expression (supplemental Figure 1, available on the Blood Web site). The Gp1ba promoter construct10 driving either a eukaryotic green fluorescent protein (eGFP) reporter or αIIb complementary DNA (cDNA)11 was cloned into the pAAVS1-SA-2A-puro-pA donor plasmid and inserted into the AAVS1 locus of iPSCs using zinc finger nuclease–mediated homologous recombination12 (supplemental Figure 2A-B). Targeted iPSCs were selected for further studies using puromycin resistance driven by the endogenous PPP1R12C promoter.12 Hemizygous insertions were confirmed by using Southern blot analysis12,13 (supplemental Figure 2B-C). iPSCs were differentiated into hematopoietic progenitor cells (HPCs) and megakaryocytes by using a previously described protocol14 and were analyzed for both quantitative gene expression and surface marker expression. The functionality of agonist-stimulated megakaryocytes was evaluated by flow cytometry using PAC-1 antibody,15 which binds specifically to the active conformation of integrin αIIbβ3 and fluorochrome-conjugated fibrinogen.16 Details regarding the methods are provided in the supplemental Methods. The study presented in this article was approved by the Children’s Hospital of Philadelphia Institutional Review Board and the Institutional Animal Care and Use Committee and the Children’s Hospital of Wisconsin Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Results and discussion
To achieve a high level of expression during megakaryopoiesis, we used a murine Gp1ba promoter construct (Figure 1A) previously shown by our group to express platelet-derived factor VIII in a transgenic mouse model.10 The Gp1ba expression cassette was inserted into a single allele of the AAVS1 locus in human iPSCs using site-specific zinc finger nucleases, a reliable and efficient targeting strategy for stable transgene expression.12,17 Figure 1 shows the initial characterization of this approach by analyzing Gp1ba promoter-driven eGFP expression in iPSCs from a normal individual. Hemizygous targeted iPSCs were differentiated into hematopoietic lineages as described.14 Pluripotent stem cells and mesodermal cells showed minimal eGFP expression by flow cytometry (Figure 1B). Surprisingly, αIIb (CD41+)/glycophorin A (CD235a+) HPCs showed eGFP expression but, were negative for surface GPIbα (CD42b) expression (data not shown). Terminal differentiation of these cells into CD41+/CD42+ megakaryocytes showed an approximate 10-fold increase in eGFP expression, while erythrocytes and myeloid cells downregulated eGFP (Figure 1C-D).
The Gp1ba promoter drives high-level expression in iPSC-derived megakaryocytes. (A) Schematic of the targeting construct. The Gp1ba expression construct (gray window) contains the murine proximal Gp1ba promoter linked to the Simian virus 40 (SV40) 5′ untranslated region (UTR) followed by a cDNA of interest (eGFP shown) followed by the SV40 3′ UTR.10 After insertion into the puromycin (puro) containing targeting plasmid (pAAVS1-SA-2A-puro-pA), the entire transgene is inserted into the AAVS1 locus (PPP1R12C intron 1) using zinc finger nuclease–mediated homologous recombination. After insertion, the endogenous PPP1R12C promoter drives puromycin resistance (gray arrow), while the Gp1ba promoter drives the transgenic message (black arrow) (SA, splice acceptor; 2A, self-cleaving peptide; pA, bovine growth hormone polyadenylation signal). (B) Control 2 with hemizygous insertion of the Gp1ba promoter driving eGFP was examined by flow cytometry during hematopoietic differentiation. (Left) Representative plots of SSEA3+/SSEA4+ iPSCs, KDR+/CD31+ mesoderm, and CD41+/CD235+ HPCs. (Right) Histograms of gated populations on left (gray rectangles) examine eGFP expression in iPSCs, mesoderm, and HPCs. (C) (Left) Representative flow cytometry plots of iPSC-derived CD45+/CD18+ myeloid cells, CD41–/CD235+ erythroid cells, and CD41+/CD42+ megakaryocytes. (Right) Histograms of gated populations on left (gray rectangles) examine eGFP expression in iPSCs, myeloid cells, erythroid cells, and megakaryocytes. (D) Mean fluorescence intensity (MFI) of eGFP expression in iPSCs, mesodermal cells (meso), HPCs, and megakaryocytes (megs). (E) Comparison of gene expression by quantitative reverse transcriptase polymerase chain reaction of endogenous GP1BA, GP1BB, and GP9 in iPSC-derived HPCs and megakaryocytes relative to cyclophilin (mean ± standard error of the mean [SEM] for 3 replicates).
The Gp1ba promoter drives high-level expression in iPSC-derived megakaryocytes. (A) Schematic of the targeting construct. The Gp1ba expression construct (gray window) contains the murine proximal Gp1ba promoter linked to the Simian virus 40 (SV40) 5′ untranslated region (UTR) followed by a cDNA of interest (eGFP shown) followed by the SV40 3′ UTR.10 After insertion into the puromycin (puro) containing targeting plasmid (pAAVS1-SA-2A-puro-pA), the entire transgene is inserted into the AAVS1 locus (PPP1R12C intron 1) using zinc finger nuclease–mediated homologous recombination. After insertion, the endogenous PPP1R12C promoter drives puromycin resistance (gray arrow), while the Gp1ba promoter drives the transgenic message (black arrow) (SA, splice acceptor; 2A, self-cleaving peptide; pA, bovine growth hormone polyadenylation signal). (B) Control 2 with hemizygous insertion of the Gp1ba promoter driving eGFP was examined by flow cytometry during hematopoietic differentiation. (Left) Representative plots of SSEA3+/SSEA4+ iPSCs, KDR+/CD31+ mesoderm, and CD41+/CD235+ HPCs. (Right) Histograms of gated populations on left (gray rectangles) examine eGFP expression in iPSCs, mesoderm, and HPCs. (C) (Left) Representative flow cytometry plots of iPSC-derived CD45+/CD18+ myeloid cells, CD41–/CD235+ erythroid cells, and CD41+/CD42+ megakaryocytes. (Right) Histograms of gated populations on left (gray rectangles) examine eGFP expression in iPSCs, myeloid cells, erythroid cells, and megakaryocytes. (D) Mean fluorescence intensity (MFI) of eGFP expression in iPSCs, mesodermal cells (meso), HPCs, and megakaryocytes (megs). (E) Comparison of gene expression by quantitative reverse transcriptase polymerase chain reaction of endogenous GP1BA, GP1BB, and GP9 in iPSC-derived HPCs and megakaryocytes relative to cyclophilin (mean ± standard error of the mean [SEM] for 3 replicates).
Normally, GPIbα surface expression during hematopoiesis is limited to maturing megakaryocytes as part of a complex requiring coexpression of GPIbβ and GPIX.18 When iPSC-derived HPCs and megakaryocytes were analyzed for messenger RNA (mRNA) expression, endogenous GPIbα and GPIX were expressed in HPCs, while endogenous GPIbβ was expressed at low levels (Figure 1E and supplemental Figure 2D). These data suggest that GPIb/GPIX surface expression is restricted by GPIbβ levels during megakaryopoiesis. Consistent with our reporter data, endogenous GPIbα mRNA expression increased ∼10-fold in megakaryocytes when compared with HPCs. These results demonstrate that the Gp1ba promoter, when targeted to the AAVS1 locus, mirrors endogenous GPIbα expression in human iPSC-derived HPCs and megakaryocytes. Our data are consistent with recently published studies showing other megakaryocyte-specific genes expressed at an earlier stage of hematopoietic development.19
To study an iPSC-based correction strategy for IPDs, we generated iPSC lines from two patients with type 1 GT, both with mutations in ITGA2B. Analysis of GTP1 platelets by flow cytometry showed <5% αIIbβ3 expression (data not shown), which was confirmed by western blot analysis (supplemental Figure 3A). Sequencing of both parental and patient genomic DNA confirmed a previously unreported homozygous IVS4(+1) G>A (g.3956G>A) mutation in ITGA2B (supplemental Figure 3B), which occurs prior to the first calcium-binding domain of αIIb.10 Analysis of the αIIb cDNA of GTP1 was consistent with activation of a cryptic splice site leading to a frameshift and early termination codon within exon 5 of ITGA2B (supplemental Figure 3C-D). GTP2 has a previously reported Gly273→Asp αIIb mutation, leading to abnormal intracellular transport of pro-αIIbβ3.20
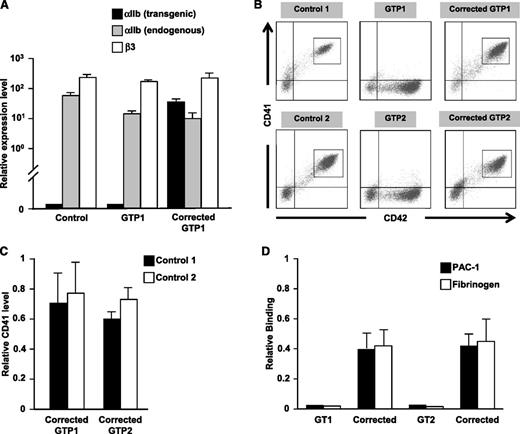
Both GTP1 and GTP2 iPSCs lack αIIbβ3 expression on derived CD42+ megakaryocytes. Upon activation with convulxin, a GPVI agonist, these thrombasthenic megakaryocytes failed to bind both PAC-1 antibody and fibrinogen above background. By replacing eGFP with human αIIb cDNA in the transgenic construct described in Figure 1, both αIIb mRNA levels and αIIbβ3 surface levels were restored in GT megakaryocytes. By using a transgene-specific primer for quantitative gene expression, Gp1ba-driven αIIb was determined to be expressed at a level comparable to endogenous αIIb (Figure 2A). Surface αIIbβ3 expression for both patients was >50% of the level of concurrently studied control iPSCs and adult CD34+ marrow-derived megakaryocytes (Figure 2B-C and supplemental Figure 3E-F). Following activation with convulxin of both uncorrected and corrected GT megakaryocytes, PAC-1 antibody and fibrinogen binding approximated surface receptor function of control megakaryocytes only on the corrected GT megakaryocytes, indicating return of αIIbβ3 biologic activity (Figure 2D).
Transgene correction of GT iPSC lines. (A) Quantitative reverse transcriptase polymerase chain reaction analysis of expression of the Gp1ba promoter construct, endogenous ITGA2B, and endogenous ITGB3 in control, uncorrected GT, and corrected GT iPSC-derived megakaryocytes relative to cyclophilin (mean ± SEM for 3 replicates). (B) Analysis by flow cytometry of CD41 vs CD42 expression in control, GT, and corrected GT iPSC lines differentiated into megakaryocytes. (C) Bar graphs of gated populations in Figure 2B (gray rectangles) examine expression of CD41 of corrected GTP1 and GTP2 relative to two different control iPSC-derived megakaryocytes (mean ± SEM for 3 independent experiments). (D) PAC-1 and fibrinogen binding in convulxin-stimulated uncorrected and corrected GT megakaryocytes relative to control megakaryocytes (mean ± SEM for 3 independent experiments).
Transgene correction of GT iPSC lines. (A) Quantitative reverse transcriptase polymerase chain reaction analysis of expression of the Gp1ba promoter construct, endogenous ITGA2B, and endogenous ITGB3 in control, uncorrected GT, and corrected GT iPSC-derived megakaryocytes relative to cyclophilin (mean ± SEM for 3 replicates). (B) Analysis by flow cytometry of CD41 vs CD42 expression in control, GT, and corrected GT iPSC lines differentiated into megakaryocytes. (C) Bar graphs of gated populations in Figure 2B (gray rectangles) examine expression of CD41 of corrected GTP1 and GTP2 relative to two different control iPSC-derived megakaryocytes (mean ± SEM for 3 independent experiments). (D) PAC-1 and fibrinogen binding in convulxin-stimulated uncorrected and corrected GT megakaryocytes relative to control megakaryocytes (mean ± SEM for 3 independent experiments).
In conclusion, these data confirm the efficiency and potency of a Gp1ba promoter construct to drive expression in human megakaryocytes. When targeted to a single allele of the AAVS1 locus, Gp1ba drives high-level transgene expression in megakaryocytes with minimal expression in erythroid or myeloid cells. This system offers a novel and reproducible tool to study megakaryocyte biology in iPSCs derived from individuals with IPDs. For GT, this study demonstrates surface reconstitution of integrin αIIbβ3 comparable to that in individuals with heterozygous mutations and normal platelet function. It also provides a proof-of-concept for a potential alternative to lentiviral-based gene therapy approaches.6 Interestingly, Amabile et al21 have recently shown multilineage engraftment of hematopoietic stem cells derived from human iPSCs in primary murine transplant recipients. While this technology is currently too inefficient for clinical applications, it does show promise for the prospect of long-term hematopoietic reconstitution from iPSC-derived HPCs. Combined with the described Gp1ba-AAVS1 targeting construct and current episomal reprogramming strategies,22 virus-free corrective therapy for patients with significant bleeding due to functional platelet defects is a possibility.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank the patients who participated in this study, Darrell Kotton and Gustavo Mostoslavsky for providing the stem cell cassette reprogramming vectors, Peter Newman for providing the SEW-8 antibody, Joel Bennett for providing tirofiban, Katherine High for access to the FACSCanto flow cytometer, and Juan Fang for technical help in characterizing GT molecular genetic defects.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants T32 HL007150-36 (S.K.S.), K12-HL-08064-06 (S.K.S.), U01 HL099656 (M.P., P.G., and D.L.F.), P01 HL064190 (M.P.), a National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK090969 (M.P. and D.L.F.); and by generous gifts from the Cure Glanzmann’s Foundation (S.K.S. and D.A.W.), Midwest Athletes Against Childhood Cancer Fund (D.A.W.), Glanzmann Research Foundation (D.A.W.), and John B. and Judith A. Gardetto (D.A.W.). S.K.S. is the recipient of a Hemostasis and Thrombosis Research Society/Novo Nordisk 2013 Mentored Research Award in Hemophilia or Rare Bleeding Disorders from the Hemostasis and Thrombosis Research Society, supported by Novo Nordisk.
Authorship
Contribution: S.K.S. designed and performed experiments and wrote the manuscript; J.A.M. designed and performed experiments and edited the manuscript; K.K.V., R.B.L., P.P., Y.W., S.K., G.Z., L.Z., and E.Z.G. assisted with experiments; L.M.S. interpreted teratoma assays; M.P.L. provided guidance and edited the manuscript; S.B.K. and D.A.W. performed gene sequencing and edited the manuscript; D.L.F., M.P., and P.G. designed experiments, edited the manuscript, and provided supervision and direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Spencer K. Sullivan, Division of Hematology, The Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, ARC 316E, Philadelphia, PA 19104; e-mail: sullivansk@email.chop.edu; and Paul Gadue, Department of Pathology and Laboratory Medicine, The University of Pennsylvania, Colket Translational Research Building, 3501 Civic Center Blvd, 5100 Suite, Philadelphia, PA 19104; e-mail: pgadue@email.chop.edu.

![Figure 1. The Gp1ba promoter drives high-level expression in iPSC-derived megakaryocytes. (A) Schematic of the targeting construct. The Gp1ba expression construct (gray window) contains the murine proximal Gp1ba promoter linked to the Simian virus 40 (SV40) 5′ untranslated region (UTR) followed by a cDNA of interest (eGFP shown) followed by the SV40 3′ UTR.10 After insertion into the puromycin (puro) containing targeting plasmid (pAAVS1-SA-2A-puro-pA), the entire transgene is inserted into the AAVS1 locus (PPP1R12C intron 1) using zinc finger nuclease–mediated homologous recombination. After insertion, the endogenous PPP1R12C promoter drives puromycin resistance (gray arrow), while the Gp1ba promoter drives the transgenic message (black arrow) (SA, splice acceptor; 2A, self-cleaving peptide; pA, bovine growth hormone polyadenylation signal). (B) Control 2 with hemizygous insertion of the Gp1ba promoter driving eGFP was examined by flow cytometry during hematopoietic differentiation. (Left) Representative plots of SSEA3+/SSEA4+ iPSCs, KDR+/CD31+ mesoderm, and CD41+/CD235+ HPCs. (Right) Histograms of gated populations on left (gray rectangles) examine eGFP expression in iPSCs, mesoderm, and HPCs. (C) (Left) Representative flow cytometry plots of iPSC-derived CD45+/CD18+ myeloid cells, CD41–/CD235+ erythroid cells, and CD41+/CD42+ megakaryocytes. (Right) Histograms of gated populations on left (gray rectangles) examine eGFP expression in iPSCs, myeloid cells, erythroid cells, and megakaryocytes. (D) Mean fluorescence intensity (MFI) of eGFP expression in iPSCs, mesodermal cells (meso), HPCs, and megakaryocytes (megs). (E) Comparison of gene expression by quantitative reverse transcriptase polymerase chain reaction of endogenous GP1BA, GP1BB, and GP9 in iPSC-derived HPCs and megakaryocytes relative to cyclophilin (mean ± standard error of the mean [SEM] for 3 replicates).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/5/10.1182_blood-2013-10-530725/4/m_753f1.jpeg?Expires=1767700203&Signature=wb4whZRRiY8T-40WIfTow5aq-izlM~g1uGtY-XahrG-esnmuTlN7XQ30L230ImuWBDmcyUgKsA2S4EeLqe4vh5OlFY2OI9y9ywBmtVP-q88qyOhY6lb8Az4j0RHDxWKxCcO-PlmC1rJFvOve0ft2iZov7kedJiYXP5PkgU0cB-uOh4RDYdYAX7-8WxW6ZUf3oqGOzFRiE3AbVRNuA6cHNYKREKaD9XS4mfeouBmOe8NnhgC2GiTYNMb4ZJWynhPclyEbUFVPEcUBzk4mOlQg4W~5yGS9JtqH-6nn6b7j5Y10ODT8A4eGO7SCSJ4ALMy-U5RVyw7I7eaS8QrQtxZHMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)