Key Points
Pyk2 plays a tumor-promoting role in MM progression via modulation of the Wnt/β-catenin signaling pathway.
Pyk2 inhibitors represent a new therapeutic option against MM.
Abstract
Proline-rich tyrosine kinase 2 (Pyk2) is a member of the focal adhesion kinase family that has been recently linked to tumor development. However, its role in modulating multiple myeloma (MM) biology and disease progression remains unexplored. We first demonstrated that patients with MM present with higher expression of Pyk2 compared with healthy individuals. By using loss-of-function approaches, we found that Pyk2 inhibition led to reduction of MM tumor growth in vivo as well as decreased cell proliferation, cell-cycle progression, and adhesion ability in vitro. In turn, overexpression of Pyk2 promoted the malignant phenotype, substantiated by enhanced tumor growth and reduced survival. Mechanistically, inhibition of Pyk2 reduced activation of Wnt/β-catenin signaling by destabilizing β-catenin, leading to downregulation of c-Myc and Cyclin D1. Furthermore, treatment of MM cells with the FAK/Pyk2 inhibitor VS-4718 effectively inhibited MM cell growth both in vitro and in vivo. Collectively, our findings describe the tumor-promoting role of Pyk2 in MM, thus providing molecular evidence for a novel tyrosine kinase inhibitor as a new therapeutic option in MM.
Introduction
Multiple myeloma (MM) represents a model of hematologic malignancy in which continuous cell dissemination and tumor progression occurs through trafficking of tumor cells in and out of the bone marrow (BM).1,2 Yet, the mechanisms by which malignant plasma cells metastasize and disseminate to different areas of the BM are not well understood. In solid tumors, focal adhesion kinase proteins are one of the master regulators of tumor metastasis and dissemination.
The focal adhesion kinase (FAK) family is composed of FAK and proline-rich tyrosine kinase 2 (Pyk2), which share homology at the structural level. It has been proposed that FAK is pressed in a large number of tumors and promotes multiple malignant processes, such as tumor cell growth, invasion, cancer stem cell self-renewal, metastasis, and angiogenesis, through integrating extracellular stimuli of integrins and growth factor receptors with downstream signaling including Akt, Erk, and nuclear factor κB.3 However, the role of the FAK homolog Pyk2 in tumors remains less explored.
Pyk2 is also known as FAK2, RAFTK, and CAKB, and it is a nonreceptor protein kinase that is structurally similar to FAK, with 48% identity of amino acids, 60% identity of sequences in the central kinase domain, and identical positions of 4 phosphorylation sites.4,5 FAK is expressed ubiquitously, indispensable for embryogenesis, and colocalized at focal contacts with integrins and growth factor receptors, whereas Pyk2 is expressed restrictedly in the endothelium, central nervous system, and hematopoietic lineages; dispensable for organ development; localized throughout the cytoplasm; and sensitive to intracellular Ca+ signaling and G-protein–coupled receptors.4,6-8 Pyk2 has been shown to interact with some of the proteins that FAK binds to, such as Src, Paxillin, and P130cas,9-11 suggesting that they may be implicated in several overlapping signaling pathways. Intriguingly, studies reported that in the context of FAK depletion, endogenous Pyk2 expression in some cell types increased in a compensatory manner to partly maintain the effects of FAK in regulating cell motility and angiogenesis.9,12,13 The specific role of Pyk2 in B cells has been shown in Pyk2−/− mice, where Pyk2-deficient B cells and macrophages exhibit impaired mobility and responsiveness to chemokines.14 A compensatory increase of FAK was not observed in these Pyk2-deficient cells. Pyk2 could be activated in FAK-deficient cells by binding to fibronectin, and it is not dependent on extracellular matrix simulation that is used to activate FAK.9,15 More interestingly, Pyk2-deficient mice present with increased bone formation due to the enhanced differentiation of osteoprogenitor cells.16 Therefore, despite sharing structural identity with FAK, Pyk2 appears to differ from FAK in regulating cellular phenotypes and signaling pathways.
Given that Pyk2 is specifically expressed in hematopoietic cells, we sought to examine the role of Pyk2 in the regulation of cell dissemination and tumor progression in MM as a representative hematologic malignancy. Aberrant upregulation of Pyk2 has been shown to correlate with poor prognosis in lung cancer and facilitate epithelial-to-mesenchymal transition in breast cancer.17,18 Nevertheless, the putative oncogenic role of Pyk2 in cancers in general and in specific hematologic malignancies has not been previously described.
In our study, we demonstrated that Pyk2 is highly expressed, at the messenger RNA (mRNA) and protein levels, in MM patients compared with healthy individuals. By using gain- and loss-of-function genetic studies together with pharmacologic studies, we confirmed the tumor-promoting role of Pyk2 both in vitro and in vivo. Mechanistically, Pyk2 protected β-catenin from GSK3β-induced degradation, thus maintaining the activation of β-catenin signaling. Overall, our findings describe the pro-oncogenic role of Pyk2 in MM, thus providing molecular evidence for a novel Pyk2-targeting therapeutic strategy in MM.
Methods
Cells
Bone marrow stromal cells (BMSCs) were isolated from BM samples from MM patients as described previously.19 Informed consent was obtained from MM patients in accordance with the Declaration of Helsinki. Approval for these studies was obtained by the Dana-Farber Cancer Institute institutional review board. The human MM cell lines MM.1S, H929, U266, OPM2, MOLP8, and RPMI8226 and the human embryonic kidney epithelial cell line HEK293 were purchased from ATCC (Manassas, VA). Cell lines and BMSCs were cultured in RPMI 1640 medium containing 2 mM/mL l-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin with 10% fetal bovine serum (FBS) for cell lines or 20% FBS for BMSCs. The GFP+/Luc+-MM.1S cell line was generated by retroviral transduction with the pGC-GFP+/Luc+ vector (a gift from Dr Andrew Kung, Dana-Farber Cancer Institute).
Generation of loss- and gain-of-function Pyk2 stable MM cell lines
Lentiviral Pyk2 short hairpin RNA (shRNA) (A2 and A4) and FAK-shRNA were obtained from The RNAi Consortium (http://www.broadinstitute.org/rnai/trc) (see sequence in supplemental Table 1, available on the Blood Web site). A scramble shRNA was used as a control. For Pyk2-expressing lentiviral particles, a Pyk2-expressing vector (Genecopoeia, Rockville, MD) containing full-length Pyk2 complementary DNA (NM_004103.4) was packaged with lentiviral plasmids using Lenti-Pac HIV Expression Packaging Systems (Genecopoeia) according to the manufacturer’s instructions. GFP+/luc+-MM.1S cells were transduced with lentiviral particles and polybrene at 8 µg/mL (Sigma-Aldrich, St. Louis, MO), followed by puromycin selection at 24 hours after the transduction. Efficiency of knockdown or overexpression was validated by immunoblotting.
Gene expression analysis
To determine the gene expression of Pyk2 in plasma cells isolated from healthy donors or MM patients in different subtypes (including monoclonal gammopathy of undetermined significance [MGUS], smoldering MM, and active MM), we used published data sets (GSE2658) from the Gene Expression Omnibus.20,21
All the intensity files were MAS5 transformed and the data normalized to the median, as previously reported.22
Cell proliferation assay
Cell proliferation of Pyk2-knockdown or Pyk2-overexpressing MM.1S cells was measured by DNA synthesis using the [3H]-thymidine uptake assay (PerkinElmer, Waltham, MA) described previously.19 For VS-4718–treated MM cells, cell proliferation was assayed using the CellTiter 96 Aqueous One Solution Proliferation assay (Promega, Madison, WI). Briefly, MM cells were seeded in 96-well regular culture plates using phenol-red–free culture media (Life Technologies, Grand Island, NY) or fibronectin-coated culture plates (BD Biosciences, San Jose, CA) in the presence of 10 ng/mL human interleukin-6 (IL-6). After overnight culture, VS-4718 was added in 1:3 serial dilutions. After a 96-hour incubation, cell proliferation was measured. Dose-response curves were generated to determine half-maximal response values of compounds using GraphPad software (GraphPad Software, La Jolla, CA).
Cell-cycle assay
Cells were stained with propidium iodine (Sigma-Aldrich), and cell cycle was determined using an Epics flow cytometry, as previously described.23
Cell adhesion assay to BMSCs and fibronectin
BMSCs were cultured overnight to confluence in 96-well plates (5 × 103 cells/well) before the adhesion assay. Indicated MM cells were starved in serum-free medium for 2 hours, prelabeled with Calcein-AM (Invitrogen, Grand Island, NY), added to the BMSC-coated well (1 × 105 cells/well), and allowed to adhere for 2 hours at 37°C. Nonadherent cells were removed, and BMSCs were washed with phosphate-buffered saline. Fluorescence intensity was measured using a fluorescent-plate reader (Ex/Em = 485/520 nm). For adhesion to fibronectin, an InnoCyte ECM Cell Adhesion Assay kit (Millipore, Billerica, MA) was used according to the manufacturer’s instructions.
Immunoblotting
Harvested cells were lysed using lysis buffer (Cell Signaling, Danvers, MA) for total cell lysates. Nuclear fraction was isolated by using NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher, Rockford, IL). Cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The antibodies used for immunoblotting included anti-Pyk2, anti-phospho-Pyk2 Tyr402, anti-phospho-Src Tyr416, anti-phospho-Paxillin Tyr118, anti-GSK3β, anti-phospho-GSK3β Ser9, anti-phospho-β-catenin ser33/37, anti-Akt, anti-phospho-Akt Ser473, anti-Erk1/2, anti-phospho-Erk1/2 Thr202/Tyr204, anti-c-Myc, anti-Cyclin D1 (Cell Signaling), and anti-β-catenin (BD Biosciences).
Immunohistochemistry
BM biopsy specimens from 14 MM patients and 6 healthy donors were fixed in Zanker formalin, embedded in paraffin blocks, and sectioned. Sections were stained with anti-Pyk2 (Abcam, Cambridge, MA). Anti-immunoglobulin G was used for negative control. Femurs of tumor-bearing mice were prepared and sectioned, followed by staining with anti-Pyk2 and anti-β-catenin (Santa Cruz Biotechnology, Dallas, TX).
Pyk2 inhibitor
VS-4718 has been previously described as a FAK inhibitor.24-26 VS-4718 was provided by Verastem (Cambridge, MA). For in vitro assays, VS-4718 was dissolved in dimethylsulfoxide and prepared as a 10 mM stock and diluted in culture medium at indicated concentrations. For in vivo experiments, VS-4718 was formulated in 0.5% carboxymethyl cellulose (Sigma-Aldrich) solution with 0.1% Tween 80 for oral gavage.
Biochemical and cellular FAK and Pyk2 kinase assays
The human leukemia cell line MV-4-11 and the breast cancer cell line SUM159 were purchased from ATCC (Manassas, VA) and Asterand (Detroit, MI), respectively. MV-4-11 cells were cultured in Iscove’s modified Dulbecco’s medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. SUM159 cells were maintained in F12 medium supplemented with 10% FBS, 5 µg/mL insulin, 1 µg/mL hydrocortisone, and penicillin (100 U/mL)/streptomycin (100 μg/mL). The biochemical potency of VS-4718 against FAK and Pyk2 was determined at Invitrogen using the SelectScreen Z′-LYTE standard assay conditions (Life Technologies). The cellular potency of VS-4718 against FAK and Pyk2 was determined using p-FAK (Y397) and p-Pyk2 (Y402) enzyme-linked immunosorbent assays (ELISAs) to assess autophosphorylation. For the p-FAK ELISA, SUM159 cells were seeded and allowed to adhere to fibronectin-coated 96-well plates (BD Biosciences) for 3 hours followed by treatment with VS-4718 in 1:3 serial dilutions for 1 hour. Cells were then lysed and subject to a phospho-FAK (Y397) assay (Millipore). For the p-Pyk2 ELISA, MV-4-11 cells were treated with VS-4718 in 1:3 serial dilutions for 1 hour followed by a 20-minute phorbol 12-myristate 13-acetate stimulation. Total Pyk2 was captured from whole-cell lysates on 96-well plates coated with an anti-Pyk2 antibody (Santa Cruz Biotechnology), and p-Pyk2 (Y402) was then detected in the wells with an anti-phospho-Pyk2 antibody (Santa Cruz Biotechnology). Following incubation with anti-mouse immunoglobulin G horseradish peroxidase and subsequent TMB substrate, plates were read at 450 nm.
In vivo studies
Animal studies were approved by Dana-Farber Cancer Institute institutional animal care and use committee. Female 6- to 8-week-old SCID-Beige mice were purchased from Taconic (Hudson, NY). A total of 3 × 106 GFP+/Luc+-MM.1S Pyk2 knockdown or overexpressing cells were injected IV into mice to generate mouse xenograft model. For bioluminescence imaging (BLI), mice were injected with 75 mg/kg of luciferin (Caliper Life Sciences, Hopkinton, MA) followed by whole-body real-time BLI (Xenogen IVIS imaging system; Caliper Life Sciences) at indicated days. For in vivo VS-4718 treatment, mice were dosed at 75 mg/kg by oral gavage twice daily. VS-4718 treatment started 5 days after cell injection and lasted 28 days (4 weeks). BLI imaging was performed at days 7, 14, 21, and 28 after the first treatment. Mice were euthanized by CO2 inhalation. The femurs were obtained for further quantitative polymerase chain reaction (qPCR) and immunohistochemistry assays.
The 6- to 8-week-old SCID/Beige mice were injected subcutaneously into the left flank with either scramble probe-infected RPMI.8226 cells (4 × 106/mouse; n = 6 mice) or RPMI.8226 Pyk-knockdown cells (4 × 106/mouse; n = 7 mice) in 200 μL Hank’s balanced salt solution (Euroclone) and Matrigel (Becton Dickinson) at a 1:1 ratio. Tumor appearance was monitored twice weekly by palpation. Tumor growth was measured twice weekly and volume (mg = mm3) calculated as (length [mm] × width2 [mm2])/2.
Statistical analysis
Data were analyzed using unpaired Student t tests comparing 2 conditions or a 1-way analysis of variance with Bonferroni or Newman-Keuls correction for multiple comparisons. P < .05 was considered significant, and 2-sided tests were performed. Data are presented as means, and error bars in the figures depict standard deviation (SD).
Results
Pyk2 is highly expressed in MM
We examined the expression of Pyk2 and its homolog, FAK, in MM patients and healthy individuals, both at the mRNA and protein levels. First, we evaluated the mRNA level of Pyk2 and FAK in CD138+ cells derived from BM of MM patients using a published gene expression data set (GSE2658). Pyk2 was significantly upregulated in plasma cells obtained from MM patients compared with the related normal cellular counterpart isolated from healthy individuals. Importantly, higher levels of Pyk2 were also detected at the MGUS or smoldering myeloma stages (Figure 1A). Conversely, FAK expression in MM patients was downregulated compared with the normal counterpart (Figure 1B). By immunohistochemistry performed on 14 MM and 6 normal BM samples, we confirmed the upregulation of Pyk2 in MM patients at the protein level (Figure 1C-D and supplemental Figure 1A). Because Pyk2 is located in the chromosomal region of 8p21, where a deletion has been reported to occur in a small subset of MM patients,27 we speculated that this deletion may affect the expression of Pyk2 in MM. We therefore analyzed the copy-number status of 111 genes located in the region from 8p21.1 to 8p22 of a total of 27 MM cell lines enrolled in the Cancer Cell Line Encyclopedia (CCLE) database28 and found that in a small subset of MM cell lines, the copy number of these genes, including Pyk2, was commonly lost (Figure 1E-F), which partly reflected the instability of the 8p21 region. Moreover, Pyk2 mRNA levels were positively associated with the copy number (Figure 1G), suggesting 8p21 stability is important for Pyk2 expression. We also examined Pyk2 expression in 6 MM cell lines by immunoblotting. The Pyk2 expression pattern at the protein level across these tested cells was consistent with their 8p21 stability. Specifically, U266 cells, which have a loss of 8p21, exhibited low/absent expression of Pyk2 (Figure 1H).
Pyk2 is highly expressed in MM. (A) Expression level of Pyk2 is significantly increased in MM patients (both smoldering MM and newly diagnosed MM) compared with healthy subjects (GSE2658). (B) Conversely, the FAK expression level is decreased in MM patients. (C) Expression of Pyk2 in BM biopsy specimens from MM patients (n = 14) and healthy donors (n = 6) was detected by immunohistochemistry staining. Three representatives in each group are shown (×20; ×100). All MM patients present with higher Pyk2 expression compared with healthy individuals. (D) Pyk2-positive cells in immunohistochemistry staining slides were quantified using ImageJ software. Three random fields at ×20 magnification were analyzed. (E) Based on analysis of the CCLE database, the copy numbers of genes harbored in the region from 8p21.1 to 8p22 are shown in the heatmap. U266 has significant loss of gene copy numbers, reflecting its 8p21 instability. (F) Pyk2 gene copy-number status of 27 MM cell lines enrolled in the CCLE database. (G) Based on analysis of the CCLE database, the Pyk2 mRNA level in MM cell lines is associated positively with its copy number (r = 0.7856; P = .032). (H) Pyk2 is expressed differentially in 6 MM cell lines using immunoblotting. Note that U266 cells express low to absent expression of Pyk2. NBM, normal bone marrow; SMOLD, smoldering MM.
Pyk2 is highly expressed in MM. (A) Expression level of Pyk2 is significantly increased in MM patients (both smoldering MM and newly diagnosed MM) compared with healthy subjects (GSE2658). (B) Conversely, the FAK expression level is decreased in MM patients. (C) Expression of Pyk2 in BM biopsy specimens from MM patients (n = 14) and healthy donors (n = 6) was detected by immunohistochemistry staining. Three representatives in each group are shown (×20; ×100). All MM patients present with higher Pyk2 expression compared with healthy individuals. (D) Pyk2-positive cells in immunohistochemistry staining slides were quantified using ImageJ software. Three random fields at ×20 magnification were analyzed. (E) Based on analysis of the CCLE database, the copy numbers of genes harbored in the region from 8p21.1 to 8p22 are shown in the heatmap. U266 has significant loss of gene copy numbers, reflecting its 8p21 instability. (F) Pyk2 gene copy-number status of 27 MM cell lines enrolled in the CCLE database. (G) Based on analysis of the CCLE database, the Pyk2 mRNA level in MM cell lines is associated positively with its copy number (r = 0.7856; P = .032). (H) Pyk2 is expressed differentially in 6 MM cell lines using immunoblotting. Note that U266 cells express low to absent expression of Pyk2. NBM, normal bone marrow; SMOLD, smoldering MM.
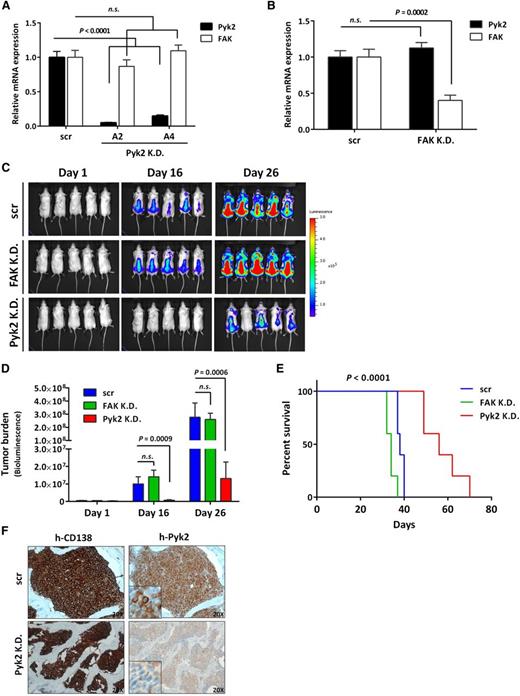
Pyk2 silencing decreases MM tumor growth and prolongs survival in vivo
To investigate whether Pyk2 is involved in MM tumor progression, we generated GFP+/Luc+-MM.1S Pyk2-knockdown cells by introducing 2 shRNA vectors (A2 and A4) targeting distinct region of Pyk2 and established a mouse MM xenograft model by injecting Pyk2-knockdown cells (shRNA A4) into SCID/Beige mice. Because FAK is a known Pyk2 homolog, we generated stable FAK knockdown GFP+/Luc+-MM.1S cells and compared the in vivo effect of both Pyk2 and FAK silencing in MM. The efficiency of Pyk2 and FAK knockdown was evaluated by qPCR, and no observed compensatory increase of the counterpart was seen (Figure 2A-B). Inhibition of Pyk2 resulted in significant reduction of MM tumor growth compared with scrambled shRNA control mice, as shown by BLI analysis, whereas inhibition of FAK did not alter tumor growth (Figure 2C-D). Accordingly, the mice bearing Pyk2-knockdown cells exhibited significantly prolonged survival, whereas FAK knockdown did not induce any effect on survival (Figure 2E). Inhibition of Pyk2 protein expression was further corroborated ex vivo by evaluating CD138+ MM tumor cells derived from the BM of Pyk2-knockdown mice (Figure 2F). Our in vivo data indicate the potential tumor-promoting role of Pyk2 in MM tumor growth, whereas FAK did not exert any functional effects on MM progression.
Pyk2 silencing decreases MM tumor growth in a mouse xenograft model. (A) Inhibition of Pyk2 induced by shRNA (A2 and A4) in GFP+/Luc+-MM.1S cells was validated using qPCR. No compensatory increase of FAK was observed. (B) shRNA-mediated FAK inhibition in GFP+/Luc+-MM.1S cells did not induce a compensatory increase of Pyk2. (C-D) SCID/Beige mice were injected IV with GFP+/Luc+-MM.1S Pyk2-knockdown (A4) or FAK-knockdown cells (3 × 106/ mouse) to establish an MM xenograft model (n = 5 per group). Tumor burden was detected and quantified using BLI. Inhibition of Pyk2 significantly reduced MM tumor burden compared with scramble control. Quantification of tumor burden has been detected by BLI (bars indicate mean ± SD; P indicates P values). (E) Mice bearing Pyk2-knockdown cells exhibited significantly prolonged survival (P < .0001), whereas inhibition of FAK did not prolong the survival of mice. (F) Inhibition of Pyk2 in CD138+ tumor cells derived from BM of Pyk2-knockdown mice was reconfirmed by immunohistochemistry. K.D., knockdown; n.s., not significant; scr, scramble control.
Pyk2 silencing decreases MM tumor growth in a mouse xenograft model. (A) Inhibition of Pyk2 induced by shRNA (A2 and A4) in GFP+/Luc+-MM.1S cells was validated using qPCR. No compensatory increase of FAK was observed. (B) shRNA-mediated FAK inhibition in GFP+/Luc+-MM.1S cells did not induce a compensatory increase of Pyk2. (C-D) SCID/Beige mice were injected IV with GFP+/Luc+-MM.1S Pyk2-knockdown (A4) or FAK-knockdown cells (3 × 106/ mouse) to establish an MM xenograft model (n = 5 per group). Tumor burden was detected and quantified using BLI. Inhibition of Pyk2 significantly reduced MM tumor burden compared with scramble control. Quantification of tumor burden has been detected by BLI (bars indicate mean ± SD; P indicates P values). (E) Mice bearing Pyk2-knockdown cells exhibited significantly prolonged survival (P < .0001), whereas inhibition of FAK did not prolong the survival of mice. (F) Inhibition of Pyk2 in CD138+ tumor cells derived from BM of Pyk2-knockdown mice was reconfirmed by immunohistochemistry. K.D., knockdown; n.s., not significant; scr, scramble control.
Pyk2 silencing reduces MM cell proliferation, adhesion, and cell-cycle progression by suppressing Wnt/β-catenin signaling
To further dissect the functional relevance of Pyk2 expression in modulating MM cell malignant phenotypes, we performed in vitro Pyk2 loss-of-function studies. Because it is known that the BM microenvironment confers growth advantage and induces drug resistance in malignant cells,29 we therefore sought to detect if inhibition of Pyk2 decreases MM cell proliferation in the context of BM milieu. Pyk2-knockdown MM.1S cells were cultured in the presence or absence of MM-derived BMSCs (MM-BMSCs) for 48 hours. As shown in Figure 3A, by using a [3H]-thymidine uptake assay, Pyk2-knockdown MM.1S cells exhibited significant reduction in cell proliferation compared with scrambled shRNA control cells in the presence or absence of MM-BMSCs, thus suggesting that loss of Pyk2 in MM cells overcomes the MM-BMSC–induced proliferation of MM cells. We next tested the effect of Pyk2 inhibition on modulating cell-cycle progression and found cell-cycle arrest, as demonstrated by an increase of the G1 phase population from 35.8% in scramble control cells to 42.5% in cells expressing Pyk2-shRNA A2 and 59.8% in those expressing Pyk2-shRNA A4 (Figure 3B). We then examined the effect of Pyk2 inhibition on modulating MM cell adhesion to MM-BMSCs or fibronectin and found that Pyk2-knockdown MM.1S cells exhibited a significant decrease in the adhesion ability of MM cells to BMSCs and fibronectin (Figure 3C-D).
Pyk2 silencing reduces MM cell proliferation, adhesion, and cell-cycle progression by suppressing Wnt/β-catenin signaling. (A) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured alone or in the presence of MM-BMSCs for 48 hours. Cell proliferation was measured using [3H]-thymidine uptake assay. Inhibition of Pyk2 significantly reduced MM cell proliferation in the presence or absence of MM-BMSCs (P < .0001). (B) The cell cycle of Pyk2-knockdown GFP+/Luc+-MM.1S cells was assessed by propidium iodine staining and flow-cytometric analysis. Inhibition of Pyk2 increased the G1 phase population from 35.82% in scramble control cells to 42.5% in cells expressing shRNA A2 and 59.8% in those expressing shRNA A4. (C-D) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured in MM-BMSC–coated or fibronectin-coated wells for 2 hours. Inhibition of Pyk2 impaired the adhesion ability of MM cells to BMSCs (P = .0004) and fibronectin (P = .0016). (E) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured alone or in the presence of MM-BMSCs. After coculture of MM cells with primary BMSCs, MM cells were separated by BMSCs, lysed, and subjected to western blot analysis. Inhibition of Pyk2 reduced β-catenin expression through inducing phosphorylated degradation of β-catenin by decreasing p-GSK3β, thus leading to downregulation of c-Myc and Cyclin D1. (F) Decreased mRNA levels of Pyk2, β-catenin, and c-Myc in BM specimens harvested from mice that were injected with Pyk2-knockdown cells. (G) MM-BMSCs enhanced Pyk2 phosphorylation and β-catenin nuclear translocation and upregulated c-Myc at the nuclear level. These effects triggered by BMSCs were abolished by inhibition of Pyk2. (H) Phosphorylation of Src and Paxillin was inhibited with the inhibition of Pyk2. K.D., knockdown; scr, scramble control.
Pyk2 silencing reduces MM cell proliferation, adhesion, and cell-cycle progression by suppressing Wnt/β-catenin signaling. (A) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured alone or in the presence of MM-BMSCs for 48 hours. Cell proliferation was measured using [3H]-thymidine uptake assay. Inhibition of Pyk2 significantly reduced MM cell proliferation in the presence or absence of MM-BMSCs (P < .0001). (B) The cell cycle of Pyk2-knockdown GFP+/Luc+-MM.1S cells was assessed by propidium iodine staining and flow-cytometric analysis. Inhibition of Pyk2 increased the G1 phase population from 35.82% in scramble control cells to 42.5% in cells expressing shRNA A2 and 59.8% in those expressing shRNA A4. (C-D) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured in MM-BMSC–coated or fibronectin-coated wells for 2 hours. Inhibition of Pyk2 impaired the adhesion ability of MM cells to BMSCs (P = .0004) and fibronectin (P = .0016). (E) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured alone or in the presence of MM-BMSCs. After coculture of MM cells with primary BMSCs, MM cells were separated by BMSCs, lysed, and subjected to western blot analysis. Inhibition of Pyk2 reduced β-catenin expression through inducing phosphorylated degradation of β-catenin by decreasing p-GSK3β, thus leading to downregulation of c-Myc and Cyclin D1. (F) Decreased mRNA levels of Pyk2, β-catenin, and c-Myc in BM specimens harvested from mice that were injected with Pyk2-knockdown cells. (G) MM-BMSCs enhanced Pyk2 phosphorylation and β-catenin nuclear translocation and upregulated c-Myc at the nuclear level. These effects triggered by BMSCs were abolished by inhibition of Pyk2. (H) Phosphorylation of Src and Paxillin was inhibited with the inhibition of Pyk2. K.D., knockdown; scr, scramble control.
We then evaluated the possible molecular mechanism responsible for Pyk2-induced MM tumor growth. We first demonstrated that Pyk2 inhibition in MM cells led to a significant increase of phosphorylated β-catenin and a subsequent decrease of total β-catenin (Figure 3E). Cyclin D1 and c-Myc, which are well-known target genes of β-catenin signaling and defined oncogenes in MM, were shown to be decreased with the inhibition of Pyk2 (Figure 3E). This observation was further confirmed ex vivo in BM specimens harvested from mice that were injected with Pyk2-knockdown cells (Figure 3F). Importantly, Pyk2 inhibition led to increased phosphorylated GSK3β responsible for increased phosphorylation of β-catenin, resulting in β-catenin degradation (Figure 3E). Moreover, we found that inhibition of Pyk2 resulted in decreased p-Akt (Figure 3E), suggesting Pyk2 may regulate β-catenin via modulating the phosphatidylinositol 3-kinase/Akt/GSK3β pathway.
Wnt/β-catenin signaling is implicated in the myeloma-BM interaction, thus promoting MM disease development.30 We therefore tested the role of Pyk2 in modulating Wnt/β-catenin signaling in MM cells in the context of the BM milieu. MM-BMSCs induced Pyk2 phosphorylation, together with upregulation of β-catenin and c-myc at the nuclear level in scramble cells. In contrast, BMSC-triggered nuclear c-Myc upregulation was abolished in Pyk2-knockdown MM cells (Figure 3G). In addition, the phosphorylation of Src and Paxillin, known as FAK-binding proteins, was decreased in MM cells upon Pyk2 silencing (Figure 3H).
Pyk2 overexpression promotes MM tumor progression and reduces survival in vivo
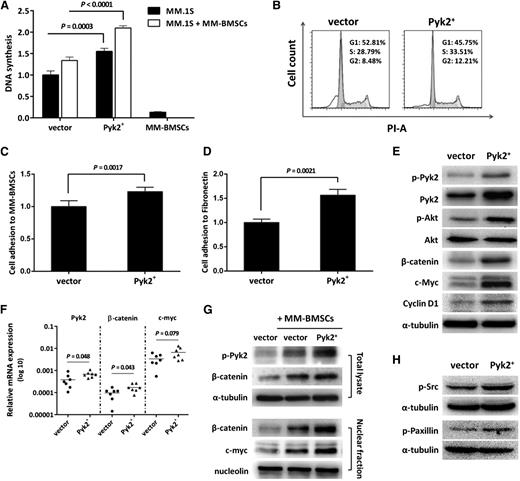
To further demonstrate the tumor-promoting role of Pyk2 in MM, we explored the effect of ectopic Pyk2 expression on MM tumor progression. GFP+/Luc+-MM.1S Pyk2-overexpressing cells (Figure 4A) were studied in vivo using a mouse MM xenograft model. Mice injected with Pyk2-overexpressing MM.1S cells showed enhanced tumor progression compared with vector control-injected mice (Figure 4B-C). Moreover, decreased median survival was demonstrated in mice injected with Pyk2-overexpressing cells vs vector control-injected mice (36 days and 44 days, respectively; P = .006; Figure 4E). These in vivo findings were confirmed in vitro, demonstrating that Pyk2-overexpressing MM.1S cells presented with increased cell proliferation in the presence or absence of MM-BMSCs (Figure 5A) and increased G2/M-phase population in cell-cycle analysis (Figure 5B), together with enhanced adherence to BMSCs and fibronectin (Figure 5C-D).
Overexpression of Pyk2 promotes MM tumor growth in vivo. (A) The Pyk2 mRNA level is increased in GFP+/Luc+-MM.1S Pyk2-overexpressing cells. (B-C) SCID/Beige mice were injected IV with GFP+/Luc+-MM.1S Pyk2-overexpressing cells (3 × 106/ mouse) to establish the MM xenograft model (n = 7 per group). Tumor burden was detected and quantified using BLI. Overexpression of Pyk2 significantly promoted MM tumor growth compared with vector control. Quantification of tumor burden has been detected by BLI (bars indicate mean ± SD; P indicates P values). (D) Mice bearing Pyk2-overexpressing cells exhibited poor survival compared with vector control mice (P = .006).
Overexpression of Pyk2 promotes MM tumor growth in vivo. (A) The Pyk2 mRNA level is increased in GFP+/Luc+-MM.1S Pyk2-overexpressing cells. (B-C) SCID/Beige mice were injected IV with GFP+/Luc+-MM.1S Pyk2-overexpressing cells (3 × 106/ mouse) to establish the MM xenograft model (n = 7 per group). Tumor burden was detected and quantified using BLI. Overexpression of Pyk2 significantly promoted MM tumor growth compared with vector control. Quantification of tumor burden has been detected by BLI (bars indicate mean ± SD; P indicates P values). (D) Mice bearing Pyk2-overexpressing cells exhibited poor survival compared with vector control mice (P = .006).
Overexpression of Pyk2 enhances MM cell proliferation, adhesion, and cell-cycle progression. (A) GFP+/Luc+-MM.1S Pyk2-overexpressing cells were cultured alone or in the presence of MM-BMSCs for 48 hours. Overexpression of Pyk2 increased MM cell proliferation in the presence or absence of MM-BMSCs. (B) Overexpression of Pyk2 promotes MM cell-cycle progression by increasing the G2/M phase population. (C-D) GFP+/Luc+-MM.1S Pyk2-overexpressing cells were cultured in MM-BMSC–coated or fibronectin-coated wells for 2 hours. Overexpression of Pyk2 enhanced the adhesion ability of MM cells to BMSCs (P = .0017) and fibronectin (P = .0021). (E) Overexpression of Pyk2 increased β-catenin, c-Myc, and Cyclin D1 expression. (F) Increased mRNA levels of Pyk2, β-catenin, and c-Myc in BM specimens harvested from mice that were injected with Pyk2-overexpressing cells. (G) Overexpression of Pyk2 further enhanced the upregulation and nuclear translocation of β-catenin in MM cells induced by interaction with BMSCs, resulting in higher nuclear c-Myc expression. (H) Src and Paxillin were activated upon increased activity of Pyk2.
Overexpression of Pyk2 enhances MM cell proliferation, adhesion, and cell-cycle progression. (A) GFP+/Luc+-MM.1S Pyk2-overexpressing cells were cultured alone or in the presence of MM-BMSCs for 48 hours. Overexpression of Pyk2 increased MM cell proliferation in the presence or absence of MM-BMSCs. (B) Overexpression of Pyk2 promotes MM cell-cycle progression by increasing the G2/M phase population. (C-D) GFP+/Luc+-MM.1S Pyk2-overexpressing cells were cultured in MM-BMSC–coated or fibronectin-coated wells for 2 hours. Overexpression of Pyk2 enhanced the adhesion ability of MM cells to BMSCs (P = .0017) and fibronectin (P = .0021). (E) Overexpression of Pyk2 increased β-catenin, c-Myc, and Cyclin D1 expression. (F) Increased mRNA levels of Pyk2, β-catenin, and c-Myc in BM specimens harvested from mice that were injected with Pyk2-overexpressing cells. (G) Overexpression of Pyk2 further enhanced the upregulation and nuclear translocation of β-catenin in MM cells induced by interaction with BMSCs, resulting in higher nuclear c-Myc expression. (H) Src and Paxillin were activated upon increased activity of Pyk2.
Overexpression of Pyk2 significantly increased β-catenin levels, thus leading to upregulation of Cyclin D1 and c-Myc in MM cells (Figure 5E). These in vitro results were further confirmed ex vivo in BM specimens obtained from mice injected with Pyk2-overexpressing cells (Figure 5F). Pyk2 overexpression significantly enhanced the BMSC-driven upregulation and nuclear translocation of β-catenin in MM cells, thus resulting in higher expression of β-catenin and c-Myc at the nuclear level (Figure 5G). In addition, Pyk2-overexpressing MM cells presented with increased phosphorylation of both Src and Paxillin (Figure 5H), thus suggesting a putative role for Pyk2 in Src-mediated signaling in MM cells.
A novel dual FAK/Pyk2 inhibitor exerts anti-MM activity both in vitro and in vivo
Based on the demonstration that Pyk2 plays a tumor-promoting role in MM, we evaluated the value of Pyk2 as a therapeutic target in MM by testing a novel FAK/Pyk2 inhibitor, VS-4718 (Verastem). As shown in Figure 6A, VS-4718 is a potent inhibitor of FAK and Pyk2 kinase activities in both biochemical and cellular assays. We then specifically treated MM cells with VS-4718 and found that VS-4718 significantly inhibited the proliferation of MM.1S, H929, and RPMI8226 cells (Figure 6B). We next compared VS-4718–dependent modulation of apoptosis in MM cells with either high-Pyk2 (MM.1S) or low-Pyk2 levels (U266) and found significant induction of apoptosis on MM.1S cells compared with U266 cells, either in the absence or presence of IL-6/fibronectin stimulation (supplemental Figure 2A-B).
Effects of FAK/Pyk2 inhibitor 74718 treatment on MM tumor growth. (A) The inhibitory activity of VS-4718 against FAK and Pyk2 was measured in in vitro kinase assays (left). Dose-response curves and 50% inhibition/inhibitory concentration (IC50) values are shown. The cellular inhibitory activity of VS-4718 against the autophosphorylation of FAK and Pyk2 was determined using p-FAK (Y397) and p-Pyk2 (Y402) ELISA assays (right). ELISA data were plotted as a function of concentration to determine half-maximal response (EC50) values. (B) MM.1S, H929, and RPMI8226 cells were cultured in suspension or under adherent conditions on fibronectin and in the presence of IL-6 were treated with VS-4718 for 96 hours. Cell proliferation and viability were assessed using a CellTiter 96 Aqueous One Solution Proliferation assay. VS-4718 inhibited the proliferation of and viability of MM cells in both the presence and absence of fibronectin and IL-6. (C) MM.1S cells were treated with VS-4718 (0, 0.5, 1, 2.5, 5, an 10 µM/L) for 4 hours. Whole-cell lysates were subjected to immunoblotting using anti-p-Pyk2 and anti-Pyk2. (D-E) Five days after being IV injected with GFP+/Luc+-MM.1S cells (3 × 106/ mouse), SCID/Beige mice were dosed with VS-4718 by oral gavage at 75 mg/kg twice daily for 4 weeks in total (n = 6 for vehicle; n = 7 for VS-4718). VS-4718 treatment effectively reduced MM tumor growth compared with vehicle control. (F) MM.1S and H929 cells were treated with VS-4718 (0, 2.5, 5, an 10 µM/L) for 24 hours. β-Catenin, c-Myc, and Cyclin D1 expression in these cells was inhibited by VS-4718. (G) SCID/Beige mice were injected subcutaneously into the left flank with either scramble probe-infected RPMI8226 cells (scr) or Pyk2-kd-RPMI8226 (PYK2 KD) cells. Tumor growth was measured twice weekly and volume (mg = mm3) calculated as (length [mm] × width2 [mm2])/2.
Effects of FAK/Pyk2 inhibitor 74718 treatment on MM tumor growth. (A) The inhibitory activity of VS-4718 against FAK and Pyk2 was measured in in vitro kinase assays (left). Dose-response curves and 50% inhibition/inhibitory concentration (IC50) values are shown. The cellular inhibitory activity of VS-4718 against the autophosphorylation of FAK and Pyk2 was determined using p-FAK (Y397) and p-Pyk2 (Y402) ELISA assays (right). ELISA data were plotted as a function of concentration to determine half-maximal response (EC50) values. (B) MM.1S, H929, and RPMI8226 cells were cultured in suspension or under adherent conditions on fibronectin and in the presence of IL-6 were treated with VS-4718 for 96 hours. Cell proliferation and viability were assessed using a CellTiter 96 Aqueous One Solution Proliferation assay. VS-4718 inhibited the proliferation of and viability of MM cells in both the presence and absence of fibronectin and IL-6. (C) MM.1S cells were treated with VS-4718 (0, 0.5, 1, 2.5, 5, an 10 µM/L) for 4 hours. Whole-cell lysates were subjected to immunoblotting using anti-p-Pyk2 and anti-Pyk2. (D-E) Five days after being IV injected with GFP+/Luc+-MM.1S cells (3 × 106/ mouse), SCID/Beige mice were dosed with VS-4718 by oral gavage at 75 mg/kg twice daily for 4 weeks in total (n = 6 for vehicle; n = 7 for VS-4718). VS-4718 treatment effectively reduced MM tumor growth compared with vehicle control. (F) MM.1S and H929 cells were treated with VS-4718 (0, 2.5, 5, an 10 µM/L) for 24 hours. β-Catenin, c-Myc, and Cyclin D1 expression in these cells was inhibited by VS-4718. (G) SCID/Beige mice were injected subcutaneously into the left flank with either scramble probe-infected RPMI8226 cells (scr) or Pyk2-kd-RPMI8226 (PYK2 KD) cells. Tumor growth was measured twice weekly and volume (mg = mm3) calculated as (length [mm] × width2 [mm2])/2.
The dose-dependent reduction of p-Pyk2 by VS-4718 in the MM.1S cell line was confirmed (Figure 6C).
We examined cell-cycle profiling in VS-4718–treated cells and found a reduction of S phase in VS-4718–treated MM.1S cells, which express high Pyk2. The same phenotype was not observed in U266 cells, which express low Pyk2. We next evaluated p21 protein expression in high-Pyk2 vs low-Pyk2 cells upon VS-4718 treatment and found that VS-4718 dose-dependently inhibited p21 in MM.1S, but not in U266 (supplemental Figure 3).
Previous studies have shown that Pyk2 leads to activation of p38 and c-Jun N-terminal kinase in several cellular systems, including MM.31-36 We confirmed that Pyk2 overexpression led to upregulation of phospho(p)-p38 in MM cells, whereas Pyk2 silencing was responsible for p-p38 downregulation together with p–c-Jun N-terminal kinase upregulation (supplemental Figure 4).
We next investigated whether Pyk2 silencing may enhance sensitivity to conventionally used anti-MM agents, such as bortezomib, and found that Pyk2-knockdown MM.1S cells presented with significantly higher cytotoxicity when exposed to bortezomib, compared with scramble probe-infected cells that were used as control (supplemental Figure 5A). Moreover, it has been previously shown that Pyk2 mediates dexamethasone-induced apoptosis in MM cells.31,32 We therefore exposed Pyk2-overexpressing cells to increasing concentrations of dexamethasone and confirmed that Pyk2 gain of function enhanced dexamethasone-dependent cytotoxicity (supplemental Figure 5B).
It was previously shown that Pyk2 mediates cell migration.14 We therefore tested the ability of VS-4718 to modulate MM cell migration using both high-Pyk2 MM.1S cells and low-Pyk2 U266 cells, in the context of primary bone marrow mesenchymal stromal cells, and found that VS-4718 significantly inhibited MM.1S cell migration toward BMSCs. This was not observed for U266 cells (supplemental Figure 6A-B).
We next evaluated the anti-MM activity of VS-4718 using primary BM-derived CD138+ MM cells as well as peripheral blood mononuclear cells from healthy individuals and found that VS-4718 exerted cytotoxicity against primary MM cells, whereas this was not observed against normal peripheral blood mononuclear cells (supplemental Figure 7A-B).
We further verified the inhibitory effect of VS-4718 on MM tumor growth in vivo. GFP+/Luc+-MM.1S cells were injected IV into SCID/Beige mice. Mice were randomized into 2 groups after cell injection to be treated with vehicle and VS-4718. VS-4718 treatment was shown to effectively reduce MM tumor growth in vivo (Figure 6D-E). We next dissected the possible mechanisms of VS-4718–dependent inhibition of MM cell growth and found that VS-4718 inhibited β-catenin expression, resulting in downregulation of Cyclin D1 and c-Myc (Figure 6F). These findings recapitulate what was previously observed in vitro and ex vivo in Pyk2 loss-of-function studies.
We finally tested additional Pyk2-silenced RPMI.8226 cells in vivo using a subcutaneous xenograft model and found that Pyk2 loss of function led to inhibited tumor growth in vivo, compared with scramble probe-infected cells (Figure 6G).
Discussion
In the present study, we demonstrated that Pyk2, but not FAK, is overexpressed in MM patients and that specific subgroups of patients with deletion of chromosomal 8p21 may not express Pyk2; however, most patients show higher expression during progression from MGUS stages to overt MM.27 In functional experiments, we show that inhibition of Pyk2 by RNA interference or enhanced expression by complementary DNA significantly regulated tumor progression in vivo and in vitro, indicating that Pyk2 acts as an oncogenic regulator in MM. These biological findings were through enhanced activity of the Wnt/β-catenin pathway as well as regulation of c-Myc and Cyclin D1, master regulators of tumor progression in MM.
Wnt/β-catenin signaling is well known to promote tumorigenesis in both solid tumor and hematologic malignancies, specifically MM.37 In MM disease progression, accumulation of β-catenin in the nucleus of MM cells has been validated to promote MM cell proliferation, migration, and invasion.38-40 Pyk2 has been previously associated with activation of β-catenin: it promotes β-catenin nuclear translocation by phosphorylating β-catenin on tyrosine residues41 and associates with the Wnt5a/Frizzled-4/LRP5 complex, leading to the downstream activation of β-catenin.42 Similarly, c-Myc and Cyclin D1 have shown oncogenic activity in MM leading to tumor progression and aggressive disease behavior.43,44 We believe that Pyk2 regulates c-Myc and Cyclin D1 through modulation of Wnt/β-catenin signaling. Indeed, our finding demonstrates that Pyk2 protects β-catenin from GSK3β-induced degradation, thus leading to accumulation and translocation of β-catenin to the nucleus, which is essential for initiating transcription of Cyclin D1 and c-Myc, known to contribute to cell survival and proliferation.
Previous reports have dissected the role of Pyk2 in MM in vitro, indicating that Pyk2 may mediate dexamethasone-induced apoptosis.31,32 By performing in vivo studies, we have further dissected the role of Pyk2 in MM, thus accounting for the effect of the BM niche that is known to play a crucial role in MM pathogenesis and disease progression. Therefore, the demonstration that Pyk2-overexpressing cells present with an enhanced in vivo tumor growth may result not only from the direct Pyk2-dependent effect on the tumor clone itself but also from the effect of the surrounding BM milieu on Pyk2-overexpressing cells.
Many studies have attempted to identify tyrosine kinase targets in MM, as it promises a viable therapeutic target. Here, we demonstrate that Pyk2 is a promising therapeutic target in MM. Pyk2 expression is limited to specific cell types, including hematopoietic cells, and therefore inhibition of Pyk2 is not expected to confer generalized toxicity. Interestingly, we found that silencing of FAK had no influence on MM tumor growth in vivo, which highlights the specific role of Pyk2 in mediating MM progression. The antitumor activity of dual FAK/Pyk2 inhibitors has been substantiated in several tumors overexpressing both FAK and Pyk2,45-47 and this study adds MM as an additional promising indication for a clinical-stage FAK/PYK2 inhibitor, such as VS-4718. VS-4718 (formerly known as PND-1186) has been previously described as a FAK inhibitor,24-26 and we are now reporting its inhibitory activity against Pyk2, which led to inhibition of MM cell growth both in vitro and in vivo. In addition, because Pyk2 inhibitors may be potentially applicable as a bone anabolic approach for osteoporosis due to enhancement of bone formation,16 we could anticipate that Pyk2 inhibitor treatment may also help in the treatment of myeloma bone disease.
In conclusion, our studies demonstrate the tumor-promoting role of Pyk2 in MM, describe the implication of Pyk2 in facilitating the oncogenic Wnt/β-catenin pathway, and support the potential clinical development of a FAK/Pyk2 inhibitor, such as VS-4718, for the treatment of MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the Multiple Myeloma Research Foundation and by the National Institutes of Health, National Cancer Institute (grants R01CA125690 and 1R01CA152607).
Authorship
Contribution: Yu Z. designed and performed the research, analyzed the data, and wrote the manuscript; M.M., Y.M., P.M., D.H., M.R., I.S., A.S., S.M., Y.A., and S.G. performed the cell biology portion of the research and analyzed the data; Y.-T.T. provided primary MM cells; Yong Z. supervised animal studies; W.Z. performed the in vitro portion of the research and analyzed the data; J.E.R., W.F.T., Q.X., and J.P. performed the VS-4718 tests and analyzed the data; N.C.M. provided primary MM cells and revised the manuscript; K.C.A. revised the manuscript; A.M.R. analyzed the data and wrote the manuscript; and I.M.G. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: J.E.R., W.F.T., Q.X., and J.P. are employees and stockholders of Verastem. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu; and Aldo M. Roccaro, Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: aldo_roccaro@dfci.harvard.edu.
References
Author notes
A.M.R. and I.M.G. contributed equally to this study.


![Figure 3. Pyk2 silencing reduces MM cell proliferation, adhesion, and cell-cycle progression by suppressing Wnt/β-catenin signaling. (A) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured alone or in the presence of MM-BMSCs for 48 hours. Cell proliferation was measured using [3H]-thymidine uptake assay. Inhibition of Pyk2 significantly reduced MM cell proliferation in the presence or absence of MM-BMSCs (P < .0001). (B) The cell cycle of Pyk2-knockdown GFP+/Luc+-MM.1S cells was assessed by propidium iodine staining and flow-cytometric analysis. Inhibition of Pyk2 increased the G1 phase population from 35.82% in scramble control cells to 42.5% in cells expressing shRNA A2 and 59.8% in those expressing shRNA A4. (C-D) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured in MM-BMSC–coated or fibronectin-coated wells for 2 hours. Inhibition of Pyk2 impaired the adhesion ability of MM cells to BMSCs (P = .0004) and fibronectin (P = .0016). (E) GFP+/Luc+-MM.1S Pyk2-knockdown cells were cultured alone or in the presence of MM-BMSCs. After coculture of MM cells with primary BMSCs, MM cells were separated by BMSCs, lysed, and subjected to western blot analysis. Inhibition of Pyk2 reduced β-catenin expression through inducing phosphorylated degradation of β-catenin by decreasing p-GSK3β, thus leading to downregulation of c-Myc and Cyclin D1. (F) Decreased mRNA levels of Pyk2, β-catenin, and c-Myc in BM specimens harvested from mice that were injected with Pyk2-knockdown cells. (G) MM-BMSCs enhanced Pyk2 phosphorylation and β-catenin nuclear translocation and upregulated c-Myc at the nuclear level. These effects triggered by BMSCs were abolished by inhibition of Pyk2. (H) Phosphorylation of Src and Paxillin was inhibited with the inhibition of Pyk2. K.D., knockdown; scr, scramble control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/17/10.1182_blood-2014-03-563981/4/m_2675f3.jpeg?Expires=1763525668&Signature=XwHs9vlNrZFbhxuCBil7Dfb-z8fvLJdbOEN3FSR5kAYQrUPVjtEDu9Sfk8LzWkuxd2qULeY-ChQZQkofj~esCkHIRP8QOSLyuio50HaoCPihrgzwkSAV~TfhOzP6Br-~D8QsEDRdoRe2eF47dQgn~U6cJHZVz2p~QKeYSjTSonNnwQBYKdNM6dY5NI2ay5MCr2QDcon61kKdocTyPWz5Lh4U8KDNUmpjldAPcQTdZuFBiBlH6NaXR~20ckYtRen4gCqNTaCcjp94ZyOLXwIqyxocBLybLg5AwHITNosYcfgT0~6L99b-VTRiOwJjOj6iJeFUAynhWzyD3Qj63DhYHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Effects of FAK/Pyk2 inhibitor 74718 treatment on MM tumor growth. (A) The inhibitory activity of VS-4718 against FAK and Pyk2 was measured in in vitro kinase assays (left). Dose-response curves and 50% inhibition/inhibitory concentration (IC50) values are shown. The cellular inhibitory activity of VS-4718 against the autophosphorylation of FAK and Pyk2 was determined using p-FAK (Y397) and p-Pyk2 (Y402) ELISA assays (right). ELISA data were plotted as a function of concentration to determine half-maximal response (EC50) values. (B) MM.1S, H929, and RPMI8226 cells were cultured in suspension or under adherent conditions on fibronectin and in the presence of IL-6 were treated with VS-4718 for 96 hours. Cell proliferation and viability were assessed using a CellTiter 96 Aqueous One Solution Proliferation assay. VS-4718 inhibited the proliferation of and viability of MM cells in both the presence and absence of fibronectin and IL-6. (C) MM.1S cells were treated with VS-4718 (0, 0.5, 1, 2.5, 5, an 10 µM/L) for 4 hours. Whole-cell lysates were subjected to immunoblotting using anti-p-Pyk2 and anti-Pyk2. (D-E) Five days after being IV injected with GFP+/Luc+-MM.1S cells (3 × 106/ mouse), SCID/Beige mice were dosed with VS-4718 by oral gavage at 75 mg/kg twice daily for 4 weeks in total (n = 6 for vehicle; n = 7 for VS-4718). VS-4718 treatment effectively reduced MM tumor growth compared with vehicle control. (F) MM.1S and H929 cells were treated with VS-4718 (0, 2.5, 5, an 10 µM/L) for 24 hours. β-Catenin, c-Myc, and Cyclin D1 expression in these cells was inhibited by VS-4718. (G) SCID/Beige mice were injected subcutaneously into the left flank with either scramble probe-infected RPMI8226 cells (scr) or Pyk2-kd-RPMI8226 (PYK2 KD) cells. Tumor growth was measured twice weekly and volume (mg = mm3) calculated as (length [mm] × width2 [mm2])/2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/17/10.1182_blood-2014-03-563981/4/m_2675f6.jpeg?Expires=1763525668&Signature=Cgttkeizb37GEW2ouaXWXxKPe6fLxh-dMW9JkuNkYgNr4wM~HeSRwt0-sr8e3j2Npu4V58EiyLJKMajK38Qrj1ZJFCC2kmfYrWCKYlsxwYUsHkBCw8SjsGPHcUNkmhu07MlesGNBFqFtDh5lAx1PfUpS-HQkyxGVx6z~afrvLRzdFB~m9U3ytbaLhb18tWDD4JSFyaCrUzov8EB25gzgf9N18zNw~Yi1NROH~~qRC3wTEbZK3GhJs1VhbRjmKvJg~RSdeFVcy12vKfF5SJ4GMT~hPnV8z0blZqnQnbDVFIe7CC7A2Toa~Yxmn1oHU5bDRM1uUCrWWfcUmoJEBv7KpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal