Abstract
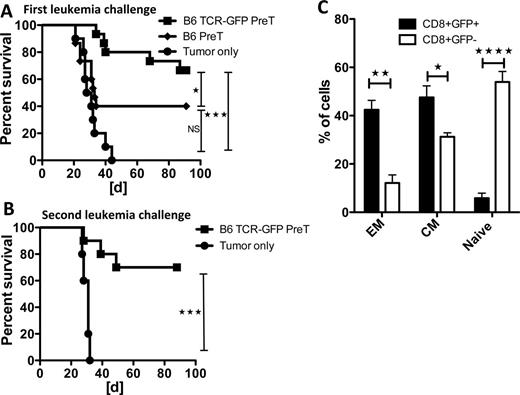
BACKGROUND: The co-transplantation of hematopoietic stem cells (HS) with those that have been engineered to express tumor-reactive T cell receptors (TCRs) and differentiated into precursor T cells (preTs) may optimize tumor reduction. Since expression of potentially self-(tumor-) reactive TCRs will lead to negative selection upon thymic maturation, we investigated whether preTs forced to express a leukemia-reactive TCR under the control of a tetracycline-inducible promoter would allow timely controlled TCR expression thereby avoiding thymic negative selection. METHODS: Using lentiviral vectors, murine LSK cells were engineered to express a Tetracycline-inducible TCR directed against a surrogate leukemia antigen. TCR-transduced LSK cells were co-cultured on T cell development-supporting OP9-DL1 cells to produce preTs. Lethally-irradiated B6/NCrl recipients received syngeneic T cell-depleted bone marrow and 8 × 106 syngeneic or allogeneic (B10.A) TCR-engineered preTs. An otherwise lethal leukemia cell (C1498) challenge was given 28 days later. RESULTS: After in vivo maturation and gene induction up to 70% leukemia free survival was achieved in recipients of syngeneic TCR-transduced preTs (p<0.001) as shown in figure 1A. Importantly, transfer of allogeneic gene-manipulated preTs increased the survival of recipients (p<0.05) without inducing graft versus host disease (GVHD). Non-transduced preTs provided significantly lower leukemia protection being not significantly superior to the PBS controls. The progenies of engineered preTs gave rise to effector and central memory cells providing protection even after repeated leukemia challenge (Figure 1B and 1C). In vitro transduction and consecutive expansion of mature T cells required at least 40 × 106 cells/recipient to mediate similar anti-leukemia efficacy, risking the development of severe GVHD if of mismatched origin, and providing no long-term protection. Importantly, while transgene induction starting immediately after transplant forced CD8+ T cell development and was required to obtain a mature T cell subset of targeted specificity, late induction favored CD4 differentiation and failed to produce a leukemia-reactive population due to missing thymic positive selection. CONCLUSION: Co-transplanting TCR gene-engineered preTs is of high clinical relevance since small numbers of even mismatched HS can be transduced at a reasonable cost, expanded in vitro, stored if needed, and provide potent and long lasting leukemia protection.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal