Abstract
Down Syndrome (DS) (Trisomy 21 – T21) is a common constitutional aneuploidy. Neonates and children with DS have a 150-fold increased risk of developing Acute Myeloid Leukaemia (DS-ML), characterized by a differentiation arrest of immature megakaryocyte-erythroid cells. In virtually all DS-ML patients, somatic mutations in the gene encoding the megakaryocyte-erythroid transcription factor GATA1 are acquired during fetal life leading to the production of a N-terminal truncated form of the GATA1 protein (GATA1s). We, and others, have previously shown that this N-terminal domain is necessary to prevent excessive megakaryocytic proliferation. However, the mechanisms by which GATA1, but not GATA1s, restrains megakaryocytes proliferation are unclear.
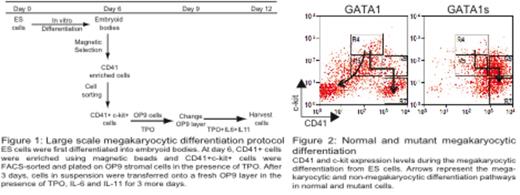
To gain mechanistic insight, we generated knock-in murine ES cell models expressing biotinylated forms of either full length GATA1 or GATA1s protein. We established large scale in vitro differentiation assays to interrogate embryonic-fetal megakaryocyte differentiation (adapted from Nishikii et al., 2008. J Exp Med; 205 (8) : 1917-27; Figure 1) to define the normal megakaryocytic differentiation pathway in GATA1-expressing cells. ES cells were differentiated into embryoid bodies (EB), which were disaggregated at D6 and CD41+c-kit+ cells were cultured on OP9 feeder layers with TPO, IL6 and IL11. Detailed examination of the differentiation kinetics of populations including FACS-sorting of specific populations followed by reculture, showed complex differentiation pathways as wild type cells differentiated into both megakaryocyte and non-megakaryocyte fates. By contrast, GATA1s-expressing cells principally differentiated into megakaryocyte fate. In addition, as immature CD41+ haemopoietic cells differentiate into the megakaryocyte lineage they lose c-kit expression and CD41 expression increases (Figure 2). In the GATA1s-expressing cells compared to GATA1-expressing cells, there is marked accumulation (5 to 10-fold) of a specific immature megakaryocyte CD41++c-kit+ population that is partially blocked in differentiation (Gate R6). Cell cycle analysis shows an increase in cells in S-phase specifically in this population in GATA1s-expressing cells compared to normal cells (44% vs 27%) together with a decrease in apoptosis (5% vs 11%).
To determine GATA1s direct and indirect target genes, we performed ChIP-sequencing and RNA-sequencing. RNA-sequencing of GATA1- and GATA1s-expressing CD41+ populations at D12 showed around 4500 differentially expressed genes (at a p-value of 0.05). Given the differences in cell cycle, it is noteworthy that cyclin D3 and cdk6 were expressed 1.7-fold and 1.5-fold higher in GATA1s- expressing cells. Chromatin in cis-regulatory regions of both genes was bound by GATA1 and GATA1s. Chemical inhibition of the Cyclin D3:Cdk4/6 complex reduced proliferation and induced partial differentiation of mutant cells, suggesting a role of this complex in regulating GATA1s-induced proliferation and differentiation inhibition. Knock-down and overexpression experiments to further test the role of Cyclin D:Cdk4/6 complex in both wild-type and mutant cells are in progress.
Taken together, these results suggest that GATA1s alters cell cycle at a specific stage in megakaryocyte differentiation causing partial differentiation arrest and that this is mediated by altered expression and function of a Cyclin D3:Cdk4/6 complex. These results may have more general implications of how mutant transcription factors cause differentiation arrest in leukemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal