Key Points
Loss of Gadd45a enhances HSC reconstitution on hematopoietic stress.
Gadd45a regulates DNA damage responses in HSCs.
Abstract
Gadd45a has been involved in DNA damage response and in many malignancies, including leukemia. However, the function of Gadd45a in hematopoietic stem cells (HSCs) remains unknown. Here, we reported that Gadd45a-deficient (Gadd45a−/−) mice showed a normal hematologic phenotype under homeostatic conditions. However, following 5-fluorouracil treatment, Gadd45a−/− HSCs exhibited a faster recovery, associated with an increase in the proliferation rate. Interestingly, young Gadd45a−/− HSCs showed enhanced reconstitution ability in serial transplantation. Following ionizing radiation (IR), young Gadd45a−/− HSCs exhibited an increased resistance to IR-induced DNA damage, associated with a decrease in the apoptosis rate and delayed DNA repair. The significantly higher level of DNA damage in Gadd45a−/− HSCs ultimately promoted B-cell leukemia in further transplanted recipient mice. In old mice, Gadd45a−/− HSCs were functionally equal to wild-type HSCs but exhibited more DNA damage accumulation and increased sensitivity to IR than wild-type HSCs. In conclusion, Gadd45a plays a significant role in HSC stress responses. Gadd45a deficiency leads to DNA damage accumulation and impairment in apoptosis after exposure to IR, which increases the susceptibility of leukemogenesis.
Introduction
The hematopoietic system stems from a rare subpopulation of hematopoietic stem cells (HSCs), which self-renew and differentiate to hematopoietic progenitor cells (HPCs) and functionally mature blood cells.1 The long-lived HSCs are confronted with intrinsic and extrinsic stress during their lifespan.2 Moreover, the accumulation of DNA damage has been proposed to compromise the maintenance and functionality of HSCs during aging.3 Therefore, proper regulation of DNA damage responses in HSCs is crucial to avoid HSC exhaustion and prevent malignant transformation.
Furthermore, appropriate regulation of the cell cycle is essential for HSC homeostasis.4 Under steady-state conditions, HSCs are mainly in dormancy to avoid exhaustion.5 On hematopoietic stress, HSCs rapidly and transiently expand and differentiate to replenish blood cells. A large amount of evidence has shed light on the significant role of DNA damage response and cell cycle control genes in the regulation of HSC function.6-15
Gadd45a has been implicated in the response to physiological and environmental stress, which can regulate cell cycle arrest, DNA repair, cell survival, senescence, and apoptosis in various cell compartments.16,17 Studies have indicated that the level of Gadd45a promoter methylation and expression of Gadd45a is related to the prognosis of several malignancies.18,19 A recent report demonstrated that Gadd45a promoter methylation predicts poor overall survival in acute myeloid leukemia.20 Although previous studies have shown that Gadd45a is involved in genotoxic stress response of bone marrow (BM) cells in vitro,21 it remains unclear whether Gadd45a plays an important role in HSCs under steady-state or stress conditions.
Here, we used Gadd45a-deficient (Gadd45a−/−) mice as a model system to explore the functional impact of Gadd45a deletion on HSCs during homeostasis maintenance, the stress response, and aging. In young mice, Gadd45a deletion caused HSCs to recover faster after 5-fluorouracil (5-FU) treatment due to an increase in proliferation. Gadd45a−/− HSCs exhibited an enhanced colony-forming ability and increased self-renewal during competitive serial transplantation. Moreover, Gadd45a deficiency promoted HSC survival after ionizing radiation (IR) by attenuating apoptosis, which increased HSC susceptibility to leukemia. Furthermore, old Gadd45a−/− HSCs exhibited impaired genomic integrity and increased sensitivity to IR stress. Together, these data revealed an essential role of Gadd45a in HSCs during stress responses and aging.
Methods
Mice
Gadd45a−/− mice were a kind gift from Professor Albert J. Fornace Jr, and were maintained in a C57BL/6 (CD45.2) background. The recipient mice used in the competitive transplantation assays were either CD45.1 mice or CD45.1/CD45.2 mice. The Animal Care and Ethics Committee at Hangzhou Normal University approved all animal experiments in our study.
Clinical samples
The ethics committee of the Institute of Hematology and Blood Disease Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College approved the human study, which was conducted in accordance with the Helsinki declaration. All patients were asked to provide written consent to participate in the study. The clinical information is presented in the supplemental Methods on the Blood Web site.
Cell culture
For liquid culture, cells were cultured in serum-free expansion medium (SFEM) (Stemcell Technologies) with 50 ng/mL stem cell factor (Pepro Tech), 50 ng/mL Thrombopoietin (Pepro Tech), and 100 U/mL penicillin/streptomycin. For semisolid culture, the methylcellulose medium with recombinant cytokines for mouse cells (M3434) was bought from Stemcell Technologies and used following the manual.
Flow cytometry
Prepared samples were analyzed on LSRFortessa (BD Biosciences) or sorted on Influx (BD Biosciences). Detailed methods and antibodies are described in the supplemental Methods.
Single-cell colony forming assay
Single cells were cultured for 14 days in liquid medium. The detailed method is in the supplemental Methods.
5-FU experiment
5-FU (150 mg/kg) was injected in mice 4 months after BM transplantation. Mice were euthanized at the indicated time point and subjected to fluorescence-activated cell sorter (FACS) analysis.
Analysis of cell cycle and apoptosis in vitro
Indicated cells were stained with antibodies (5-Bromo-2′-deoxyuridine [BrdU], Ki67, Annexin V, or Caspase 3), followed by FACS analysis. The detailed methods are provided in the supplemental Methods.
Immunofluorescence
Sorted cells were fixed, permeabilized, and stained with antibodies. The detailed methods are provided in the supplemental Methods.
Comet assay
The alkaline comet assay was performed to evaluate the degree of DNA damage as previously described.22 A brief description of the methods is provided in the supplemental Methods.
Quantitative reverse transcriptase-polymerase chain reaction
Real-time polymerase chain reaction (PCR) was performed using KAPA SYBR FAST qPCR kits (Kapa Biosystems) or the TaqMan assay (Applied Biosystems) on the CFX 96 real-time System (Bio-Rad). The detailed methods are provided in the supplemental Methods.
Lentivirus production
Lentivirus was produced in 293T cells after transfection of 12 mg cDNA plasmid, 9 mg pspAX2 helper plasmid, and 3 mg pMD2G.23 The virus was concentrated by centrifugation at 27 000 rpm for 3.0 hours at 4°C, and the virus pellet was resuspended in sterile SFEM medium.
Statistical analysis
Data are presented as mean ± standard deviation. The statistical significance of the differences between groups was calculated using the unpaired Student t test, and the survival curve was analyzed using a log-rank (Mantel-Cox) test.
Results
Gadd45a−/− mice showed a normal hematologic phenotype under homeostatic conditions
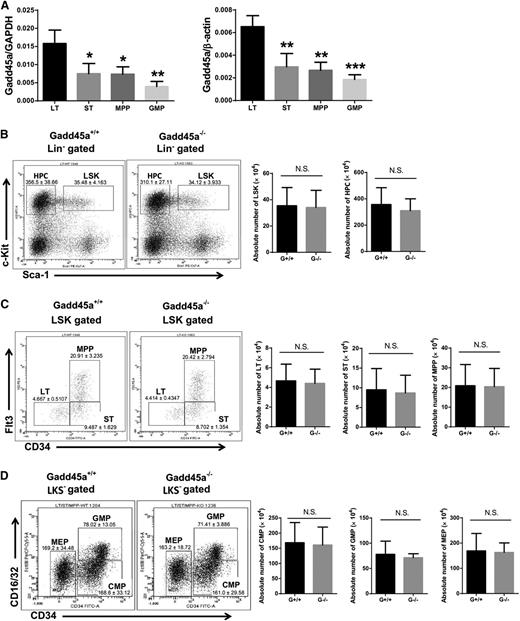
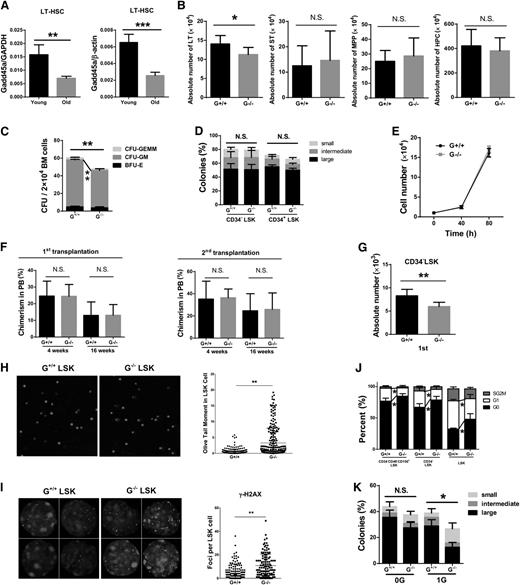
To assess the role of Gadd45a in HSCs and HPCs, we first determined the expression level of Gadd45a in HSCs and their progeny from young wild-type mice (2 months old). Real-time PCR revealed a significantly higher level of Gadd45a expression in primitive long-term HSCs (LT-HSCs) compared with short-term HSCs (ST-HSCs), multipotent progenitors (MPPs), and granulocyte/macrophage progenitors (GMPs) (Figure 1A and supplemental Figure 1A), suggesting a potential role for Gadd45a in primitive HSCs.
Gadd45a−/−mice showed a normal hematologic phenotype under homeostatic condition. (A) Expression of Gadd45a mRNA in the indicated subpopulations of BM from young wild-type mice (2 months old) was measured by qRT-PCR (SYBR green assay). The relative expression of Gadd45a was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin. Each subpopulation was compared with LT-HSCs for statistical analysis (n = 4). (B-D) The absolute number of LT-HSCs (CD34−Flt3−Lin−c-Kit+Sca-1+), ST-HSCs (CD34+Flt3−Lin−c-Kit+Sca-1+), MPPs (CD34+Flt3+Lin−c-Kit+Sca-1+), LSK (Lin−c-Kit+Sca-1+) cells, HPCs (Lin−c-Kit+Sca-1−, LKS−), common myeloid progenitors (CD34+CD16/32−LKS−), GMPs (CD34+CD16/32+LKS−), and megakaryocyte/erythroid progenitors (CD34−CD16/32−LKS−) in young mice was determined by FACS (n = 11) (*P < .05, **P < .01, ***P < .001). N.S., not significant.
Gadd45a−/−mice showed a normal hematologic phenotype under homeostatic condition. (A) Expression of Gadd45a mRNA in the indicated subpopulations of BM from young wild-type mice (2 months old) was measured by qRT-PCR (SYBR green assay). The relative expression of Gadd45a was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin. Each subpopulation was compared with LT-HSCs for statistical analysis (n = 4). (B-D) The absolute number of LT-HSCs (CD34−Flt3−Lin−c-Kit+Sca-1+), ST-HSCs (CD34+Flt3−Lin−c-Kit+Sca-1+), MPPs (CD34+Flt3+Lin−c-Kit+Sca-1+), LSK (Lin−c-Kit+Sca-1+) cells, HPCs (Lin−c-Kit+Sca-1−, LKS−), common myeloid progenitors (CD34+CD16/32−LKS−), GMPs (CD34+CD16/32+LKS−), and megakaryocyte/erythroid progenitors (CD34−CD16/32−LKS−) in young mice was determined by FACS (n = 11) (*P < .05, **P < .01, ***P < .001). N.S., not significant.
However, no significant difference was found in the peripheral blood (PB) count and the frequency of leukocytes in Gadd45a−/− mice compared with Gadd45a+/+ mice (supplemental Figure 1B-C). In addition, the absolute number of LT-HSCs, ST-HSCs, MPPs, LSK cells, HPCs, common myeloid progenitors, GMPs, and megakaryocyte/erythroid progenitors in the BM of Gadd45a−/− mice was comparable to Gadd45a+/+ mice (Figure 1B-D). Furthermore, in the steady state, no significant difference was observed in the cell cycle status of Gadd45a−/− HSCs compared with Gadd45a+/+ HSCs (supplemental Figure 1D).
Gadd45a deficiency enhances HSC proliferation after 5-FU treatment
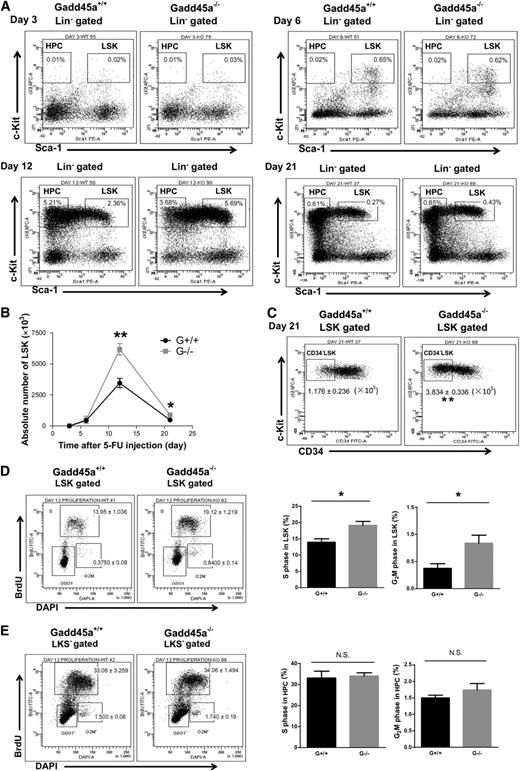
To evaluate the influence of Gadd45a deficiency on the regenerative capacity of HSCs under hematopoietic stress, we analyzed HSCs at different time points following 5-FU treatment. To exclude the potential effects of 5-FU on nonhematopoietic tissues, we first transplanted either Gadd45a+/+ or Gadd45a−/− BM cells into lethally irradiated wild-type recipient mice. Four months later, the recipient mice were treated with 5-FU (day 0). Before the 5-FU experiment, we confirmed that >99% of the LSK cells in the recipient mice were donor derived, and we analyzed the apoptosis and cell cycle status of donor-derived HSCs by Annexin V/4′,6 diamidino-2-phenylindole (DAPI) staining and BrdU incorporation assay. No significant difference was found in apoptosis and cell cycle of Gadd45a−/− donor-derived HSCs compared with Gadd45a+/+ controls (supplemental Figure 2A). After treatment with 5-FU, LSK cells were drastically depleted in both the Gadd45a+/+ and Gadd45a−/− mice at day 3 and slightly recovered at day 6 (Figure 2A-B). At day 12, the number of LSK cells in the Gadd45a−/− mice was significantly higher than those in the Gadd45a+/+ mice (Figure 2A-B). At day 21, the number of LSK cells returned to a lower level, whereas a greater number of LSK and CD34−LSK cells were still present in Gadd45a−/− mice (Figure 2A-C). These data indicated that Gadd45a deficiency increased the recovery of HSCs after 5-FU treatment.
Gadd45a deficiency enhances HSC proliferation after 5-FU treatment. (A) The representative FACS plots of the LSK population at the indicated time points after 5-FU treatment. (B) The dynamic change of absolute number of LSK cells after 5-FU injection detected by flow cytometry (n = 3-5). (C) The representative FACS plots and absolute number of CD34-LSK cells in BM at day 21 after 5-FU injection are shown (G+/+, n = 5; G−/−, n = 3). (D-E) BrdU was delivered by intraperitoneal injection 1 hour before euthanasia at day 12 after 5-FU injection. The representative FACS plots and percentage of S or G2M phases in LSK cells and HPCs are shown (G+/+, n = 4; G−/−, n = 5) (*P < .05, **P < .01). N.S., not significant.
Gadd45a deficiency enhances HSC proliferation after 5-FU treatment. (A) The representative FACS plots of the LSK population at the indicated time points after 5-FU treatment. (B) The dynamic change of absolute number of LSK cells after 5-FU injection detected by flow cytometry (n = 3-5). (C) The representative FACS plots and absolute number of CD34-LSK cells in BM at day 21 after 5-FU injection are shown (G+/+, n = 5; G−/−, n = 3). (D-E) BrdU was delivered by intraperitoneal injection 1 hour before euthanasia at day 12 after 5-FU injection. The representative FACS plots and percentage of S or G2M phases in LSK cells and HPCs are shown (G+/+, n = 4; G−/−, n = 5) (*P < .05, **P < .01). N.S., not significant.
To determine the underlying mechanisms, we performed a BrdU incorporation assay to examine the cell cycle status of HSCs at day 12 after 5-FU administration. We found a remarkable increase in the populations of S and G2M phases in Gadd45a−/− LSK cells compared with Gadd45a+/+ LSK cells (Figure 2D). However, the cell cycle profile in HPCs showed no difference between the Gadd45a+/+ and Gadd45a−/− mice (Figure 2E). In addition, we found no significant difference in apoptosis in HSCs and HPCs between the Gadd45a+/+ and Gadd45a−/− mice (supplemental Figure 2B). Together, these data indicated that Gadd45a deficiency enhanced the ability of HSCs to repopulate, likely due to an increase in cell cycle progression.
Gadd45a−/− HSCs show enhanced reconstitution ability
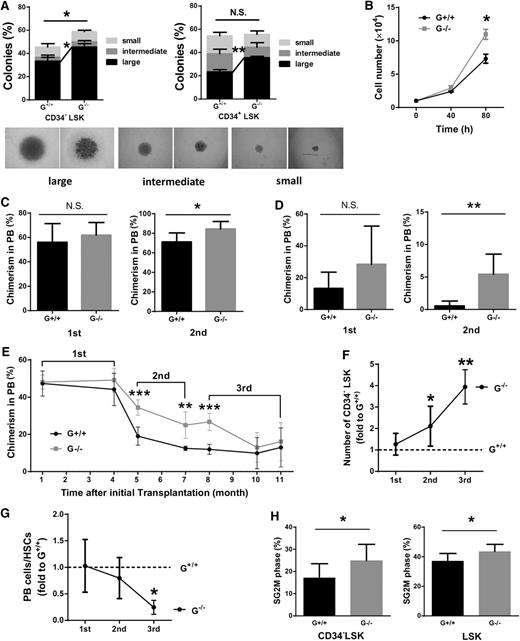
To evaluate the proliferation and differentiation ability of HSCs in vitro, we performed a single-cell colony forming assay and found that Gadd45a−/− HSCs formed more colonies (Figure 3A). To further assess the proliferation capacity of HSCs, 10 000 CD34−LSK cells were cultured in SFEM, and the cells were counted at specific time points. After 80 hours, a significant increase in cell number was found in Gadd45a−/− HSCs compared with Gadd45a+/+ HSCs (Figure 3B). These data suggested that Gadd45a−/− HSCs and/or their downstream progenitor cells have higher proliferative capacity compared with Gadd45a+/+ controls.
Gadd45a−/−HSCs show enhanced reconstitution ability. (A) Single CD34−LSK and CD34+LSK cells from young mice were sorted in a 96-well plate and cultured for 14 days in vitro. The percentage of colonies was calculated by dividing the number of colonies by the original number of single cells seeded (n = 3). (B) Ten thousand CD34−LSK cells from young mice were sorted and expanded in liquid culture. Cell numbers were counted at various time points (n = 3). (C) BM cells from young mice were mixed in a 1:1 ratio with competitors (CD45.1) and injected into lethally irradiated recipients (CD45.1/2). Subsequently, the chimeric BM cells were retransplanted into the secondary recipient mice (CD45.1/2) 4 months later. The chimerism in PB was shown 16 weeks after transplantation (first, n = 10; second, n = 5). (D) One hundred fifty CD34−LSK cells along with 1 × 106 BM cells (competitor) were injected into lethally irradiated recipients. Four months later, 150 donor-derived CD34−LSK cells were purified and retransplanted with fresh competitors into secondary recipients. The chimerism in PB is shown at 16 weeks after transplantation (first: G+/+, n = 5; G−/−, n = 6; second: G+/+, n = 11; G−/−, n = 10). (E) Three-round serial transplantation was conducted using 4000 purified LSK cells along with 1 × 106 fresh competitors each time. Chimerism in PB was shown at the indicated time after transplantation (first, n = 5; second, n = 6; third, n = 3). (F) The absolute number of donor-derived CD34−LSK cells was calculated 3 or 4 months after transplantation. The number of Gadd45a−/− donor-derived CD34−LSK cells was divided by that of Gadd45a+/+ donor-derived CD34−LSK cells (first, n = 5; second, n = 6; third, n = 3). (G) The ratio of PB cells/HSCs was calculated as the PB contribution divided by the absolute number of donor-derived CD34−LSK cells (first, n = 5; second, n = 6; third, n = 3). (H) Cell cycle analysis was performed on donor-derived HSCs 3 weeks after the second round of transplantation by staining with DAPI. The percentage of cycling cells in SG2M phases is shown (G+/+, n = 8; G−/−, n = 10) (*P < .05, **P < .01, ***P < .001). N.S., not significant.
Gadd45a−/−HSCs show enhanced reconstitution ability. (A) Single CD34−LSK and CD34+LSK cells from young mice were sorted in a 96-well plate and cultured for 14 days in vitro. The percentage of colonies was calculated by dividing the number of colonies by the original number of single cells seeded (n = 3). (B) Ten thousand CD34−LSK cells from young mice were sorted and expanded in liquid culture. Cell numbers were counted at various time points (n = 3). (C) BM cells from young mice were mixed in a 1:1 ratio with competitors (CD45.1) and injected into lethally irradiated recipients (CD45.1/2). Subsequently, the chimeric BM cells were retransplanted into the secondary recipient mice (CD45.1/2) 4 months later. The chimerism in PB was shown 16 weeks after transplantation (first, n = 10; second, n = 5). (D) One hundred fifty CD34−LSK cells along with 1 × 106 BM cells (competitor) were injected into lethally irradiated recipients. Four months later, 150 donor-derived CD34−LSK cells were purified and retransplanted with fresh competitors into secondary recipients. The chimerism in PB is shown at 16 weeks after transplantation (first: G+/+, n = 5; G−/−, n = 6; second: G+/+, n = 11; G−/−, n = 10). (E) Three-round serial transplantation was conducted using 4000 purified LSK cells along with 1 × 106 fresh competitors each time. Chimerism in PB was shown at the indicated time after transplantation (first, n = 5; second, n = 6; third, n = 3). (F) The absolute number of donor-derived CD34−LSK cells was calculated 3 or 4 months after transplantation. The number of Gadd45a−/− donor-derived CD34−LSK cells was divided by that of Gadd45a+/+ donor-derived CD34−LSK cells (first, n = 5; second, n = 6; third, n = 3). (G) The ratio of PB cells/HSCs was calculated as the PB contribution divided by the absolute number of donor-derived CD34−LSK cells (first, n = 5; second, n = 6; third, n = 3). (H) Cell cycle analysis was performed on donor-derived HSCs 3 weeks after the second round of transplantation by staining with DAPI. The percentage of cycling cells in SG2M phases is shown (G+/+, n = 8; G−/−, n = 10) (*P < .05, **P < .01, ***P < .001). N.S., not significant.
To evaluate the self-renewal and differentiation capacity of Gadd45a+/+ and Gadd45a−/− HSCs in vivo, we performed a competitive BM transplantation experiment. BM cells from young Gadd45a+/+ or Gadd45a−/− mice (CD45.2) were mixed in a 1:1 ratio with competitor BM cells (CD45.1) and injected into lethally irradiated recipient mice (CD45.1/2). Four months after transplantation, the chimeric BM cells were retransplanted into the secondary recipient mice. The chimerism in PB was examined monthly after transplantation. Gadd45a−/− BM cells showed a comparable contribution to the PB with Gadd45a+/+ BM cells in the primary recipients but a significantly higher contribution in the secondary recipients than Gadd45a+/+ BM cells (Figure 3C). To reinforce this observation, we conducted a competitive transplantation experiment with CD34−LSK cells. Similarly, no significant difference in reconstitution was observed between Gadd45a+/+ and Gadd45a−/− CD34−LSK cells in the primary recipients, whereas Gadd45a−/− donor-derived PB chimerism showed a 10-fold increase compared with Gadd45a+/+ donor-derived PB chimerism in the secondary recipients (Figure 3D). These data indicated that Gadd45a−/− HSCs had an enhanced ability to reconstitute the PB at the secondary transplantation, suggesting that Gadd45a deficiency enhanced reconstitution capacity of HSCs in serial transplantation.
We next performed a three-round competitive serial transplantation using 4000 LSK cells from young Gadd45a+/+ and Gadd45a−/− mice. For the consecutive transplantation, 4000 donor-derived LSK cells were isolated, mixed with fresh competitor cells, and retransplanted into recipient mice. The donor-derived multilineage reconstitution in PB was analyzed 4 months after transplantation (Figure 3E and supplemental Figure 3A). Consistently, Gadd45a−/− donors exhibited greater reconstruction in secondary recipient mice (Figure 3E and supplemental Figure 3B); however, in tertiary recipients, Gadd45a−/− donor-derived PB chimerism gradually decreased to the level of Gadd45a+/+ donors after transplantation (Figure 3E). Further analysis of BM in recipient mice revealed that Gadd45a deficiency resulted in a significant increase of LT-HSCs after the second and third transplantation, suggesting that Gadd45a−/− HSCs have enhanced self-renewal ability (Figure 3F). However, the ratio of donor-derived PB cell number divided by HSC number was remarkably reduced in Gadd45a−/− mice at tertiary transplantation, indicating a compromised potential of Gadd45a−/− HSC and progenitors to generate PB cells after serial transplantation (Figure 3G). To reveal the underlying mechanisms, we examined the cell cycle and apoptosis in the donor-derived HSCs. Cell cycle analysis revealed that Gadd45a−/− HSCs exhibited a notably increased proliferation in the secondary transplantation (Figure 3H) but a reduced cell cycle entry in the tertiary transplantation (supplemental Figure 3C). Annexin V staining showed no significant difference between Gadd45a+/+ and Gadd45a−/− donor-derived HSCs (supplemental Figure 3D). Together, these data indicate that Gadd45a deficiency prevents HSC depletion during serial transplantation by enhancing HSC self-renewal.
Gadd45a−/− HSCs exhibit impaired apoptosis induction and delayed DNA repair
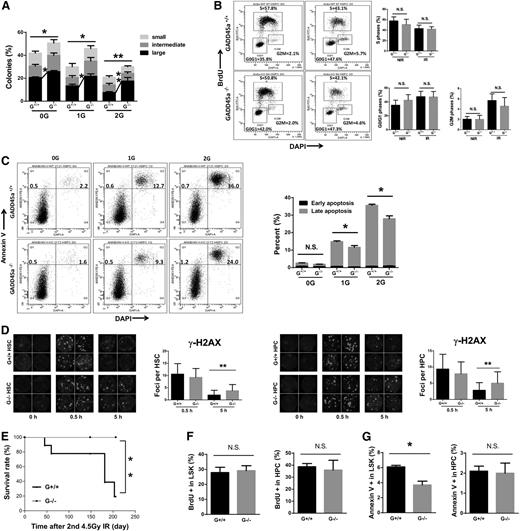
Gadd45a is mainly involved in DNA damage response. We first detected Gadd45a expression in HSCs (Flt3−LSK) and GMPs from wild-type mice 4 hours after IR. The induction of Gadd45a expression was significant higher in HSCs than GMPs after IR (supplemental Figure 4A). To test the effect of Gadd45a deficiency on the DNA damage response in HSCs, we conducted a single-cell colony forming assay on HSCs treated with 1- to 2-Gy IR. The suppression of single-cell colony forming ability by IR was significantly less in Gadd45a−/− HSCs than Gadd45a+/+ HSCs (Figure 4A), indicating that Gadd45a deficiency results in a significant IR resistance in HSCs. To confirm this, we cultured 200 HSCs and GMPs in methylcellulose semisolid medium after 1.5-Gy IR for 6 days and found Gadd45a−/− HSCs were more resistant to IR compared with Gadd45a+/+ HSCs, whereas Gadd45a−/− GMPs were more sensitive to IR compared with Gadd45a+/+ GMPs (supplemental Figure 4B).
Gadd45a−/−HSCs exhibit impaired apoptosis and delayed DNA repair after IR. (A) Single HSCs (CD34−LSK) were sorted in 96-well plates, followed by 1- to 2-Gy IR and culture for 14 days. Colonies were counted in each group (n = 4). (B) The cell cycle profile of HSCs (Flt3−LSK) was analyzed using BrdU incorporation assay 24 hours after 2-Gy IR (n = 3). (C) IR-induced apoptosis was detected with Annexin V/DAPI staining 24 hours after 1- to 2-Gy IR. The early apoptosis (Annexin V+/DAPI−) and late apoptotic cells (Annexin V+/DAPI+) are shown (n = 4). (D) γ-H2AX foci per cell were counted at the indicated time point post-IR (HSC, n = 40; HPC, n = 60). Representative immunofluorescence staining for γ-H2AX and the nucleus is shown. (E) Survival curves showed the survival rate of mice subjected to 4.5-Gy IR twice (at a 3-month interval) for the indicated genotypes (n = 9). (F-G) The cell cycle status and apoptosis were analyzed 4.5 months after the second 4.5-Gy IR (n = 3) (*P < .05, **P < .01). N.S., not significant.
Gadd45a−/−HSCs exhibit impaired apoptosis and delayed DNA repair after IR. (A) Single HSCs (CD34−LSK) were sorted in 96-well plates, followed by 1- to 2-Gy IR and culture for 14 days. Colonies were counted in each group (n = 4). (B) The cell cycle profile of HSCs (Flt3−LSK) was analyzed using BrdU incorporation assay 24 hours after 2-Gy IR (n = 3). (C) IR-induced apoptosis was detected with Annexin V/DAPI staining 24 hours after 1- to 2-Gy IR. The early apoptosis (Annexin V+/DAPI−) and late apoptotic cells (Annexin V+/DAPI+) are shown (n = 4). (D) γ-H2AX foci per cell were counted at the indicated time point post-IR (HSC, n = 40; HPC, n = 60). Representative immunofluorescence staining for γ-H2AX and the nucleus is shown. (E) Survival curves showed the survival rate of mice subjected to 4.5-Gy IR twice (at a 3-month interval) for the indicated genotypes (n = 9). (F-G) The cell cycle status and apoptosis were analyzed 4.5 months after the second 4.5-Gy IR (n = 3) (*P < .05, **P < .01). N.S., not significant.
To understand the underlying mechanisms, we examined the cell cycle profile in HSCs and GMPs 24 hours after 2-Gy IR treatment by BrdU incorporation assay. No significant difference was observed in the cell cycle profile of Gadd45a+/+ HSCs and Gadd45a−/− HSCs (Figure 4B), and a similar result was observed in the GMPs (supplemental Figure 4C). Next, we examined the apoptosis rate by Annexin V/DAPI staining. Interestingly, the induction of apoptosis in Gadd45a−/− HSCs was much less than Gadd45a+/+ HSCs 24 hours after IR, whereas Gadd45a−/− GMPs showed more IR-induced apoptosis than Gadd45a+/+ GMPs (Figure 4C and supplemental Figure 4D-E). Moreover, we also detected the active Caspase 3 after IR by FACS analysis to confirm this result. Consistently, Gadd45a−/− HSCs expressed less active Caspase 3 than Gadd45a+/+ HSCs 24 hours after IR, whereas Gadd45a−/− GMPs expressed more active Caspase 3 than Gadd45a+/+ GMPs (supplemental Figure 4F). These results indicated that Gadd45a deficiency suppresses apoptosis in HSCs after IR but promotes that in GMPs.
Next, we used the phosphorylated histone H2A variant H2AX at Ser139 (γ-H2AX) staining to examine the DNA repair efficiency of HSCs and HPCs after IR. The γ-H2AX foci in Gadd45a−/− HSCs showed no apparent difference from Gadd45a+/+ HSCs 0.5 hours after IR. However, a large amount of γ-H2AX foci remained visible in Gadd45a−/− HSCs and HPCs 5 hours after IR, when the majority of Gadd45a+/+ HSCs and HPCs repaired the lesion (Figure 4D), indicating that Gadd45a deficiency impaired the DNA repair capacity in HSCs and HPCs. To confirm this observation, 53BP1 staining together with a counterstaining of γ-H2AX was performed during a time course of 1 to 12 hours after IR. In both Gadd45a+/+ and Gadd45a−/− HSCs, 53BP1 foci mostly colocalized with γ-H2AX after IR (supplemental Figure 4G). In Gadd45a−/− HSCs, DNA damage foci remained visible until 7 hours after IR, when very few foci could be seen in Gadd45a+/+ HSCs (supplemental Figure 4G). All the DNA damage foci disappeared 12 hours after IR (supplemental Figure 4G-H).
Next, we performed a rescue experiment with ectopic expression of murine Gadd45a in wild-type and Gadd45a−/− HSCs by lentiviral transduction. To analyze the HSC phenotype with ectopic Gadd45a expression, we transplanted the lentiviral-transduced HSCs into recipient mice. Nearly the same infection efficiency was observed in the overexpression and control groups 48 hours after infection, tested by FACS analysis. However, the Gadd45a-overexpressed HSCs were largely diminished in recipient mice 1 month after transplantation (supplemental Figure 4I), which is associated with a drastic increase of apoptosis in the Gadd45a-overexpressed BM cells compared with control (supplemental Figure 4I). To confirm whether Gadd45a overexpression could result in cell death, we ectopically expressed murine Gadd45a in wild-type HSCs by lentiviral transduction in a short-term culture. Shortly after lentiviral transduction, the cells were subjected to 1- to 2-Gy IR. Gadd45a-overexpressed HSCs were more sensitive to IR-induced cell death (supplemental Figure 4J), indicating a role of Gadd45a in apoptosis induction after IR.
To validate these findings in vivo, we subjected recipient mice (reconstituted with either Gadd45a+/+ or Gadd45a−/− BM cells) to 2 rounds of 4.5-Gy IR. Strikingly, most of the Gadd45a+/+ mice died within 203 days, whereas none of the Gadd45a−/− mice died within the same time frame (Figure 4E). We examined cell cycle status and apoptosis 4.5 months after the second-round IR. A comparable cell cycle profile was observed in both HSCs and HPCs between Gadd45a+/+ and Gadd45a−/− donor-derived BM (Figure 4F), whereas a significantly reduced apoptosis was detected in Gadd45a−/− HSCs but not in Gadd45a−/− HPCs (Figure 4G). These data suggested that the long-term beneficial effect of Gadd45a deficiency on the survival of mice after IR might involve a decrease in HSC apoptosis.
Accumulation of IR-induced DNA damage impairs the function of Gadd45a−/− HSCs and promotes leukemogenesis
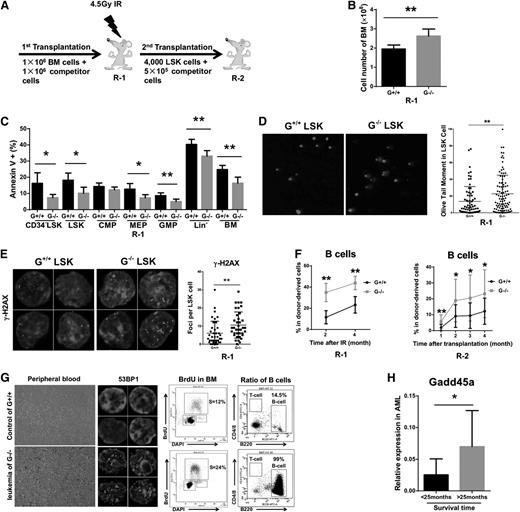
The attenuated apoptosis and delayed DNA repair after IR might impair the genomic integrity of Gadd45a−/− HSCs. To explore the potential deleterious consequences, we transplanted Gadd45a+/+ or Gadd45a−/− BM cells into primary Recipients-1 (R-1) mice, and 4 months after transplantation, R-1 mice were treated with 4.5-Gy IR, followed by secondary transplantation to Recipients-2 (R-2) mice 4 months after IR (Figure 5A). One month after 4.5-Gy IR, Gadd45a−/− R-1 mice showed a dramatic increase in the total number of BM cells and a significant decrease in apoptosis compared with Gadd45a+/+ R-1 mice (Figure 5B-C), whereas no significant difference in cell cycle status was detected in these mice (supplemental Figure 5A). Furthermore, a comet assay and γ-H2AX staining were performed to evaluate the level of DNA damage in the HSCs of R-1 mice 3 months after IR. The results showed that Gadd45a−/− HSCs had significantly more DNA breaks and DNA damaged foci (Figure 5D-E and supplemental Figure 5B).
Accumulation of IR-induced DNA damage impairs the function of Gadd45a−/−HSCs and promotes leukemogenesis. (A) Schematic diagram for study design. Wild-type mice were fully reconstituted with either Gadd45a+/+ or Gadd45a−/− BM and were named as R-1, which were exposed to 4.5-Gy IR 4 months after transplantation. Four thousand donor-derived LSK cells, along with 0.5 × 106 competitors, were subjected to secondary transplantation into R-2 mice 4 months after IR. (B) The cell number of total BM cells in R-1 mice 1 month after 4.5-Gy IR is shown (G+/+, n = 5; G−/−, n = 6). (C) Apoptosis in the indicated cell populations of the R-1 mice was detected with Annexin V/DAPI staining 1 month after 4.5-Gy IR (G+/+, n = 5; G−/−, n = 6). (D) Isolated LSK cells from R-1 mice 3 months after 4.5-Gy IR were subjected to the alkaline comet assay. The DNA was stained with DAPI, and representative examples are shown. The olive tail moment represents DNA damage level. (E) Isolated LSK cells from R-1 mice 3 months after 4.5-Gy IR were stained by γ-H2AX antibody and DAPI. (F) The percentage of donor-derived B cells in PB of R-1 (post-IR) or R-2 mice is shown (R-1, n = 5; R-2, n = 16). (G) Representative image showing the differences between Gadd45a+/+ donor-derived control and Gadd45a−/− donor-derived leukemia: hemogram of PB, 53BP1 foci, BrdU incorporation, and percentage of B cells in BM. (H) Gadd45a expression (relative to GAPDH) was measured in BM cells of AML patients, who were separated by survival time of 25 months (<25 months, n = 10; >25 months, n = 7) (*P < .05, **P < .01).
Accumulation of IR-induced DNA damage impairs the function of Gadd45a−/−HSCs and promotes leukemogenesis. (A) Schematic diagram for study design. Wild-type mice were fully reconstituted with either Gadd45a+/+ or Gadd45a−/− BM and were named as R-1, which were exposed to 4.5-Gy IR 4 months after transplantation. Four thousand donor-derived LSK cells, along with 0.5 × 106 competitors, were subjected to secondary transplantation into R-2 mice 4 months after IR. (B) The cell number of total BM cells in R-1 mice 1 month after 4.5-Gy IR is shown (G+/+, n = 5; G−/−, n = 6). (C) Apoptosis in the indicated cell populations of the R-1 mice was detected with Annexin V/DAPI staining 1 month after 4.5-Gy IR (G+/+, n = 5; G−/−, n = 6). (D) Isolated LSK cells from R-1 mice 3 months after 4.5-Gy IR were subjected to the alkaline comet assay. The DNA was stained with DAPI, and representative examples are shown. The olive tail moment represents DNA damage level. (E) Isolated LSK cells from R-1 mice 3 months after 4.5-Gy IR were stained by γ-H2AX antibody and DAPI. (F) The percentage of donor-derived B cells in PB of R-1 (post-IR) or R-2 mice is shown (R-1, n = 5; R-2, n = 16). (G) Representative image showing the differences between Gadd45a+/+ donor-derived control and Gadd45a−/− donor-derived leukemia: hemogram of PB, 53BP1 foci, BrdU incorporation, and percentage of B cells in BM. (H) Gadd45a expression (relative to GAPDH) was measured in BM cells of AML patients, who were separated by survival time of 25 months (<25 months, n = 10; >25 months, n = 7) (*P < .05, **P < .01).
To explore the ultimate outcomes of Gadd45a−/− HSCs with unrepaired DNA lesions induced by IR, we calculated the percentage of hematopoietic reconstruction (PB chimerism > 1%) 3 months afte transplantation in R-2 mice. Gadd45a−/− R-2 mice showed a much lower percentage than Gadd45a+/+ R-2 mice (52.9% vs 87.5%, P = .05), suggesting the DNA damage accumulation impaired the reconstitution capacity of Gadd45a−/− HSCs. Surprisingly, a higher percentage of B lymphocytes was observed in the donor-derived PB of Gadd45a−/− R-1 (after IR) and R-2 mice (leukemic mice were excluded), suggesting IR-induced damage potentiates a lineage skewing toward B cells in the Gadd45a−/− donor-derived PB cells (Figure 5F). Interestingly, 6 months after the secondary transplantation, 3 Gadd45a−/− R-2 mice developed donor-derived B-cell acute lymphoblastic leukemia, whereas B-cell acute lymphoblastic leukemia was not observed in Gadd45a+/+ R-2 mice. The leukemic mice showed a blast of leukemic cells expressing B220 (B-cell surface marker) in the PB and BM cells and exhibited drastically enhanced proliferation and a massive accumulation of DNA damage (Figure 5G).
To understand the clinical relevance of the aforementioned results, we examined the expression level of Gadd45a in acute myeloid leukemia (AML) patients using quantitative reverse transcriptase (qRT)-PCR. Interestingly, we found patients with poor survival rate (>25 months) expressed a lower level of Gadd45a (Figure 5H), suggesting the level of Gadd45a expression might influence the prognosis of AML patients.
Accumulation of DNA damage impairs HSC maintenance in old Gadd45a−/− mice
We next investigated the influence of Gadd45a deficiency on the maintenance of HSCs during aging. First, we compared the expression level of Gadd45a in old (24 months old) and young (2 months old) wild-type mice. Compared with young LT-HSCs, the expression of Gadd45a was remarkably lower in old LT-HSCs (Figure 6A and supplemental Figure 6A). In addition, the old mice exhibited comparable level of Gadd45a expression in LT-HSCs and HPCs (supplemental Figure 6B). We then analyzed the hematopoietic phenotypes in old Gadd45a+/+ mice and age-matched Gadd45a−/− mice. In PB, old Gadd45a−/− mice had a greater number of erythrocytes (supplemental Figure 6C) and a higher percentage of T cells (supplemental Figure 6D). In BM, a significant decrease in the absolute number of LT-HSCs was observed in old Gadd45a−/− mice (Figure 6B), suggesting a deleterious effect of Gadd45a deficiency on the long-term maintenance of primitive HSCs.
Accumulation of DNA damage impairs HSCs maintenance in old Gadd45a−/−HSCs. (A) Gadd45a expression (relative to GAPDH or β-actin) in young (2 months old) and old LT-HSCs (24 months old) was measured by qRT-PCR (SYBR green assay) (n = 4). (B) The absolute number of LT-HSCs, ST-HSCs, MPPs, and HPCs in old mice was measured by FACS (n = 8). (C) Twenty thousand BM cells from old mice were cultured with cytokines in methylcellulose semisolid medium for 9 days. The number of colonies were counted (n = 4). The colony forming unit-granulocyte/macrophage colonies include the colony forming unit-granulocyte and colony forming unit-macrophage colonies. (D) Single CD34−LSK and CD34+LSK cells were sorted in a 96-well plate and cultured for 14 days in vitro. Colonies were counted in each group (G+/+, n = 6; G−/−, n = 8). (E) Ten thousand CD34−LSK cells from old mice were cultured in SFEM with stem cell factor (50 ng/mL) and Thrombopoietin (50 ng/mL). Cell numbers were counted at particular time points (n = 4). (F) Competitive transplantation of aging LT-HSCs was conducted by injecting 500 CD34−LSK cells along with 1 × 106 competitor cells into lethally irradiated recipients. Four months later, the chimeric BM cells were retransplanted into the secondary recipient mice. The chimerism in PB is shown at 4 or 16 weeks after transplantation (first, n = 7-8; second, n = 7). (G) The absolute number of donor-derived CD34−LSK cells was calculated 4 months after the first transplantation using FACS (n = 6). (H-I) LSK cells were isolated from old mice and subjected to the comet assay and γ-H2AX staining. DAPI was used to stain the nucleus. (J) Cell cycle analysis was performed on CD34−CD48−CD150+LSK, CD34−LSK, and LSK fractions with Ki67/DAPI staining. The percentages of cells in the G0, G1, and S/G2/M phases were analyzed using flow cytometry (G+/+, n = 4; G−/−, n = 5). (K) Single CD34−LSK cells were sorted in a 96-well plate and were exposed to 1Gy IR; colonies were counted after 14-day culture. The percentage of colonies (determined by dividing the number of colonies by the original number of seeded single cells) in each group is shown (G+/+, n = 3; G−/−, n = 4) (*P < .05, **P < .01, ***P < .001). N.S., not significant.
Accumulation of DNA damage impairs HSCs maintenance in old Gadd45a−/−HSCs. (A) Gadd45a expression (relative to GAPDH or β-actin) in young (2 months old) and old LT-HSCs (24 months old) was measured by qRT-PCR (SYBR green assay) (n = 4). (B) The absolute number of LT-HSCs, ST-HSCs, MPPs, and HPCs in old mice was measured by FACS (n = 8). (C) Twenty thousand BM cells from old mice were cultured with cytokines in methylcellulose semisolid medium for 9 days. The number of colonies were counted (n = 4). The colony forming unit-granulocyte/macrophage colonies include the colony forming unit-granulocyte and colony forming unit-macrophage colonies. (D) Single CD34−LSK and CD34+LSK cells were sorted in a 96-well plate and cultured for 14 days in vitro. Colonies were counted in each group (G+/+, n = 6; G−/−, n = 8). (E) Ten thousand CD34−LSK cells from old mice were cultured in SFEM with stem cell factor (50 ng/mL) and Thrombopoietin (50 ng/mL). Cell numbers were counted at particular time points (n = 4). (F) Competitive transplantation of aging LT-HSCs was conducted by injecting 500 CD34−LSK cells along with 1 × 106 competitor cells into lethally irradiated recipients. Four months later, the chimeric BM cells were retransplanted into the secondary recipient mice. The chimerism in PB is shown at 4 or 16 weeks after transplantation (first, n = 7-8; second, n = 7). (G) The absolute number of donor-derived CD34−LSK cells was calculated 4 months after the first transplantation using FACS (n = 6). (H-I) LSK cells were isolated from old mice and subjected to the comet assay and γ-H2AX staining. DAPI was used to stain the nucleus. (J) Cell cycle analysis was performed on CD34−CD48−CD150+LSK, CD34−LSK, and LSK fractions with Ki67/DAPI staining. The percentages of cells in the G0, G1, and S/G2/M phases were analyzed using flow cytometry (G+/+, n = 4; G−/−, n = 5). (K) Single CD34−LSK cells were sorted in a 96-well plate and were exposed to 1Gy IR; colonies were counted after 14-day culture. The percentage of colonies (determined by dividing the number of colonies by the original number of seeded single cells) in each group is shown (G+/+, n = 3; G−/−, n = 4) (*P < .05, **P < .01, ***P < .001). N.S., not significant.
To examine the influence of Gadd45a deficiency on HSC function during aging, we performed a colony-forming assay with BM cells in methylcellulose medium. The total number of colonies and the number of colony forming unit-granulocyte/macrophage colonies (including colony forming unit-granulocyte and colony forming unit-macrophage) was significantly lower in the Gadd45a−/− group (Figure 6C), suggesting a decreased number of HSCs and/or HPCs in Gadd45a−/− mice during aging. To test the function of HSCs from old Gadd45a+/+ and Gadd45a−/− mice at a single-cell level, we performed a single-cell colony forming assay in liquid culture medium. Old Gadd45a+/+ and Gadd45a−/− HSCs showed a comparable ability to form colonies (Figure 6D). To further evaluate the proliferation capacity of HSCs, we cultured 10 000 CD34−LSK cells from old Gadd45a+/+ and Gadd45a−/− mice and recorded the growth curve. After 80 hours of culture, old Gadd45a+/+ and Gadd45a−/− HSCs showed very similar growth rates (Figure 6E).
To determine the influence of Gadd45a deficiency on the function of old HSCs in vivo, a competitive transplantation experiment was conducted using 500 CD34−LSK cells from old Gadd45a+/+ or Gadd45a−/− mice, along with 1 × 106 competitor cells. After 4 months, the chimeric BM cells were retransplanted into the secondary recipient mice. There was no significant difference in the PB and BM chimerism derived from old Gadd45a+/+ and Gadd45a−/− HSCs (Figure 6F and supplemental Figure 6E). The Gadd45a−/− donor-derived PB cells showed a comparable multilineage distribution compared with Gadd45a+/+ donor-derived PB cells (supplemental Figure 6F). Further analysis of BM cells in the primary recipient mice revealed a significant decrease in Gadd45a−/− donor-derived HSCs compared with Gadd45a+/+ controls (Figure 6G) Together, these data indicated that Gadd45a deficiency leads to a decrease in the number of HSCs during aging, despite the function still remaining on a per cell basis.
Next, we examined the level of DNA damage in old Gadd45a+/+ and Gadd45a−/− HSCs by comet assay and γ-H2AX staining. The data showed a much higher level of DNA damage in old Gadd45a−/− HSCs compared with Gadd45a+/+ HSCs (Figure 6H-I), indicating a substantial accumulation of DNA damage in Gadd45a−/− HSCs during aging. Furthermore, we detected a reduced cell cycle entry in old Gadd45a−/− HSCs (Figure 6J). Together, these data suggested that the reduced pool of HSCs in old Gadd45a−/− mice was likely due to compromised cell proliferation, which was caused by excessive DNA damage accumulation during aging. To further test this presumption, CD34−LSK cells were isolated from old, Gadd45a+/+, and Gadd45a−/− mice treated with 1-Gy IR and subjected to a single-cell colony forming assay. In contrast to the young HSCs (Figure 4A), the old Gadd45a−/− HSCs were more sensitive to IR, which suppressed the formation of colonies (Figure 6K). This reinforced the presumption that increased DNA damage diminishes the pool of functional HSCs in old Gadd45a−/− mice. To reveal the underlying mechanisms, we tested the DNA damage response in old mice. Gadd45a could be induced by IR in old HSCs. However, the level of Gadd45a induction in old HSCs was significantly lower than that in young HSCs (supplemental Figure 6G). No significant difference was found in the cell cycle profile 24 hours after 1- to 2-Gy IR between HSCs from old Gadd45a+/+ and Gadd45a−/− mice (supplemental Figure 6H). Interestingly, old Gadd45a−/− HSCs exhibited a higher level of apoptosis than age-matched Gadd45a+/+ HSCs 24 and 72 hours after IR (supplemental Figure 6I), which suggested that a higher level of DNA damage accumulation in old Gadd45a−/− HSCs activates a Gadd45a-independent mechanism to induce cell death after IR
Discussion
Gadd45a has been implicated in many biological processes and functions as a key tumor suppressor in many types of malignancies.17 Although the function of Gadd45a in the genotoxic-stress response has been studied in BM cells, its role in the HSC homeostasis and stress responses is still unknown. In this study, we found that deletion of Gadd45a resulted in an increased regeneration capacity and enhanced resistance to IR-induced apoptosis in HSCs while leaving more HSCs with unrepaired DNA damage. This accumulation of DNA damage ultimately compromised the HSC function and increased their susceptibility to malignant transformation. Interestingly, during aging, Gadd45a−/− HSCs also accumulated more DNA damage than the age-matched Gadd45a+/+ mice, which progressively reduced the pool of HSCs and increased their sensitivity to IR.
In the present study, our data suggested a distinct function of Gadd45a in HSCs and progenitors. In response to IR-induced DNA damage, Gadd45a appeared to promote apoptosis in HSCs but suppress cell death in GMPs. The contrast phenotype between HSCs and GMPs could be due to the different level of Gadd45a expression in HSCs and GMPs. In steady state, Gadd45a+/+ GMPs exhibited a lower level of Gadd45a expression compared with HSCs (Figure 1A). After IR, the level of Gadd45a expression was also significantly lower in GMPs than in HSCs (supplemental Figure 4A). Therefore, in response to IR, the role of Gadd45a in the regulation of apoptosis might be less significant in GMPs compared with HSCs, whereas the presence of Gadd45a is still important for DNA repair in GMPs. As a consequence, the unrepaired DNA damage in Gadd45a−/− GMPs (Figure 4D and supplemental Figure 4H) mainly activates Gadd45a-independent apoptosis pathways to eliminate damaged cells (supplemental Figure 4E-F). In contrast, HSCs exhibited higher level of Gadd45a expression induced by IR, which directly promotes the induction of apoptosis in addition to its role in DNA repair. Therefore, loss of Gadd45a significantly impaired the activation of IR-induced apoptosis in HSCs (Figure 4C). According to our data and a previous publication,24 we speculate that a higher level of Gadd45a might be required for exerting its role in apoptosis induction, whereas the basal level of Gadd45a mainly involves in DNA repair. This is concordant with the perspectives that HSCs use distinctive mechanisms in DNA damage response from their mature progeny.25-27
In line with this speculation, our data also showed a distinct impact of Gadd45a loss on the young and old HSCs. Old Gadd45a−/− HSCs were more sensitive to IR-induced apoptosis than old Gadd45a+/+ HSCs (supplemental Figure 6I), which is in contrast to their young counterparts (Figure 4C). A possible explanation could be that the Gadd45a-mediated apoptosis might be significant in young HSCs but not in old HSCs, considering the lower expression level in old HSCs at steady state and after IR compared with their young counterparts (Figure 6A and supplemental Figure 6G). In fact, our data showed that the IR-induced apoptosis was much lower in the old HSCs than the young HSCs in both Gadd45a+/+ and Gadd45a−/− mice. In addition, loss of Gadd45a led to an impairment in DNA repair (Figure 4D), which progressively increased the accumulation of DNA damage in old Gadd45a−/− HSCs (Figure 6H-I). This increased basal level of DNA damage in old Gadd45a−/− HSCs aggravated the IR-induced apoptosis, which appeared to involve Gadd45a-independent apoptosis pathways. Taken together, our data suggested that Gadd45a has a dual role in response to DNA damage: facilitating DNA repair at the basal level to prevent cell death and inducing apoptosis at a higher level to eliminate damaged HSCs. This finding is particularly significant for its clinical relevance because the DNA damage response and genomic integrity of HSCs is specifically essential for the lifelong maintenance of the blood system and leukemia prevention.28
In a previous study, Gupta et al21 showed the Gadd45a−/− BM cells were more sensitive to genotoxic agents than Gadd45+/+ BM cells. Consistently, in our study, Gadd45a−/− GMPs showed more IR-induced apoptosis than Gadd45a+/+ GMPs, although the phenotype may not be as drastic as Gupta et al found. Possible explanations for this discrepancy could be as follows. (1) Different cell populations were analyzed. Gupta et al studied the function of Gadd45a using BM cells, whereas we used FACS-purified GMPs. (2) Different culture conditions might influence the sensitivity to DNA damage. Gupta et al cultured BM cells for 48 hours prior to treatment with stress agents, whereas we used freshly isolated GMPs. (3) A different type of DNA damage could influence the results, as different stress could activate Gadd45a through different signaling pathways.16 Gupta et al studied the function of Gadd45a using ultraviolet radiation–, VP-16–, and daunorubicin-induced stress, whereas we investigated Gadd45a function with IR-induced DNA damage.
In conclusion, the results of the present study demonstrated that Gadd45a has an important function in regulating HSC stress responses by preventing excessive proliferation after 5-FU treatment or serial transplantation and promoting DNA damage repair and eliminating damaged HSCs after IR-induced DNA damage. Our findings from a genetic mouse model have significant implications for not only the understanding of the regulation of HSCs under stress conditions but also the development of strategies in clinical therapies for age-related hematopoietic disorders and leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported by the National Basic Research Program of China (2011CB964800 and 2012CB911203), the National Natural Science Foundation of China (81130074), the Strategies for Engineered Negligible Senescence Research Foundation (to Z.J.), and the National Basic Research Program of China (2012CB966604) (to W.Y.).
Authorship
Contribution: Y.C. and Z.J. initiated the research, developed the concept of the paper, designed the study, and analyzed and interpreted the data; Y.C., X.M., M.Z., X.W., C.W., H.W., and P.G. performed the experiments; and Y.C. and Z.J. wrote the manuscript with contributions from K.L.R., Q.Z., and W.Y.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhenyu Ju, Institute of Aging Research, School of Medicine, Hangzhou Normal University, Wen Yi Xi Lu 1378, Hangzhou, Zhejiang, China; e-mail: zhenyuju@163.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal