Key Points
Prophylactic ECP protects against GVHD in a murine BMT model.
ECP provides apoptotic signals that promote tolerance through dendritic cells and Tregs.
Abstract
Acute graft-versus-host disease (GVHD) is induced by alloreactivity of donor T cells toward host antigens presented on antigen-presenting cells (APCs). Apoptotic cells are capable of inducing tolerance by altering APC maturation. Apoptosis can be induced by extracorporeal photopheresis (ECP). We demonstrate that the use of ECP as a prophylaxis prior to conditioning significantly improves survival (P < .0001) after bone marrow transplantation (BMT) by inhibiting the initiation phase of acute GVHD in a murine BMT model. ECP-treated autologous splenocytes resulted in immune tolerance in the host, including reduced dendritic cell activation with decreased nuclear factor-κB engagement, increased regulatory T-cell (Treg) numbers with enhanced expression of cytolytic T lymphocyte-associated antigen 4, potentiating their suppressive function. The protective effect required host production of interleukin-10 and host Tregs. Conventional T cells that entered this tolerant environment experienced reduced proliferation, as well as a reduction of tissue homing and expression of activation markers. The induction of this tolerant state by ECP was obviated by cotreatment with lipopolysaccharide, suggesting that the inflammatory state of the recipient prior to treatment would play a role in potential clinical translation. The use of prophylactic ECP may provide an alternative and safe method for immunosuppression in the bone marrow transplant setting.
Introduction
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality after allogeneic bone marrow transplantation (BMT). GVHD occurs when donor T cells recognize and respond to alloantigens on antigen-presenting cells (APCs).1,2 The pathophysiology of acute GVHD has been described as a 3-step phenomenon: (1) activation of host APCs; (2) the effector phase, characterized by donor T-cell activation, proliferation, and differentiation; and (3) target tissue destruction.3 All three steps continue to interact as GVHD progresses.
Strategies to prevent GVHD focus primarily on the effector phase by inhibiting donor T-cell activation and proliferation. The most common approach is to administer immunosuppressive drugs that act in a nonspecific fashion without inducing true tolerance. Although effective, these drugs result in significant toxicity and risk for opportunistic infections.4,5 An alternative approach is to engineer the graft including T-cell depletion or administration of various subsets of T cells, such as regulatory T cells (Tregs).6-8 Despite the knowledge of the impact of host APCs on GVHD induction, only a few preclinical studies have focused on targeting APCs to prevent GVHD induction.9-12
Professional APCs internalize antigens, display peptides on their surface, and express costimulatory molecules that guide T-cell activation. Dendritic cells (DCs), the most potent APC population, play important roles in induction of immunity and in the maintenance of tolerance.13 Whether DCs act in an immunogenic vs a tolerogenic fashion depends on the maturation state and context in which the antigen is acquired.14 Although immature DCs efficiently take-up antigens but poorly activate T cells, mature DCs shut down antigen acquisition and upregulate costimulatory molecules to effectively prime T cells.15 During steady state, immature DCs continuously encounter apoptotic cells from normal tissue turnover. Phagocytosis of apoptotic cells inhibits the upregulation of costimulatory molecules, converting immature DCs into tolerogenic DCs (TOL-DCs).16,17 Consequently, T-cell activation in the absence of costimulatory molecules results in T-cell anergy and induction of Tregs.18,19
Apoptosis can be induced by extracorporeal photopheresis (ECP), a therapy based on exposure of cells to photoactivatable 8-methoxypsoralen (8MOP) and ultraviolet light A (UVA) irradiation.20 ECP is successfully used to treat established acute and chronic GVHD especially in patients unresponsive to conventional immunosuppressive drugs and has been proven successful in prevention of solid organ rejection.21-23
We hypothesized that administration of apoptotic cells prior to transplantation would induce unresponsiveness in the majority of immature DCs, consequently limit donor T-cell activation, and hence reduce GVHD induction. Our data show that the novel use of ECP as a prophylactic therapy during conditioning prevents GVHD by inducing tolerance and immunosuppression from immature DCs in response to proapoptotic signals. These signals increase host Tregs that also upregulate cytolytic T lymphocyte-associated antigen 4 (CTLA4). Alloreactive T-cell proliferation is impaired and mortality due to GVHD is significantly reduced. Graft-versus-tumor (GVT) effects are maintained.
Materials and methods
Mice
C57BL/6 (H2kb), BALB/c (H2kd), FVB (H2kq), C57BL/6 IL10−/− (B6.129P2-Il10tm1Cgn/J; H2b), BALB/c IL10−/− (C.129P2(B6)-Il10tm1Cgn/J; H2d), and AKR/J (H2k) mice were purchased from The Jackson Laboratory. Luciferase-expressing (luc+) C57BL/6-L2G85 (H2kb) mice were created as previously described.24,25 C57BL/6-Foxp3DTR mice were a kind gift from Dr A. Rudensky (Howard Hughes Medical Institute, Memorial Sloan-Kettering Cancer Center, NY) and BALB.K (H2k) from Dr J. A. Shizuru (Stanford University, Stanford, CA). Mice were 8 to 12 weeks old, cared for according to the Guidelines for Laboratory Animals and studies were approved by Stanford University Administrative Panel on Laboratory Animal Care.
BM transplantation
Mice were conditioned with total body irradiation (BALB/c: 800 cGy, BALB.K: 700 cGy, C57BL/6: 1000 cGy, split dose). BALB/c mice received an IV infusion of 5 × 106 T-cell–depleted BM (TCD-BM) plus 7.5 × 105 positively selected CD4+/CD8+ conventional T cells (Tcons) from allogeneic C57BL/6 or syngeneic BALB/c mice. In some experiments, conditioned mice were injected with luc+ C57BL/6-L2G85 Tcons to allow for bioluminescent imaging (BLI) imaging. In some experiments, BALB/c mice received 1 × 105 fluorescence-activated cell sorter (FACS)–purified Tregs (CD4+CD25high) from ECP-treated or nontreated BALB/c 24 hours prior to BMT.
BALB.K mice received 5 × 103 purified hematopoietic stem cells (HSCs: c-Kit+, Thy1.1lo-int, Sca-1+, CD3ε−, CD4−, CD5−, CD8α−, B220−, Gr1−, Mac1−, TER-119−) plus 105 Tcons from allogeneic AKR/J mice.
C57BL/6 and C57BL/6-Foxp3DTR mice received 5 × 106 TCD-BM plus 1.5 × 106 Tcons from FVB donors. Treg depletion in C57B6-Foxp3DTR mice was performed by intraperitoneal (IP) injection of 50 μg/g diphtheria toxin (DT) at day −1 and −2 prior to BMT.
Mice were housed in autoclaved cages with antibiotic water (sulfamethoxazole and trimethoprim; Hi-Tech Pharmacal) for a minimum of 30 days.
ECP treatment
Splenocytes were incubated for 30 minutes with 0.2 mg/mL 8MOP (UVADEX; Therakos) and exposed to 2 J/cm2 UVA light (UVAR Light Set; Therakos). After 2 washes, 107 cells were injected IV. For some experiments, whole splenocytes were incubated with 8MOP but no UV irradiation. If not indicated otherwise, IV injection of apoptotic cells was performed 48 hours prior to BMT.
Cell isolation and flow cytometry reagents
Positive selection or depletion for CD4+ and CD8+ T cells was performed using anti-CD4 monoclonal antibody (mAb) (clone L3T4) and anti-CD8 mAb (Ly-2) and magnet column separation techniques (Miltenyi Biotec). Cells were stained and analyzed or sorted using the following reagents: CD4 (GK1.5 or RM4-5), CD8α (53-6.7), CD25 (PC61.5), H-2Kb (AF6-88.5), Foxp3 (FJK-16s), CD80 (16-10A1), CD86 (GL1), CD40 (HM40-3), MHCII-IAd (39-10-8), CD69 (H1-2F3), CD44 (IM7), P-Selectin (K02.12), α4β7 (DATK32), CD11c (HL3), CTLA4 (UC10-4B9), NF-kB (pS529), AnnexinV, Sca-1+ (D7), CD3ε (17A2), CD5 (53-7.3), B220 (RA3-6B2), Gr1 (RB6-8C5), Mac1 (M1/70) and TER-119 (TER-199), live/dead fixable Aqua (BD Biolegend, Invitrogen, or eBiosciences).
Phospho-flow
Cells were stimulated with 5 μg/mL lipopolysaccharide (LPS; 30 minutes, 37°C), fixed with 1.5% paraformaldehyde (PFA) and cold methanol 100%, followed by intracellular staining. Samples were analyzed and sorted in a Stanford shared FACS facility (LSRII, FACSAria; Becton Dickinson). FlowJo software (TreeStar 8.8) was used for data analysis.
In vitro mixed lymphocyte reaction (MLR) culture
BALB/c splenocytes were cultured with or without ECP-treated BALB/c splenocytes and used as stimulators. After 48 hours, stimulators were irradiated (30 cGy) followed by addition of carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled C57BL/6 Tcon responders (stimulators:responders ratio: splenocytes 4:1; DCs 1:2). After 96 hours, CFSE (Vybrant CFSE) dilution was quantified or cells were pulsed with 1 µCi/well [3H]-thymidine and incorporation was measured with a Wallac Betaplate counter (PerkinElmer).
In vivo BLI
BLI was performed with an IVIS spectrum charge-coupled device imaging system as previously described (Xenogen)26 ; images were analyzed with LivingImage 3.0 software (Xenogen).
CFSE labeling and pulsed in vivo BrdU labeling
Tcons were labeled with a 2.5 μM CFSE Tracer kit (Invitrogen) in phosphate-buffered saline (5 minutes, 37°C). Reaction was quenched by cold RPMI 1640 with 10% fetal calf serum and washed 3 times to remove excess CFSE. 5-Bromo-2′-deoxyuridine (BrdU) was injected IP at a dose of 1 mg/mouse every second day, starting day +2 post-BMT.
Cytokine analysis
Supernatants of in vitro MLR assays (96 hours) or serum of experimental animals from day +5 were stained with Milliplex Immunoassay according to the manufacturer’s instruction (Millipore) and analyzed using Cytokine Bead Array (Luminex 200; Bio-Rad). Intracellular cytokines were assessed by restimulating with phorbol 12-myristate 13-acetate (40 ng/mL) and ionomycin (2 μM) in presence of monensin (2 μM; Sigma-Aldrich) for 5 hours. Cells were fixed, permeabilized, and assessed for interferon γ (INFγ) and tumor necrosis factor α (TNFα).
Cytotoxicity assay and in vivo tumor model
Bcl1 and A20 target cell lines (ATCC) were labeled with 300 μCi/mL 51Cr (PerkinElmer) at a dose of 2 × 104 (2 hours, 37°C, 5% CO2). CD8+ T cells, isolated 10 days post-BMT, served as effector cells (E) at effector-to-target (E:T) ratios of 20:1 and 5:1. Radioactivity released into supernatants was measured after 4 hours of incubation in a scintillation counter. Negative controls (spontaneous release) were supernatants from 3H-labeled target cell culture without effector cells.
In vivo Bcl1 tumor model
BALB/c mice were injected IV with 5 × 103 Bcl1 Luc+ (H2d) tumor cells. After tumor establishment, defined as BLI signal >2 × 106 photons per second per tumor, mice were transplanted and followed by BLI imaging.
Statistic analysis
Animal survival was analyzed by the log-rank test. To compare donor T-cell expansion, cytokine levels, surface marker expressions, and cytotoxicity assay, a 2-tailed Student t test was used (GraphPad Prism). In some cases, results were pooled from several experiments displaying the fold change based on Tcon as reference group.
Results
Host-type apoptotic cells infused prior to transplantation prolong survival
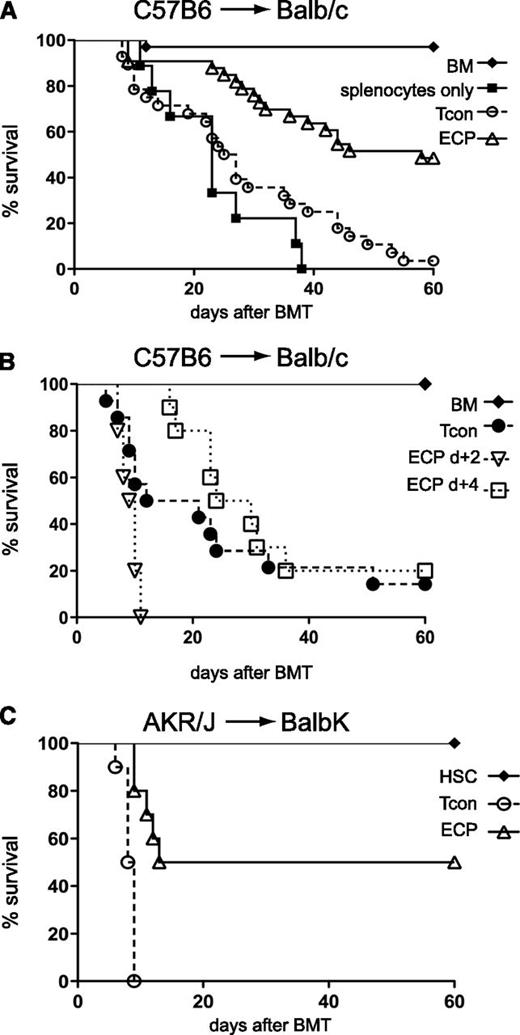
To investigate whether the tolerance-inducing effect of apoptotic cells can be exploited to reduce GVHD, BALB/c recipients received 107 ECP-treated BALB/c splenocytes, accounting for 5% to 10% of the whole splenic pool. Lower cell numbers (2 × 106) showed similar effects (data not shown). When apoptotic cells were cleared from recipients (supplemental Figure 1A, see supplemental Data available at the Blood Web site) and DCs have captured and processed apoptotic cells after 48 hours recipient mice underwent BMT. ECP-treated mice had improved survival (median survival 59 vs 26 days) with a delayed onset of GVHD compared with control mice treated with only TCD-BM, Tcons, and 8MOP (Figure 1A). Administration of ECP-treated cells prior to BMT at day −2 and −5 resulted in similar survival benefit without further improvement (data not shown). In contrast, administration of ECP-treated cells following BMT at day +2 or +4 showed no improvement in survival (Figure 1B). To exclude strain-specific effects, we performed additional experiments across minor histocompatibility barriers (AKR/j→BALB.K). The ECP-treated group similarly showed a significant improvement in survival (median survival 37 vs 8 days) with surviving mice showing no signs of GVHD (Figure 1C). To exclude a specific effect of splenocytes as opposed to peripheral blood cells we performed an MLR using peripheral blood cells as the apoptotic source. T-cell proliferation was significantly reduced (supplemental Figure 1B).
Exposure to host-type apoptotic cells 48 hours prior, but not after BMT improves survival in GVHD models. (A) ECP treatment 48 hours prior to BMT in C57BL/6→BALB/c mice improves survival. BALB/c mice injected with C57BL/6 TCD-BM plus Tcon only (○, n = 29) or with prior injection of ECP-treated BALB/c cells (△, n = 32) or with prior injection of 8MOP but no UV light-treated BALB/c cells (▪, n = 9). ○ vs △, P < .0001 (median survival, 26 vs 59 days). (B) ECP treatment 2 or 4 days after BMT does not improve survival. BALB/c with TCD-BM plus Tcon only (●, n = 10) or followed by ECP treatment with C57BL/6 cells at day +2 (▿, n = 10) or day +4 (□, n = 10). (C) ECP treatment 48 hours prior to BMT across minor histocompatibility barriers in AKR/J→BALB.K improves outcome. BALB.K injected with purified AKR/J HSC and Tcon alone (○, n = 10) or with prior injection of ECP-treated BALB.K cells (△, n = 10). ○ vs △, P = .0002 (median survival, 8 vs 37 days). Significance was assessed using the log-rank test.
Exposure to host-type apoptotic cells 48 hours prior, but not after BMT improves survival in GVHD models. (A) ECP treatment 48 hours prior to BMT in C57BL/6→BALB/c mice improves survival. BALB/c mice injected with C57BL/6 TCD-BM plus Tcon only (○, n = 29) or with prior injection of ECP-treated BALB/c cells (△, n = 32) or with prior injection of 8MOP but no UV light-treated BALB/c cells (▪, n = 9). ○ vs △, P < .0001 (median survival, 26 vs 59 days). (B) ECP treatment 2 or 4 days after BMT does not improve survival. BALB/c with TCD-BM plus Tcon only (●, n = 10) or followed by ECP treatment with C57BL/6 cells at day +2 (▿, n = 10) or day +4 (□, n = 10). (C) ECP treatment 48 hours prior to BMT across minor histocompatibility barriers in AKR/J→BALB.K improves outcome. BALB.K injected with purified AKR/J HSC and Tcon alone (○, n = 10) or with prior injection of ECP-treated BALB.K cells (△, n = 10). ○ vs △, P = .0002 (median survival, 8 vs 37 days). Significance was assessed using the log-rank test.
For comparative purposes, we injected donor-type ECP-treated cells 2 days prior to BMT that resulted in equivalent improvement in survival and in vitro suppression (supplemental Figure 2).
Uptake of apoptotic cells during steady state reduces NF-κB activation and costimulatory molecule expression in host DCs and diminishes trogocytosis in donor T cells
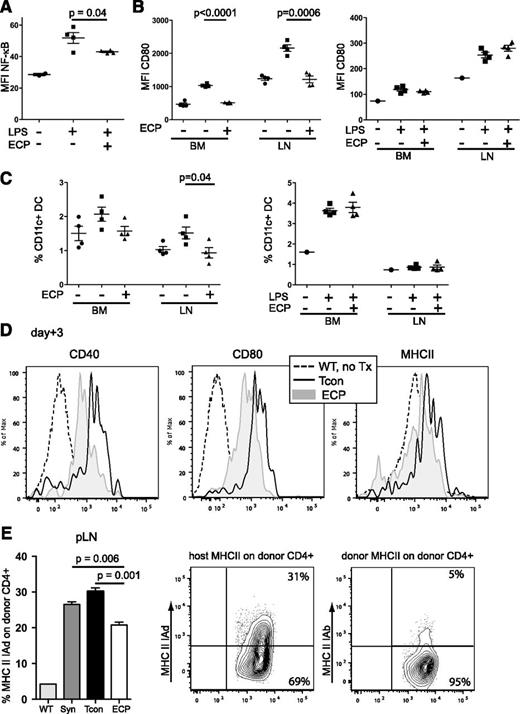
Because nuclear factor-κB (NF-κB) plays an important role in APC maturation,27 we assessed NF-κB activation by phospho-flow analysis and observed a significantly lower expression in host DCs in ECP-treated mice as compared with untreated mice (Figure 2A). Furthermore, both the expression of the costimulatory molecules CD80/CD86 as a characteristic of mature DCs and the frequency of DCs were significantly reduced. In contrast, when mice received LPS injections simultaneously with ECP treatment, DC maturation was restored with similar CD80/CD86 expression in ECP-treated and nontreated animals (Figure 2B-C, CD86, data not shown). The expression of CD40, CD80, and major histocompatibility complex II (MHCII) on host-type DCs remained significantly lower 3 days post-BMT in ECP-treated mice (Figure 2D). Together, the exposure to apoptotic cells favored TOL-DCs17 with low NF-κB activation and costimulatory molecule expression.
Uptake of apoptotic cells reduces NF-κB activation and costimulatory molecule expression in host DCs and diminishes MHCII uptake in donor T cells. (A) NF-κB activation in LPS-challenged BM-DCs is reduced in ECP-treated mice. Non-ECP-treated (▪) or ECP-treated mice (▲) were challenged with LPS (10 μg/mouse IV) 48 hours after ECP treatment and NF-κB activation was measured by phospho-flow staining. BM cells from unchallenged nontreated mice served as baseline control (●). MFI, Non-ECP-treated (▪) vs ECP-treated (▲), P = .04. (B) CD80 expression of DCs is reduced after ECP treatment but restored in the presence of danger signals during ECP treatment. Left, DCs in non-ECP-treated (▪) or ECP-treated (▲) mice were activated with LPS (10 μg/mouse IV) 48 hours after ECP treatment and harvested 18 hours after activation. MFI of CD80: (▪) vs (▲) in BM, P < .0001, or pLN: P = .0006. Right, Non-ECP-treated (▪) or ECP-treated (▲) mice received additional LPS (10 μg/mouse IV) at time of ECP treatment 48 hours prior to harvest. CD80 expression is restored in BM and pLNs. Unchallenged BM cells from untreated mice served as baseline control in both experiments (●). (C) DC frequency is reduced after ECP treatment but restored in the presence of additional danger signals during time of ECP treatment. Proportional contributions to cellular content of BM-DCs and pLN DCs from mice in panel B are shown. (D) Expression of costimulatory molecules in host-type DCs (H2d) is reduced at day +3 after BMT in ECP-treated mice. Histograms with MFI of CD40, CD80, and MHCII on host-type DCs (H2d) from representative animals are shown. (E) Trogocytosis is lower in ECP-treated mice. CD4+ T cells were isolated from pLN at day +5 post-BMT from untreated mice (WT), syngeneic BMT (BALB/c CD45.1→BALB/c, CD45.2; Syn) and allogeneic BMT (C57BL/6, H2b→BALB/c, H2d) with Tcon alone (Tcon) or plus ECP treatment (ECP) and analyzed for uptake of host MHCII-IAd (H2d) or donor MHCII-IAb (H2b). pLN: Tcon vs ECP, P = .001. WT represents MHCII expression on CD4+ T cells during steady state in untreated mice. (A-E) One representative experiment with at least 4 mice per group of 2 independent experiments is shown. Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test. MFI, mean fluorescence intensity; SEM, standard error of the mean.
Uptake of apoptotic cells reduces NF-κB activation and costimulatory molecule expression in host DCs and diminishes MHCII uptake in donor T cells. (A) NF-κB activation in LPS-challenged BM-DCs is reduced in ECP-treated mice. Non-ECP-treated (▪) or ECP-treated mice (▲) were challenged with LPS (10 μg/mouse IV) 48 hours after ECP treatment and NF-κB activation was measured by phospho-flow staining. BM cells from unchallenged nontreated mice served as baseline control (●). MFI, Non-ECP-treated (▪) vs ECP-treated (▲), P = .04. (B) CD80 expression of DCs is reduced after ECP treatment but restored in the presence of danger signals during ECP treatment. Left, DCs in non-ECP-treated (▪) or ECP-treated (▲) mice were activated with LPS (10 μg/mouse IV) 48 hours after ECP treatment and harvested 18 hours after activation. MFI of CD80: (▪) vs (▲) in BM, P < .0001, or pLN: P = .0006. Right, Non-ECP-treated (▪) or ECP-treated (▲) mice received additional LPS (10 μg/mouse IV) at time of ECP treatment 48 hours prior to harvest. CD80 expression is restored in BM and pLNs. Unchallenged BM cells from untreated mice served as baseline control in both experiments (●). (C) DC frequency is reduced after ECP treatment but restored in the presence of additional danger signals during time of ECP treatment. Proportional contributions to cellular content of BM-DCs and pLN DCs from mice in panel B are shown. (D) Expression of costimulatory molecules in host-type DCs (H2d) is reduced at day +3 after BMT in ECP-treated mice. Histograms with MFI of CD40, CD80, and MHCII on host-type DCs (H2d) from representative animals are shown. (E) Trogocytosis is lower in ECP-treated mice. CD4+ T cells were isolated from pLN at day +5 post-BMT from untreated mice (WT), syngeneic BMT (BALB/c CD45.1→BALB/c, CD45.2; Syn) and allogeneic BMT (C57BL/6, H2b→BALB/c, H2d) with Tcon alone (Tcon) or plus ECP treatment (ECP) and analyzed for uptake of host MHCII-IAd (H2d) or donor MHCII-IAb (H2b). pLN: Tcon vs ECP, P = .001. WT represents MHCII expression on CD4+ T cells during steady state in untreated mice. (A-E) One representative experiment with at least 4 mice per group of 2 independent experiments is shown. Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test. MFI, mean fluorescence intensity; SEM, standard error of the mean.
We next examined trogocytosis, a phenomenon characterized by inflammation-dependent incorporation of cell surface proteins including MHCII by T cells.28 The MHCII uptake on T cells in ECP-treated mice was significantly reduced compared with T cells from non-ECP-treated mice after allogeneic and syngeneic BMT (Figure 2E). Interestingly, allogeneic donor T cells showed a specific uptake of host MHCII (IAd) but not donor MHCII (IAb).
Reduced T-cell activation and proliferation rather than peripheral T-cell deletion is responsible for improved survival
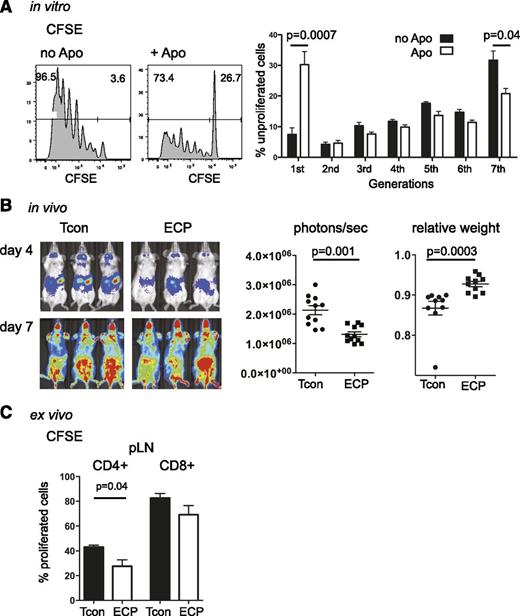
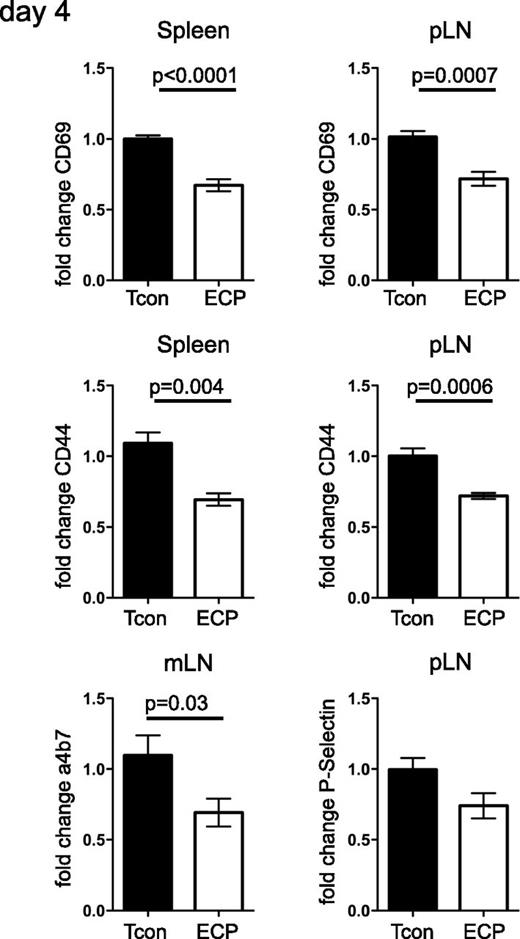
Splenocytes were cultured with or without syngeneic apoptotic cells and served as stimulators in an MLR. Allogeneic T-cell response to these stimulators was significantly reduced in cultures containing apoptotic cells (Figure 3A). BALB/c mice treated with ECP showed significantly lower T-cell proliferation as assessed by BLI 4 days after transplantation with C57Bl6 luc+ T cells compared with untreated mice (Figure 3B). ECP-treated mice had less weight loss associated with GVHD and a significantly prolonged overall survival. Interestingly, the BLI proliferation signals in ECP and untreated mice became the same at day +7 after BMT during the effector phase (Figure 3B). We confirmed the BLI results using CFSE-labeled donor Tcons. We found significantly lower T-cell proliferation in ECP-treated mice especially among CD4+ T cells (Figure 3C). The diminished proliferation could be due to decreased survival or reduced activation of donor Tcon cells. Because ECP-treated and untreated mice had the same level of apoptosis in donor T cells as assessed by equivalent Annexin V staining (data not shown), it is probable that decreased proliferation is the result of reduced activation rather than peripheral deletion. We further observed lower expression of activation markers CD69 and CD44, skin-homing marker p-selectin and gut-homing marker α4β7 in spleen, peripheral lymph nodes (pLNs), and mesenteric lymph nodes (mLNs) in ECP-treated animals (Figure 4). Reduced activation and slower trafficking of allogeneic T cells to target organs during the GVHD initiation phase contributed to the delayed onset of GVHD.
Apoptotic cells reduce T-cell proliferation in vitro and in vivo. (A) In vitro, T-cell proliferation is significantly reduced in MLR cultures of whole splenocytes, cultured with or without apoptotic cells for 48 hours, irradiated with 30 cGy and followed by coculture with CFSE-labeled allogeneic T cells for 96 hours at a ratio 2:1. FACS histogram of CFSE dilution profile (left) and bar graphs displaying the proportions of unproliferated T cells (right) are shown. (B) In vivo, Quantification of T-cell proliferation shows significant reduction in ECP-treated mice. BLI on day +4 (left, upper panel) shows a significant reduction in T-cell proliferation among ECP-treated mice, no difference in BLI signal is observed at day +7 between both groups (left, lower panel). Quantification of photons per second per mouse (middle; P = .001) and relative weight loss (right; P = .0003) of transplanted mice at day +4 is shown. (C) Ex vivo, Proportion of T-cell proliferation in reisolated CFSE-labeled Tcon at day +4 is shown. CD4+ and CD8+ T cells show lower proliferation index in ECP-treated mice. Results were done in triplicates (A) and represent 3 independent experiments or (B) are a composite of 2 independent experiments or (C) are representative for 2 individual experiments with a total of 8 to 11 mice per group. Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test.
Apoptotic cells reduce T-cell proliferation in vitro and in vivo. (A) In vitro, T-cell proliferation is significantly reduced in MLR cultures of whole splenocytes, cultured with or without apoptotic cells for 48 hours, irradiated with 30 cGy and followed by coculture with CFSE-labeled allogeneic T cells for 96 hours at a ratio 2:1. FACS histogram of CFSE dilution profile (left) and bar graphs displaying the proportions of unproliferated T cells (right) are shown. (B) In vivo, Quantification of T-cell proliferation shows significant reduction in ECP-treated mice. BLI on day +4 (left, upper panel) shows a significant reduction in T-cell proliferation among ECP-treated mice, no difference in BLI signal is observed at day +7 between both groups (left, lower panel). Quantification of photons per second per mouse (middle; P = .001) and relative weight loss (right; P = .0003) of transplanted mice at day +4 is shown. (C) Ex vivo, Proportion of T-cell proliferation in reisolated CFSE-labeled Tcon at day +4 is shown. CD4+ and CD8+ T cells show lower proliferation index in ECP-treated mice. Results were done in triplicates (A) and represent 3 independent experiments or (B) are a composite of 2 independent experiments or (C) are representative for 2 individual experiments with a total of 8 to 11 mice per group. Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test.
CD4+ T cells in ECP-treated mice show reduced expression of homing and activation markers. Donor CD4+ T cells (H2b) were reisolated at day +4 post-BMT and fold change based on Tcon as reference group is shown. Expression of activation markers CD69 and CD44 in spleen and pLN and gut homing marker α4β7 in mLN are significantly reduced, with skin homing marker p-selectin in pLN showing a similar trend. Data are pooled from 2 independent experiments with 7 mice per group. Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test.
CD4+ T cells in ECP-treated mice show reduced expression of homing and activation markers. Donor CD4+ T cells (H2b) were reisolated at day +4 post-BMT and fold change based on Tcon as reference group is shown. Expression of activation markers CD69 and CD44 in spleen and pLN and gut homing marker α4β7 in mLN are significantly reduced, with skin homing marker p-selectin in pLN showing a similar trend. Data are pooled from 2 independent experiments with 7 mice per group. Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test.
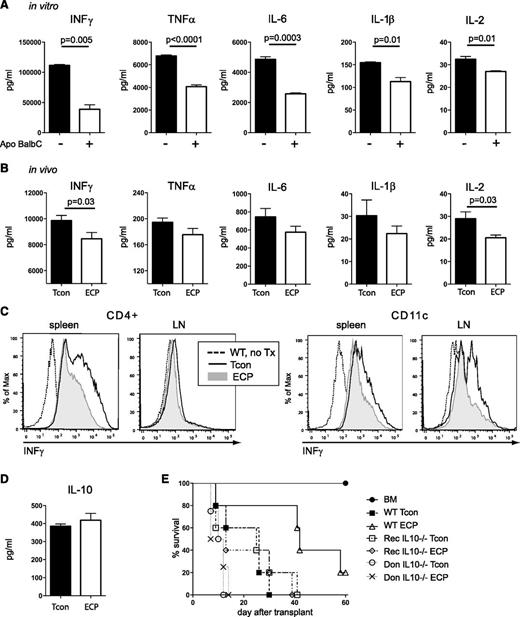
Uptake of apoptotic cells reduces proinflammatory cytokine secretion
To determine whether ECP treatment had an impact on proinflammatory cytokine profiles, we evaluated supernatants from MLR cultures of T cells coincubated with or without ECP-treated cells and found that coincubation with apoptotic cells resulted in a highly significant reduction of INFγ, TNFα, interleukin-6 (IL-6), IL-1β, and IL-2 (Figure 5A). Next, we compared cytokine profiles in serum of transplanted mice 5 days post-BMT and found a significant reduction in the levels of INFγ and IL-2 in the ECP group with a similar trend in TNFα, IL-6, and IL-1β (Figure 5B). Intracellular INFγ production was reduced in CD4 T cells and DCs indicating the impact of ECP not only on T cells but also on APCs (Figure 5C) whereas TNFα reduction was only observed in T cells in LNs during this early time point after BMT (supplemental Figure 3).
ECP treatment reduces proinflammatory cytokine secretion in vitro and in vivo and requires host type IL-10 for its beneficial effect. (A) Proinflammatory cytokine secretion into supernatants of MLR cultures is reduced in cells cocultured with apoptotic cells. Purified DCs were cultured with or without apoptotic cells for 48 hours, stimulated with LPS (2 μg/mL) and cocultured with freshly isolated allogeneic Tcons. Supernatants were harvested after 96 hours. (B) Proinflammatory cytokines are reduced in serum of mice treated with ECP, although statistical significance was only reached for INFγ and IL-2. Serum was obtained 5 days post-BMT. (C) Intracellular INFγ production is reduced in mice treated with ECP 4 days after transplantation. Single-cell suspension from LN and spleen were restimulated for 5 hours with PMA and Ionomycin in the presence of Monensin. (D) Serum analysis of IL-10 at day +5 shows marginal increase in ECP-treated group. (E) Host-type IL-10 is required for beneficial effect of ECP treatment. WT BALB/c recipients received either WT donor C57BL/6 Tcon (WT-Tcon, WT-ECP) or IL10-deficient donor C57BL/6 Tcon (Don IL10−/−Tcon; Don IL10−/−ECP) and IL10-deficient BALB/c recipient mice received WT C57BL/6 donor Tcon (Rec IL10−/−Tcon; Rec IL10−/−ECP). ECP was performed with WT BALB/c splenocytes. Rec IL10−/−ECP group showed no beneficial effect of ECP as compared with WT-Tcon or Rec IL10−/−Tcon group. Don IL10−/−Tcon and Don IL10−/−ECP showed accelerated death as compared with all other groups. Results were done in triplicates (A) and are representative of 3 individual experiments or (B,D) are representative of 2 individual experiments with 10 mice per group or 4 mice per group (C) or are representative of 2 independent experiments with n = 5 mice per group (E). Don, donor; Rec, recipient.
ECP treatment reduces proinflammatory cytokine secretion in vitro and in vivo and requires host type IL-10 for its beneficial effect. (A) Proinflammatory cytokine secretion into supernatants of MLR cultures is reduced in cells cocultured with apoptotic cells. Purified DCs were cultured with or without apoptotic cells for 48 hours, stimulated with LPS (2 μg/mL) and cocultured with freshly isolated allogeneic Tcons. Supernatants were harvested after 96 hours. (B) Proinflammatory cytokines are reduced in serum of mice treated with ECP, although statistical significance was only reached for INFγ and IL-2. Serum was obtained 5 days post-BMT. (C) Intracellular INFγ production is reduced in mice treated with ECP 4 days after transplantation. Single-cell suspension from LN and spleen were restimulated for 5 hours with PMA and Ionomycin in the presence of Monensin. (D) Serum analysis of IL-10 at day +5 shows marginal increase in ECP-treated group. (E) Host-type IL-10 is required for beneficial effect of ECP treatment. WT BALB/c recipients received either WT donor C57BL/6 Tcon (WT-Tcon, WT-ECP) or IL10-deficient donor C57BL/6 Tcon (Don IL10−/−Tcon; Don IL10−/−ECP) and IL10-deficient BALB/c recipient mice received WT C57BL/6 donor Tcon (Rec IL10−/−Tcon; Rec IL10−/−ECP). ECP was performed with WT BALB/c splenocytes. Rec IL10−/−ECP group showed no beneficial effect of ECP as compared with WT-Tcon or Rec IL10−/−Tcon group. Don IL10−/−Tcon and Don IL10−/−ECP showed accelerated death as compared with all other groups. Results were done in triplicates (A) and are representative of 3 individual experiments or (B,D) are representative of 2 individual experiments with 10 mice per group or 4 mice per group (C) or are representative of 2 independent experiments with n = 5 mice per group (E). Don, donor; Rec, recipient.
Host IL-10 is required for effective ECP treatment
IL-10 has been shown to play a key role in conventional ECP treatment but the role of IL-10 in preemptive ECP treatment is unclear.29,30 Although we could not detect differences in serum levels of IL-10 in ECP-treated vs non-ECP-treated mice, possibly due to utilization of IL-10 by Tregs (Figure 5D), the survival benefit after ECP treatment was completely abrogated when BALB/c IL-10-KO were used as recipients (Figure 5E). These results evidence that host IL10 is needed in our model. We evaluated whether IL-10 production is required by donor cells utilizing IL10-KO animals as donors. We found that mice that received IL-10-KO donor T cells showed equivalently accelerated GVHD and death irrespective of ECP treatment even when compared with the wild-type (WT) Tcon group (Figure 5E). Although not necessary, we cannot exclude the possibility that donor IL10 could contribute to ECP protection.
Host-type Foxp3+ Treg are required to improve survival but are not solely responsible for beneficial outcomes
Tregs are major determinants in the success of conventional ECP treatment.30,31 In our model of preemptive ECP, we observed a significant increase of host but not donor-type Tregs in ECP-treated mice as compared with untreated mice 5 days post-BMT (Figure 6A). It is known that Tregs are relatively radio-resistant32 and we hypothesized that the increase in host Tregs after BMT reflected a persistence of Tregs that were present before irradiation because host Tregs from ECP and untreated mice did not proliferate after BMT, as shown by BrdU incorporation assay (Figure 6B). Indeed, we found a significant increase in Tregs in ECP-treated mice with significantly higher surface expression of CTLA4 as compared with untreated control mice (Figure 6C-D). Importantly, these Tregs displayed a higher suppressive capability than WT Tregs in an MLR assay (Figure 6E).
ECP treatment induces host-type Foxp3+ Tregs that substantially contribute to but are not solely responsible for improved outcome. (A) Preemptive ECP treatment specifically increases host-type Tregs in C57BL/6→BALB/c. FACS gating strategy for Tregs isolated at day +4 (left), total percentage of Treg, fold increase of host-type Treg in spleen and pLNs based on Tcon group, and absolute numbers of host Foxp3+ cells per spleen are shown (right). (B) Proliferation capacity of Treg after BMT. Transplanted mice received BrdU injection (1 mg per mouse per IP) every second day followed by pLN harvest at day +7. No proliferation of host-type Treg was observed in mice transplanted with TCD-BM plus Tcon (left, middle). In mice receiving TCD-BM alone, host and donor-Treg proliferated (right). (C) ECP increases Tregs within 48 hours. Mice with or without ECP treatment were challenged with LPS (10 μg per mouse per IV) 48 hours after injection of ECP-treated cells and Tregs were analyzed in pLNs. Mice treated with ECP had significantly higher proportions of CD4+/Foxp3+ cells, compared with WT mice challenged with LPS only. Unchallenged mice served as a baseline. (D) CD4+ T cells from mice in panel C were evaluated for surface CTLA4 expression. ECP-treated mice had significantly higher levels of CTLA4 expression within the CD4+ population (left) and CD4+/Foxp3+ subpopulation, compared with untreated mice challenged with LPS only. (E) Treg from ECP-treated mice more actively suppressed T-cell proliferation than WT Tregs. [3H]-Thymidine incorporation of WT C57BL/6 responder Tcons (R) to Balb/c stimulators (S) in the presence of WT Tregs or ECP Tregs at different Treg to responder ratios is shown, P = .035. (F) Specific depletion of host Tregs prior to BMT in FVB→Foxp3DTR-C57BL/6. WT-C57BL/6 recipients received FVB Tcon (Tcon, ▲; ECP, ▪), as control for DT toxicity 1 group received 50 μg/kg DT at day −2 and −1 (WT Tcon+DT, ♦; WT ECP+DT, ▼). To specifically deplete host Tregs, C57BL/6-Foxp3DTR recipients were injected with 50 μg/kg DT at day −2 and −1 and transplanted with FVB Tcon (Foxp3DTR Tcon, ○; Foxp3DTR ECP, □). ECP group showed significant survival improvement in comparison with Tcon group, ▪ vs ▲, P = .03. Mice injected with DT showed exacerbated GVHD due to DT toxicity in all groups. Foxp3DTR ECP had no survival benefit in comparison with Foxp3DTR Tcon whereas benefit was maintained in WT ECP + DT vs WT Tcon + DT. (G) Adoptive transfer of host-type Tregs into untreated recipients prior to BMT failed to inhibit donor T-cell proliferation. Recipient mice received 105 sort-purified Tregs (CD4+CD25high) 24 hours prior to BMT. Tregs originated either from WT BALB/c (WT Treg) or from mice treated with ECP (ECP Treg). Recipients were transplanted with luc+Tcon and followed by BLI imaging. Data are representative (A,B,C,F,G) of at least 2 individual experiments with 3 to 10 mice per group, or (D) are a composite of 2 experiments with 10 mice per group or are done in triplicates with 3 independent mice (E). Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test and log-rank test.
ECP treatment induces host-type Foxp3+ Tregs that substantially contribute to but are not solely responsible for improved outcome. (A) Preemptive ECP treatment specifically increases host-type Tregs in C57BL/6→BALB/c. FACS gating strategy for Tregs isolated at day +4 (left), total percentage of Treg, fold increase of host-type Treg in spleen and pLNs based on Tcon group, and absolute numbers of host Foxp3+ cells per spleen are shown (right). (B) Proliferation capacity of Treg after BMT. Transplanted mice received BrdU injection (1 mg per mouse per IP) every second day followed by pLN harvest at day +7. No proliferation of host-type Treg was observed in mice transplanted with TCD-BM plus Tcon (left, middle). In mice receiving TCD-BM alone, host and donor-Treg proliferated (right). (C) ECP increases Tregs within 48 hours. Mice with or without ECP treatment were challenged with LPS (10 μg per mouse per IV) 48 hours after injection of ECP-treated cells and Tregs were analyzed in pLNs. Mice treated with ECP had significantly higher proportions of CD4+/Foxp3+ cells, compared with WT mice challenged with LPS only. Unchallenged mice served as a baseline. (D) CD4+ T cells from mice in panel C were evaluated for surface CTLA4 expression. ECP-treated mice had significantly higher levels of CTLA4 expression within the CD4+ population (left) and CD4+/Foxp3+ subpopulation, compared with untreated mice challenged with LPS only. (E) Treg from ECP-treated mice more actively suppressed T-cell proliferation than WT Tregs. [3H]-Thymidine incorporation of WT C57BL/6 responder Tcons (R) to Balb/c stimulators (S) in the presence of WT Tregs or ECP Tregs at different Treg to responder ratios is shown, P = .035. (F) Specific depletion of host Tregs prior to BMT in FVB→Foxp3DTR-C57BL/6. WT-C57BL/6 recipients received FVB Tcon (Tcon, ▲; ECP, ▪), as control for DT toxicity 1 group received 50 μg/kg DT at day −2 and −1 (WT Tcon+DT, ♦; WT ECP+DT, ▼). To specifically deplete host Tregs, C57BL/6-Foxp3DTR recipients were injected with 50 μg/kg DT at day −2 and −1 and transplanted with FVB Tcon (Foxp3DTR Tcon, ○; Foxp3DTR ECP, □). ECP group showed significant survival improvement in comparison with Tcon group, ▪ vs ▲, P = .03. Mice injected with DT showed exacerbated GVHD due to DT toxicity in all groups. Foxp3DTR ECP had no survival benefit in comparison with Foxp3DTR Tcon whereas benefit was maintained in WT ECP + DT vs WT Tcon + DT. (G) Adoptive transfer of host-type Tregs into untreated recipients prior to BMT failed to inhibit donor T-cell proliferation. Recipient mice received 105 sort-purified Tregs (CD4+CD25high) 24 hours prior to BMT. Tregs originated either from WT BALB/c (WT Treg) or from mice treated with ECP (ECP Treg). Recipients were transplanted with luc+Tcon and followed by BLI imaging. Data are representative (A,B,C,F,G) of at least 2 individual experiments with 3 to 10 mice per group, or (D) are a composite of 2 experiments with 10 mice per group or are done in triplicates with 3 independent mice (E). Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test and log-rank test.
To determine the functional impact of host Tregs, we applied specific Treg depletion, utilizing a C57BL/6-Foxp3-DTR knock-in strain as recipients (Figure 6F), where Foxp3+ Treg can specifically be depleted by DT. We observed a significant survival improvement in the WT-ECP as compared with the WT-Tcon group in the FVB→C57BL/6 strain combination. In contrast, after host-Treg depletion in C57BL/6-Foxp3DTR mice, ECP treatment failed to improve survival as compared with the Tcon group. Control WT-C57BL/6 recipients also treated with DT showed accelerated death due to DT toxicity, however, the survival benefit was maintained in the ECP group. Although induction of Tregs using the ECP approach was associated with a clear survival benefit throughout all experiments, we sought to clarify the role of host Treg specifically. To do this, we infused host-type Tregs from WT or ECP-treated mice into WT recipients prior to BMT, expecting higher Treg numbers would reduce GVHD. However, additional host Tregs had no effect on donor T-cell proliferation (Figure 6G).
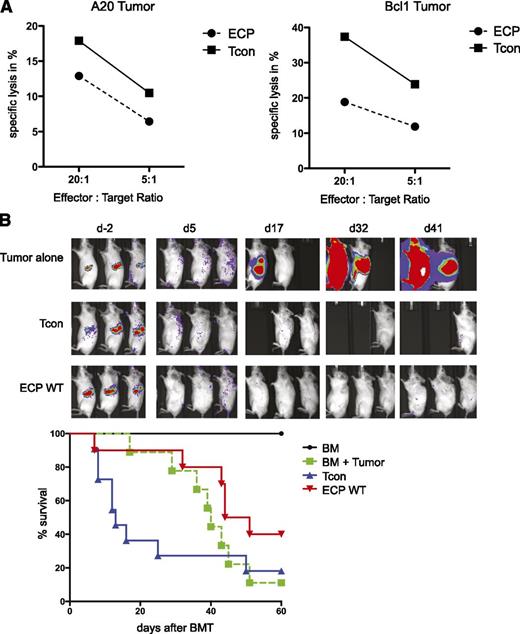
GVT effect is maintained after ECP treatment
Approaches to inhibit GVHD can sometimes reduce or prevent GVT.33 To address this issue, we evaluated the impact of ECP treatment on GVT effects. In vitro cytotoxicity assays using A20 and Bcl1 tumor cell lines as target cells and donor CD8+ T cells purified 10 days after BMT, as effector cells demonstrated persistence of lysis in both ECP and Tcon groups (Figure 7A); however, the degree of killing in the ECP group was reduced against both target cell lines. To evaluate whether this killing capacity in the ECP group is sufficient to maintain GVT in vivo, we established the Bcl1 tumor in recipient mice prior to BMT. All recipients of T-cell-replete grafts showed no recurrence of the tumor irrespective of ECP treatment, whereas mice transplanted with TCD-BM alone died of tumor progression (Figure 7B). Thus, ECP treatment did not impair antitumor responses by allogeneic Tcons in this tumor model.
GVT effect is maintained after ECP treatment. (A) Specific killing of target (T) tumor cell lines A20 (left) and Bcl1 (right) by effector CD8+ T cells (E) is shown. CD8+ T cells were isolated from ECP-treated (ECP) or nontreated (Tcon) mice 10 days after BMT and were added to 51Cr-labeled target cells at an E:T ratio of 20:1 and 5:1. Specific killing occurred in both target cells, but with reduced efficiency in ECP-treated mice. (B) GVT effects in vivo are maintained in ECP-treated mice in a Bcl1 tumor model. Recipients were injected with 5 × 103luc+Bcl1 cells 9 days prior to BMT. Tumor-bearing recipients were transplanted with Tcon alone (blue, ▲), or additionally received ECP-treated splenocytes from healthy mice (red, ▼) 48 hours prior to BMT. Results are done in triplicates and are representative of 2 individual experiments (A) or are compiled from 2 experiments with 10 mice per group (B).
GVT effect is maintained after ECP treatment. (A) Specific killing of target (T) tumor cell lines A20 (left) and Bcl1 (right) by effector CD8+ T cells (E) is shown. CD8+ T cells were isolated from ECP-treated (ECP) or nontreated (Tcon) mice 10 days after BMT and were added to 51Cr-labeled target cells at an E:T ratio of 20:1 and 5:1. Specific killing occurred in both target cells, but with reduced efficiency in ECP-treated mice. (B) GVT effects in vivo are maintained in ECP-treated mice in a Bcl1 tumor model. Recipients were injected with 5 × 103luc+Bcl1 cells 9 days prior to BMT. Tumor-bearing recipients were transplanted with Tcon alone (blue, ▲), or additionally received ECP-treated splenocytes from healthy mice (red, ▼) 48 hours prior to BMT. Results are done in triplicates and are representative of 2 individual experiments (A) or are compiled from 2 experiments with 10 mice per group (B).
Discussion
Our work demonstrates that a single dose of host-type ECP-treated cells prior to transplantation diminishes GVHD and significantly improves survival in murine BMT models. The benefit of ECP-treated cells depends upon immunosuppressive signals inherent to apoptotic cells that inhibit maturation of host DCs. Such active tolerance induction requires host IL-10 production and host Treg cells. The effectiveness of host-type ECP-treated cells is important because obtaining host cells is perhaps more amenable to clinical translation than using donor-type cells.
We evaluated biological attributes of apoptotic cells to gain insights into the mechanism(s) underlying the beneficial effect of infusing host ECP-treated cells prior to transplantation. Apoptotic cells flip phosphatidylserine, normally located on the inner membrane, to the outer bilayer and binding to MerTK receptors on APCs induces active downregulation of NF-κB.34,35 NF-κB activation in DCs is critical for the upregulation of proinflammatory cytokines and costimulatory molecules, and its inhibition is a promising approach to reduce T-cell activation in GVHD.15,36-41 In our model, the uptake of ECP-induced apoptotic cells significantly reduced NF-κB activation in host DCs. The inhibition of NF-κB activation blocked the maturation of DCs in terms of upregulation of MHC and costimulatory molecules at the time of transplantation and resulted in significantly lower proinflammatory cytokine production. Consequently, donor T-cell activation was significantly reduced in these mice, which in turn contributed to the reduced secretion of proinflammatory signals. Additionally, the frequency of recipient DCs was lower in the ECP-treated group with an increase in apoptotic signals, thus further reducing potential sites of donor T-cell priming.
Reducing DC antigen presentation to donor T cells is unlikely the only mechanism to reduce GVHD in ECP-treated animals. Shlomchik et al recently demonstrated that selective depletion of host DCs did not ameliorate GVHD.42 It has been shown that uptake of apoptotic cells by DCs induces Tregs, which when triggered by specific antigens, can act back via a “feedback loop” on immature DCs to further block the upregulation of costimulatory molecules. Additionally, Tregs are able to directly inhibit T-cell activation by inhibition of CD28 signaling.17,43,44 Previous studies have shown that Tregs play an essential role in mediating immune suppression following ECP. Donor Tregs were important to reverse established GVHD in a murine model and Tregs were found to be increased in patients with acute and chronic GVHD when successfully treated with ECP.30,45,46 In contrast to these studies, we observed a significant increase of host-type Tregs while leaving donor Tregs unaffected in ECP-treated mice. More importantly, these ECP-induced Tregs had significantly higher surface expression of the suppressor molecule CTLA4, potentiating their suppressive capacity.47
The impact of host Tregs was necessary because their specific depletion abrogated the beneficial outcome of infusion of ECP-treated cells. However, the isolated adoptive transfer of host-type Tregs 24 hours prior to transplantation with the aim of increasing Treg numbers failed to reduce T-cell proliferation, indicating these cells alone are likely not sufficient for protection but require the interplay with other immune-tolerogenic processes or require activation for functional activity. This result shows that the benefit of ECP treatment is not solely based on Tregs and highlights the importance of the immunologic context of these cells.31
Previous reports have demonstrated the importance of donor-derived IL-10 to augment BM engraftment by ECP.29 In our model, mice transplanted with IL-10–deficient donor cells died rapidly irrespective of ECP treatment, thus not allowing for an evaluation of the impact of donor IL-10 on prophylactic ECP treatment. However, when recipient mice were deficient in IL-10 production the beneficial effect of ECP was completely abrogated. The effect could neither be rescued by IL-10 released from apoptotic leukocytes nor by transplantation of IL-10–producing donor cells 48 hours later, indicating the necessity of IL-10 in the recipient at the time of ECP.29,31
The role of trogocytosis has not yet been well evaluated in ECP and BMT. Under inflammatory conditions, T cells incorporate cell surface proteins including MHCII molecules from DCs and other APCs that enable them to serve as APC-like cells hence driving local inflammation.48 We observed significantly lower MHCII expression on donor T cells in ECP-treated mice as compared with control groups, most likely due to the repressed proinflammatory milieu in ECP-treated mice. We hypothesize that the inhibition of trogocytosis by ECP contributes to reduce local inflammation. Further experiments to block trogocytosis are needed, however, approaches that can be used in vivo in the setting of transplantation are lacking.
Importantly, despite the tolerogenic environment created by ECP, GVT was not impaired in our model. This is consistent with 2 retrospective clinical studies where ECP was part of the preparative regimen. Although overall and disease-free survival was significantly improved and incidence of severe acute GVHD (grade III-IV) was lower than expected in these high-risk patients, it did not appear to increase relapse rates or the risk of opportunistic infections that have been observed in other attempts to prevent GVHD.10,49,50 Furthermore, T- and B-cell responses to novel and recall antigens remained intact in patients treated with ECP.51
We have shown for the first time that GVHD is reduced when ECP is performed prior to transplant conditioning. ECP treatment early after transplantation (day +2 or +4) failed to protect mice from GVHD, a finding that contrasts previous reports on the protective function of early posttransplant ECP in mice.29 This discrepancy could be explained by differences in model systems where posttransplant ECP may be protective with less intense conditioning and allogenicity because the tolerizing effect can be overcome by additional danger signals in a dose-dependent manner.29,52-54 In our model, when we injected LPS simultaneously with ECP-treated cells, we observed restoration of CD80/86 expression on DCs as an indicator for DC maturation, and mice treated with ECP shortly after conditioning showed no survival benefit. These results suggest that DC sensitivity to ECP is highly contextual and the micromilieu during DC maturation is the key variable for the functional activity of DCs to induce tolerance vs immunity. Danger signals, inherent to conditioning and transplantation, such as uric acid or adenosin triphosphate, could therefore reduce the efficacy of early posttransplant ECP despite the induction of high amounts of apoptotic cells.29,53,54
Prophylactic ECP did not completely eliminate the presentation of host antigens to donor effector cells but significantly delayed the induction of donor T-cell priming. Later onset of acute GVHD may itself have a beneficial effect, as it permits more recovery time from the preparative regimen, resulting in better immune reconstitution and less organ damage.55 Controlling this delay marks an important strategy that is the underlying rationale for newer immunosuppressive and nonmyeloblative strategies such as posttransplant cyclophosphamide.56
It is arguable that more frequent courses of ECP prior to BMT may enhance the benefit, however, in our murine model additional ECP treatment at day −5 did not improve outcomes further. The timing might relate to the effective generation of new DCs capable of antigen presentation and the short lifespan of DCs once they captured apoptotic cells.13 Increasing apoptotic cell numbers is another strategy but this may be constrained by the saturation of HMGB1,52 which reverses the DC response by leading to immune induction, and as noningested apoptotic cells undergo secondary necrosis they provide additional danger signals.57
In conclusion, we show that prophylactic ECP prior to BMT significantly improved survival in a murine BMT model. Because ECP treatment is safer than many other immunosuppressive approaches and widely used, our study supports the evaluation of prophylactic ECP in reducing transplant complications like GVHD in future prospective clinical studies.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yuqiong Pan for technical assistance and Maite Alvarez for critical review of this manuscript. Furthermore, they thank Therakos for providing vital equipment and reagents.
This work was supported by the National Institutes of Health: P01 CA49605 from the National Cancer Institute and P01 HL57442 from the National Heart, Lung and Blood Institute.
Authorship
Contribution: M.F. designed research, performed experiments, analyzed data, and wrote the paper; E.I.S., D.B.L.-G., J.B., D.S., and A.M.S.M. performed experiments; E.M. wrote the paper; and R.S.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Division of Blood and Marrow Transplantation, Stanford Medical School, Stanford University, CCSR, Room 2205, 269 W Campus Dr, Stanford, CA 94305; e-mail: negrs@stanford.edu.

![Figure 6. ECP treatment induces host-type Foxp3+ Tregs that substantially contribute to but are not solely responsible for improved outcome. (A) Preemptive ECP treatment specifically increases host-type Tregs in C57BL/6→BALB/c. FACS gating strategy for Tregs isolated at day +4 (left), total percentage of Treg, fold increase of host-type Treg in spleen and pLNs based on Tcon group, and absolute numbers of host Foxp3+ cells per spleen are shown (right). (B) Proliferation capacity of Treg after BMT. Transplanted mice received BrdU injection (1 mg per mouse per IP) every second day followed by pLN harvest at day +7. No proliferation of host-type Treg was observed in mice transplanted with TCD-BM plus Tcon (left, middle). In mice receiving TCD-BM alone, host and donor-Treg proliferated (right). (C) ECP increases Tregs within 48 hours. Mice with or without ECP treatment were challenged with LPS (10 μg per mouse per IV) 48 hours after injection of ECP-treated cells and Tregs were analyzed in pLNs. Mice treated with ECP had significantly higher proportions of CD4+/Foxp3+ cells, compared with WT mice challenged with LPS only. Unchallenged mice served as a baseline. (D) CD4+ T cells from mice in panel C were evaluated for surface CTLA4 expression. ECP-treated mice had significantly higher levels of CTLA4 expression within the CD4+ population (left) and CD4+/Foxp3+ subpopulation, compared with untreated mice challenged with LPS only. (E) Treg from ECP-treated mice more actively suppressed T-cell proliferation than WT Tregs. [3H]-Thymidine incorporation of WT C57BL/6 responder Tcons (R) to Balb/c stimulators (S) in the presence of WT Tregs or ECP Tregs at different Treg to responder ratios is shown, P = .035. (F) Specific depletion of host Tregs prior to BMT in FVB→Foxp3DTR-C57BL/6. WT-C57BL/6 recipients received FVB Tcon (Tcon, ▲; ECP, ▪), as control for DT toxicity 1 group received 50 μg/kg DT at day −2 and −1 (WT Tcon+DT, ♦; WT ECP+DT, ▼). To specifically deplete host Tregs, C57BL/6-Foxp3DTR recipients were injected with 50 μg/kg DT at day −2 and −1 and transplanted with FVB Tcon (Foxp3DTR Tcon, ○; Foxp3DTR ECP, □). ECP group showed significant survival improvement in comparison with Tcon group, ▪ vs ▲, P = .03. Mice injected with DT showed exacerbated GVHD due to DT toxicity in all groups. Foxp3DTR ECP had no survival benefit in comparison with Foxp3DTR Tcon whereas benefit was maintained in WT ECP + DT vs WT Tcon + DT. (G) Adoptive transfer of host-type Tregs into untreated recipients prior to BMT failed to inhibit donor T-cell proliferation. Recipient mice received 105 sort-purified Tregs (CD4+CD25high) 24 hours prior to BMT. Tregs originated either from WT BALB/c (WT Treg) or from mice treated with ECP (ECP Treg). Recipients were transplanted with luc+Tcon and followed by BLI imaging. Data are representative (A,B,C,F,G) of at least 2 individual experiments with 3 to 10 mice per group, or (D) are a composite of 2 experiments with 10 mice per group or are done in triplicates with 3 independent mice (E). Error bars indicate mean ± SEM and significance was assessed by 2-tailed Student t test and log-rank test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/11/10.1182_blood-2014-02-555128/4/m_1832f6.jpeg?Expires=1769276450&Signature=ifn-aHomBCwDMg3oLsf1e2Hc0CoPyxw27irnarlzO1~f1t23aLmIEA-q~aBUy-tgudrhbwNbbkCFulcDq7CeiSBdW0veJanBE42iP26lBj1iR5qW~Ni-wYgPLoIa53GV~aRtCmFfBmtU0tJKBJS2-wnM57FgcMHQ7V3TXyqCuPLqRS87ZO0z0LHWHH6J3~tmNB2JA4fjDswSAT0IjKV9T47OWB83LiIei1RRL~Z1Mz18ViaQ9tZ05mwJ58hEUaSmZaCfiSgH0FJB57NKTA45P8iDX3nFkgywYXvxUpEKFVc5NZM4u~dpzXQCyzkfSMk2vO7g25aaPmZARyzzIVseyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal