Key Points
Echinomycin can selectively kill the leukemia-initiating cell in relapsed AML without normal stem cell toxicity.
In vivo delivery of echinomycin can induce long-term complete remission in a murine model of relapsed AML.
Abstract
Acute myeloid leukemia (AML) often relapses following chemotherapy-induced remission and is generally chemo-resistant. Given the potential role for cancer stem cells in relapse, targeting of the leukemia-initiating cell (LIC) in AML may provide improved outcome following remission induction. However, due to overlap in their self-renewal program with normal hematopoietic stem cells (HSCs), therapeutic targeting of the LIC may have an adverse effect on long-term hematopoietic recovery. Here we used a mouse model of relapsed AML to explore whether the hypoxia-inducible factor (HIF)1α inhibitor echinomycin can be used to treat relapsed AML without affecting host HSCs. We show that echinomycin cured 40% to 60% of mice transplanted with relapsed AML. Bone marrow cells from the cured mice displayed normal composition of HSCs and their progenitors and were as competent as those isolated from nonleukemic mice in competitive repopulation assays. Importantly, in mice with complete remission, echinomycin appeared to completely eliminate LICs because no leukemia could be propagated in vivo following serial transplantation. Taken together, our data demonstrate that in a mouse model of relapsed AML, low-dose echinomycin selectively targets LICs and spares normal hematopoiesis.
Introduction
The outcome of patients with acute myeloid leukemia (AML), one of the most common forms of adult leukemia, remain poor, with only 30% to 40% of them achieving long-term survival.1 Currently, clinical practice includes induction chemotherapy following by high-dose chemotherapy consolidation and/or allogeneic bone marrow transplantation (BMT) for those patients who achieve complete remission. The majority of patients in complete remission however, eventually relapse. Therefore, a challenging issue in AML therapy is the development of a successful postremission strategy that improves the fraction of patients cured.2 Possible mechanisms leading to disease relapse include an intrinsic chemoresistance of leukemia-initiating cells (LICs)3,4 that are likely protected from drug toxicity by residing in the bone marrow (BM) niche and through other stemness-related biological functions.4,5
AML was the model used by Lapidot et al when they revived the LIC concept >20 years ago.6 The LIC concept posits that the survival of LICs is an underlying cause for drug resistance and recurrence associated with antileukemia therapy. It has been suggested that effective targeting of LICs may overcome the ultimate obstacle to successful therapy.7,8 However, the similarity in self-renewal programs between LICs and normal hematopoietic stem cells (HSCs)9-12 poses a major challenge for selective targeting of LICs. Therefore, a successful LIC-targeting therapy not only requires selectivity toward LICs over bulk AML blasts but also selectivity over normal HSCs.
Current experimental approaches that target LICs include monoclonal antibodies against cell surface targets,13-15 cytokine-induced cycling of LICs,16 and inhibition of nuclear factor κB.17 More recently, we observed hypoxia-inducible factor (HIF)1α signaling was selectively activated in the LICs of mouse acute lymphoblastic leukemia (ALL) and human AML under normoxia.18 Subsequent studies by others confirmed that the same pathway is also critical for the maintenance of chronic myeloid LICs.19 The HIF1α inhibitor echinomycin efficiently eradicated LICs for mouse ALL and human AML with great selectivity over the bulk of leukemic blasts.18 Remarkably, in 7 independent primary AML samples tested, we observed ∼100-fold increased sensitivity of AML LICs over the bulk of AML blasts.18 The unprecedented selectivity of echinomycin for LICs prompted us to explore whether the drug can be useful for treatment of relapsed AML and whether targeting AML LICs can be achieved without affecting normal HSC function.
A major challenge to the cancer stem cell concept is the use of xenogeneic models with both immunological and cytokine growth barriers.20,21 To avoid this caveat, we took advantage of a mouse model of spontaneous AML that results from the double heterozygous knock-in of the MLL- partial tandem duplication (PTD)22 and the internal tandem duplication (ITD) of FLT323 alleles reported by one of our groups and found in a fraction of AML patients.24 This model presents clinical, cytogenetic, and molecular features that match the human counterpart,2,25,26 thereby representing a suitable model to study novel leukemogeneic mechanisms and therapeutic approaches. It is also particularly suitable for testing echinomycin as we have previously shown the drug to be effective in surrogate leukemia stem cell assays with 7 cases of human AML, 3 of which harbor FLT3 mutations.18 The MllPTD/WT:Flt3ITD/WT AML mouse uniformly relapses with resistant disease following treatment with a DNA hypomethylating agent and/or a histone deacetylase (HDAC) inhibitor.27 Here we report that echinomycin administered as a single agent cured a significant fraction of mice challenged with relapsed MllPTD/WT:Flt3ITD/WT AML. More importantly, echinomycin effectively targets the AML LIC without affecting normal HSC function.
Materials and methods
Experimental animals
All procedures involving experimental animals were approved by Institutional Animal Care and Use Committees of the University of Michigan where this work was performed. CD45.1+ and CD45.2+ mice are obtained from Charles River Laboratories through a contract of National Cancer Institute. MllPTD/WT:Flt3ITD/WT AML mice have been described recently.24
Lentiviral vectors and transduction
All lentiviral vectors, including those used for Hif1α silencing and reporter of HIF activities, as well as the protocol for transduction of leukemia cells, have been described previously.18
Syngeneic grafting of relapsed AML in the mice
Approximately 1.5 million spleen cells obtained from secondary transplants of CD45.2+MllPTD/WT:Flt3ITD/WT murine AML were transplanted into sublethally irradiated (450 Gy) syngeneic CD45.1+ mice (Jackson Laboratories, Bar Harbor, ME). AML was documented in the moribund state at ∼36 days in 100% of mice. Treatment was started on day 21 after transplant, when mice had a white blood cell count >105/μL and engraftment was >70% donor cells in the blood. Treatment consisted of a DNA hypomethylating agent, 5′-aza-2′-deoxycytidine (0.2 mg/kg daily intraperitoneal injection for a total of 4 doses), followed by 50 mg/kg of (S)-(+)-N-hydroxy-4-(3-methyl-2-phenyl-butyrylamino)-benzamide (an HDAC inhibitor28 ) given orally every other day for 3 doses. This regimen has been shown to be temporarily effective in the MllPTD/WT:Flt3ITD/WT AML murine model and therefore suitable to test our hypothesis. Although 100% of recipient mice responded to of (S)-(+)-N-hydroxy-4-(3-methyl-2-phenyl-butyrylamino)-benzamide and 5′-aza-2′-deoxycytidine, as documented by normalization of the white blood cells and spleen weight, all mice relapsed with AML and were killed when moribund at a median of 52 days following engraftment.27 Spleen cells were then collected when the mice became moribund after relapse and were serially transplanted intravenously into wild-type irradiated recipient mice that subsequently were killed when moribund, at which time splenocytes (96% AML blasts) were harvested and frozen. These cells were then thawed viably for use as tertiary AML donor cells for the current studies.
Treatment of relapsed AML with echinomycin
Congenic CD45.1+ mice received 106 relapsed tertiary CD45.2+MllPTD/WT:Flt3ITD/WT AML splenocytes intravenously. On day 8, when the AML blasts reached ∼20% of peripheral blood leukocytes (PBLs), the recipients received 5 intraperitoneal injections of echinomycin, once every other day at 10 μg/kg. The second round of treatment with the same dose was initiated on day 27. The mice were observed for up to 200 days.
Flow cytometric analysis of hematopoiesis
Fluorochrome-conjugated antibodies for identification of HSCs, progenitor cells, and differentiated blood cells have been described recently.29-31 Flow cytometric identification of the cell types were based on the following markers: long-term (LT) HSCs defined as Flk2−Lin−Sca-1+c-Kit+CD34−CD150+CD48−; short-term (ST) HSCs defined as Flk2−Lin−Sca-1+c-Kit+CD150+CD48+; LSK defined as Lin−Sca-1+c-Kit+; myeloid progenitors (MP) defined as Lin−CD127−c-Kit+Sca-1−; multipotent progenitors (MPPs) defined as Flk2−Lin−Sca-1+c-Kit+CD150−CD48+; common myeloid progenitors (CMPs) defined as Lin−Sca1−c-Kit+CD34+CD16/32mi; granulocyte/monocyte progenitors (GMPs) defined as Lin−Sca1−c-Kit+CD34+CD16/32hi; and megakaryocyte/erythroid progenitors (MEPs) defined as Lin−Sca1−c-Kit+CD34+CD16/32−.
BMT
Eight- to 10-week-old congenic recipient mice were lethally irradiated for a combined 1150 rads delivered 4 hours apart. BM cells of donor type were mixed with competitive recipient-type BM cells and were then transplanted into recipients by injection through tail vein. Reconstitution was measured by flow cytometry of blood from the tail vein, spleen, and BM. The red blood cells were lysed by ammonium chloride/potassium bicarbonate buffer before staining.
Colony-forming unit assay
For the colony-forming unit (CFU) assay, 5 × 104/well of relapsed MllPTD/WT:Flt3ITD/WT AML cells (96% blasts) were treated with either vehicle control or a given concentration of echinomycin overnight and seeded in MethoCult M3434 (Cat No.03434; Stemcell Technology). Seven days later, the number of colonies formed was counted under the microscope, according to the manufacturer’s instructions.
Cell viability assay
The relapsed MllPTD/WT:Flt3ITD/WT AML cells (5 × 104/well) were treated with different concentrations of echinomycin for 24 hours. Ten microliters of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) in phosphate-buffered saline was added to each well. Plates were returned to the cell culture incubator for 2 to 4 hours. The resulting MTT formazan crystals were dissolved by adding 150 μL dimethylsulfoxide. Absorbance was measured at a wavelength of 490 nm.
Statistical methods
Statistical significance in survival was determined by the log-rank test. A 2-tailed Student t test was used to determine statistical significance in differences between 2 groups.
Results
LIC-selective echinomycin induces long-lasting remission in syngeneic hosts transplanted with relapsed MllPTD/WT:Flt3ITD/WT AML
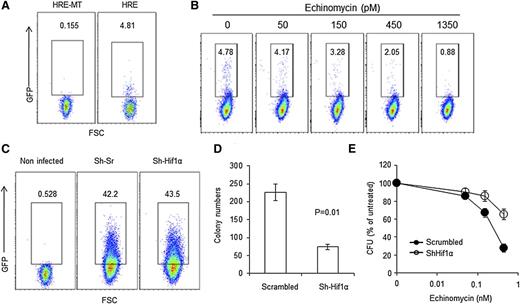
Our previous study showed that stem cell activity in mouse acute T-lymphoblastic leukemia and human AML depends on constitutive HIF activity under normoxia and is highly sensitive to echinomycin.18 To determine whether relapsed MllPTD/WT:Flt3ITD/WT AML also contains a subset of cells with HIF activity under normoxia, we used the previously described lentiviral reporter that expresses green fluorescent protein (GFP) when the cell exhibits HIF activity (called HRE for use of wild-type hypoxia responsive element).18 A lentiviral vector with mutation in the HIF-responsive element (HRE-MT) was used as negative control. As shown in Figure 1A, the relapsed AML contain a small subset (4.8%) of GFP+ cells. The expression of GFP is abrogated when the HRE is mutated (Figure 1B). More than 50% reduction of the HIF+ cells was achieved using as little as 450 pM of echinomycin. To determine whether HIF1α is required for LIC activities, we used short hairpin RNA (shRNA) to silence the Hif1α gene and tested the impact of gene silencing on CFU activity, the surrogate in vitro assay for LIC activities. Because the silencing lentiviral vector contains a GFP marker (Figure 1C), we focused on the GFP+ CFU. As shown in Figure 1D, in comparison with the scramble control, Hif1α silencing reduced the CFU by two-thirds. Therefore, HIF1α plays a critical role in LIC activities of the relapsed MllPTD/WT:Flt3ITD/WT AML. To test whether the HIF1α activities can be targeted by echinomycin, we transduced the relapsed MllPTD/WT:Flt3ITD/WT AML with scramble and Hif1α shRNA vectors and treated them with different doses of echinomycin. To facilitate a comparison between the AML cells transduced with the 2 different vectors, we used the untreated AML samples as 100% CFU activity (ie, normalization) for both. As shown in Figure 1E, silencing Hif1α reduced the CFU sensitivity to echinomycin. These data demonstrate that echinomycin inhibits CFU activities of the relapsed MllPTD/WT:Flt3ITD/WT AML by targeting Hif1α. The inhibition of CFU from HIF1a shRNA-transduced leukemia cells may be either due to incomplete silencing of HIF1a or due to an off-target effect.
HIF1α and colony-forming properties of the relapsed AML cells. (A) Relapsed MllPTD/WTFlt3ITD/WT AML cells contain of a small subset of cells with constitutive HIF activity under normoxia. Relapsed AML cells were transduced with a lentiviral reporter in which the expression of GFP is under the control of hypoxia-responsible element (HRE, 5′-GTA CGT GAC CAC-3′); a lentiviral vector in which the GFP expression is controlled by a mutated HRE (HRE-MT, 5′-GTA AAA GAC CAC-3′) was used as control. The leukemia cells were analyzed by fluorescence-activated cell sorter at 72 hours after transduction. (B) Echinomycin effectively eliminates the subset of relapsed AML cells with HIF activity under normoxia. As in A except the relapsed AML cells were cultured in the presence of given concentration of echinomycin as indicated. (C) Transduction efficacy of scrambled shRNA (Sh-Srambled) or Hif1α shRNA (Sh-Hif1α) for the relapsed AML cells. Lentiviral vectors expressing either Sh-Scrambled or Sh-Hif1α in conjunction with GFP were transduced into the leukemia cells, and the transduction efficiencies were assayed at 72 hours after transduction. (D) Sh-Scrambled or Sh-Hif1α transduced cells from relapsed MllPTD/WTFlt3ITD/WT AML described in C were assayed for LIC activities by CFU assay. Hif1α shRNA silencing of HIF1α reduces LIC activity within relapsed MllPTD/WTFlt3ITD/WT AML, as measured by CFU activity and compared with control. (E) Sh-Scrambled or Sh-Hif1α transduced blasts from relapsed MllPTD/WTFlt3ITD/WT AML described in C were assayed for LIC activities by CFU assay. In this dose escalation experiment, silencing Hif1α reduces the sensitivity of relapsed MllPTD/WTFlt3ITD/WT AML LIC to echinomycin as the dose of echinomycin is increased. The relapsed AML cells from C were treated for 24 hours with the noted concentrations of echinomycin before plating the cells into metrogels for CFU assay. Data shown in D and E are means ± standard deviation (SD) of triplicate cultures. All data in this figure have been reproduced twice.
HIF1α and colony-forming properties of the relapsed AML cells. (A) Relapsed MllPTD/WTFlt3ITD/WT AML cells contain of a small subset of cells with constitutive HIF activity under normoxia. Relapsed AML cells were transduced with a lentiviral reporter in which the expression of GFP is under the control of hypoxia-responsible element (HRE, 5′-GTA CGT GAC CAC-3′); a lentiviral vector in which the GFP expression is controlled by a mutated HRE (HRE-MT, 5′-GTA AAA GAC CAC-3′) was used as control. The leukemia cells were analyzed by fluorescence-activated cell sorter at 72 hours after transduction. (B) Echinomycin effectively eliminates the subset of relapsed AML cells with HIF activity under normoxia. As in A except the relapsed AML cells were cultured in the presence of given concentration of echinomycin as indicated. (C) Transduction efficacy of scrambled shRNA (Sh-Srambled) or Hif1α shRNA (Sh-Hif1α) for the relapsed AML cells. Lentiviral vectors expressing either Sh-Scrambled or Sh-Hif1α in conjunction with GFP were transduced into the leukemia cells, and the transduction efficiencies were assayed at 72 hours after transduction. (D) Sh-Scrambled or Sh-Hif1α transduced cells from relapsed MllPTD/WTFlt3ITD/WT AML described in C were assayed for LIC activities by CFU assay. Hif1α shRNA silencing of HIF1α reduces LIC activity within relapsed MllPTD/WTFlt3ITD/WT AML, as measured by CFU activity and compared with control. (E) Sh-Scrambled or Sh-Hif1α transduced blasts from relapsed MllPTD/WTFlt3ITD/WT AML described in C were assayed for LIC activities by CFU assay. In this dose escalation experiment, silencing Hif1α reduces the sensitivity of relapsed MllPTD/WTFlt3ITD/WT AML LIC to echinomycin as the dose of echinomycin is increased. The relapsed AML cells from C were treated for 24 hours with the noted concentrations of echinomycin before plating the cells into metrogels for CFU assay. Data shown in D and E are means ± standard deviation (SD) of triplicate cultures. All data in this figure have been reproduced twice.
To test whether the relapsed AML and untreated AML are sensitive to echinomycin, we harvested MllPTD/WT:Flt3ITD/WT AML blasts after leukemic mice were either untreated or treated with a DNA hypomethylating agent followed by an HDAC inhibitor, and then relapsed and had cells passaged twice in vivo. The untreated and relapsed AML cells (>95% blasts) were incubated in vitro with different doses of echinomycin overnight. After washing out the echinomycin, the same aliquots were tested for viability by MTT assay and were plated for CFU as a surrogate assay for AML LIC activity. As shown in Figure 2A, the untreated and relapsed AML show essentially identical responses to echinomycin in both the MTT and CFU assays. Therefore, relapsed AML has not gained resistance to echinomycin.
Echinomycin induces long-lasting remission in syngeneic hosts transplanted with relapsed MllPTD/WTFlt3ITD/WT AML. (A) Relapsed and untreated AML cells were equally sensitive to echinomycin. The untreated and relapsed AML cells were thawed and allowed to recover overnight in vitro. After treatment with vehicle control or different concentrations of echinomycin for 48 hours, the drugs were washed away, and the cells were placed in a CFU assay or a MTT assay to determine viability. The drug doses are 0, 50, 150, 450, and 1350 pM. Data shown are means ± SD of colony numbers plated in triplicate or assessment of overall viability by MTT assay in triplicate. The data are representative of 2 independent experiments. (B-E) Therapeutic efficacy of a low dose of echinomycin in vivo for relapsed AML. (B) Diagram of experimental design. One million CD45.2+ relapsed AML cells were injected intravenously into each of 20 sublethally irradiated CD45.1+ recipients. On day 8, when the CD45.2+ AML cells were detectable in all recipient mice, 2 5-dose cycles of echinomycin treatments were initiated. Arrows indicate dosing and arrowheads indicate date of flow cytometry of blood cells. (C) Flow cytometry of PBL for CD45.2+ leukemia cells in the blood of vehicle-treated (top 2 rows) or echinomycin-treated (bottom 2 rows) mice at 23 days after transplantation. Each histogram represents 1 mouse, and data show reduction in the number of leukemic cells in the blood following 1 cycle of echinomycin treatment. (D) Complete remission in a significant fraction of Echinomycin-treated mice shown in [c] harboring relapsed AML. The lower panel shows percent of leukemic cells in the 10 vehicle-treated mice throughout their life span and the point at which death was recorded in all 10, while the upper panel shows percent of leukemic cells in the 10 Echinomycin-treated mice over a 12-week period. Of the 10 Echinomycin-treated mice harboring relapsed AML, 4 had a complete remission at 12 weeks (as defined by lack of leukemia cell in the PBLs) and never relapsed over the 300 days of observation or serial transplantation. No mice in the vehicle-treated group experienced remission. Similar results were observed in another independent experiment involving 5 mice per group. (E) Echinomycin treatment significantly increased the life span of mice with transplanted relapsed AML. Data shown are Kaplan-Meier survival curves of 2 independent experiments.
Echinomycin induces long-lasting remission in syngeneic hosts transplanted with relapsed MllPTD/WTFlt3ITD/WT AML. (A) Relapsed and untreated AML cells were equally sensitive to echinomycin. The untreated and relapsed AML cells were thawed and allowed to recover overnight in vitro. After treatment with vehicle control or different concentrations of echinomycin for 48 hours, the drugs were washed away, and the cells were placed in a CFU assay or a MTT assay to determine viability. The drug doses are 0, 50, 150, 450, and 1350 pM. Data shown are means ± SD of colony numbers plated in triplicate or assessment of overall viability by MTT assay in triplicate. The data are representative of 2 independent experiments. (B-E) Therapeutic efficacy of a low dose of echinomycin in vivo for relapsed AML. (B) Diagram of experimental design. One million CD45.2+ relapsed AML cells were injected intravenously into each of 20 sublethally irradiated CD45.1+ recipients. On day 8, when the CD45.2+ AML cells were detectable in all recipient mice, 2 5-dose cycles of echinomycin treatments were initiated. Arrows indicate dosing and arrowheads indicate date of flow cytometry of blood cells. (C) Flow cytometry of PBL for CD45.2+ leukemia cells in the blood of vehicle-treated (top 2 rows) or echinomycin-treated (bottom 2 rows) mice at 23 days after transplantation. Each histogram represents 1 mouse, and data show reduction in the number of leukemic cells in the blood following 1 cycle of echinomycin treatment. (D) Complete remission in a significant fraction of Echinomycin-treated mice shown in [c] harboring relapsed AML. The lower panel shows percent of leukemic cells in the 10 vehicle-treated mice throughout their life span and the point at which death was recorded in all 10, while the upper panel shows percent of leukemic cells in the 10 Echinomycin-treated mice over a 12-week period. Of the 10 Echinomycin-treated mice harboring relapsed AML, 4 had a complete remission at 12 weeks (as defined by lack of leukemia cell in the PBLs) and never relapsed over the 300 days of observation or serial transplantation. No mice in the vehicle-treated group experienced remission. Similar results were observed in another independent experiment involving 5 mice per group. (E) Echinomycin treatment significantly increased the life span of mice with transplanted relapsed AML. Data shown are Kaplan-Meier survival curves of 2 independent experiments.
It is of interest to note that, although only 100 pM of echinomycin caused nearly 50% reduction of CFU (ie, LIC activity; supplemental Figure 1 available on the Blood Web site), 3 nM of echinomycin is required to achieve similar reduction in viability of total AML blasts. A similar, although less dramatic, trend can be discerned by comparing the left and right panel in Figure 2A when different isolates of cells were tested. Thus, the CFU or LIC activity is much more sensitive to echinomycin than the viability of total AML cells. Because only ∼5% of the leukemia cells have HIF activity under nomoxia and because echinomycin eliminated essentially all AML blasts at 5 nM (Figure 2A), echinomycin also targets AML blasts that show no detectable HIF activity under nomoxia at steady state due to an off-target effect.
To test whether echinomycin can be used to treat relapsed AML, we transplanted the relapsed CD45.2+MllPTD/WT:Flt3ITD/WT AML blasts into CD45.1+ congenic hosts. As shown in supplemental Figure 2, all recipient mice developed AML within 1 week. On day 8 after transplantation, when ∼20% of the PBL are of donor origin, the AML-bearing mice were randomly divided into 2 groups for treatment with either vehicle or echinomycin (Figure 2B). Two weeks after the first 5-day treatment, all but 1 (90%) vehicle-treated mouse had >60% AML blasts in PBLs, whereas 5 of 10 (50%) echinomycin-treated mice had ≤20% AML blasts in PBLs (Figure 2C). At this time point, all vehicle-treated mice had a higher percentage of AML blasts in PBL than pretreatment and became moribund, whereas 5 of 10 treated mice were healthy and showed a decrease in the percentage of AML blasts in PBL compared with pretreatment (Figure 2D). To ensure the continued decrease of AML blasts in PBLs, another 5-day course of echinomycin was undertaken (Figure 2B). Robust reduction of AML blasts in PBLs was observed in the 5 mice alive on day 39 (supplemental Figure 3). In fact, essentially no AML blasts in PBLs were detected in 4 of the 5 echinomycin-treated mice alive at this time point (Figure 2D). The absence of AML blasts in PBLs in the 4 mice was confirmed by another analysis on day 84 (Figure 1D). The fifth mouse in the echinomycin-treated group had a nearly 50% reduction in the percent of AML blasts in PBLs following the second cycle of echinomycin (mouse 2 in supplemental Figure 3) but became moribund on day 39.
Surprisingly, we did not observe any measurable response in about half of the echinomycin-treated mice (Figure 2D). To determine whether the AML in the echinomycin-treated mice that did not respond had acquired resistance to the drug, we compared the relapsed AML obtained from vehicle or echinomycin-treated mice for their sensitivity to echinomycin using the CFU assay. As shown in supplemental Figure 4, CFU from echinomycin-treated AML are more resistant to echinomycin. These data suggest that in vivo echinomycin therapy had selected for drug-resistant leukemia clones in select mice. The apparent lack of any discernible response in this subset of echinomycin-treated mice may have been due to infrequent monitoring of the leukemia content in the PBLs. However, it is also possible that stochastically, echinomycin-resistant clones have a differential contribution to leukemia in different mice.
Corresponding with the dramatic reduction of relapsed AML blasts in PBL, 4 of the 10 (40%) echinomycin-treated mice remained healthy throughout the entire observation period (>250 days), whereas all 10 of the vehicle-treated mice experienced mortality before day 30 (Figure 2E, upper; P = .015). In a second independent experiment, 3 of the 5 (60%) echinomycin-treated mice showed long-term survival of >225 days compared with 100% mortality in the vehicle-treated mice (Figure 2E, lower; P = .009). These data demonstrate that in the immune competent syngeneic system, echinomycin is highly effective against relapsed MllPTD/WT:Flt3ITD/WT AML, which represents a combination of mutations also present in a fraction of AML patients.
Echinomycin treatment eliminates LICs without affecting normal HSCs and progenitor cells in the host
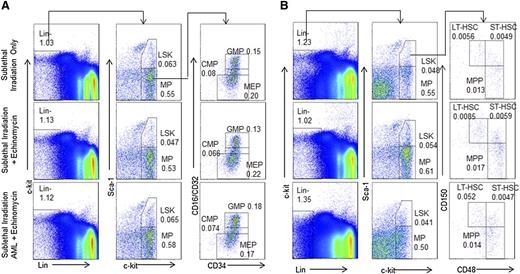
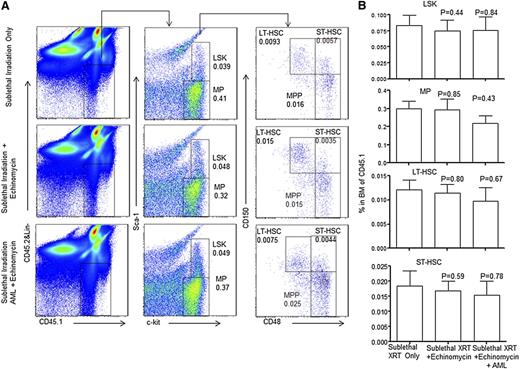
The similarity in self-renewal programs in cancer and normal stem cells9-12 poses a major challenge for selective targeting of LICs without affecting normal HSCs. We took 2 approaches to evaluate whether effective echinomycin treatment in vivo adversely affected the function of the normal HSCs. First, we analyzed composition of both HSCs and progenitors in pooled BM from mice that have been cured of relapsed AML by echinomycin. As shown in Figure 3, as well as supplemental Figures 5 and 6, CD45.1+ BM from mice that received sublethal irradiation only, or sublethal irradiation plus 2 cycles of echinomycin treatment, or sublethal irradiation, CD45.2+ relapsed AML, and 2 cycles of echinomycin treatment, have a similar frequency of HSCs, as well as all subsets of progenitors at >20 weeks after the initial treatments.
Echinomycin treatment did not reduce normal hematopoietic progenitor cells and HSCs. Wild-type CD45.1+ mice received (1) sublethal irradiation alone (top lane); (2) sublethal irradiation and 2 cycles of echinomycin therapy starting same day as started in group 3 (middle lane), or (3) sublethal irradiation, intravenous injection of tertiary relapsed CD45.2+ AML blasts (1 × 106/mouse), and 2 cycles of echinomycin therapy starting day 8 after infusion of AML (bottom). Mice were then observed for 150 or 165 days and killed, and harvested BM cells were stained as defined in the Materials and methods to assess frequency of CD45.1+ (A) LSK, MP, CMP, GMP, and MEP and (B) LSK, MP, LT-HSCs, MPC, MPP, and ST-HSCs. The data were reproduced in 2 independent experiments. An additional example of A is shown in supplemental Figure 3; an additional example of B is shown in supplemental Figures 5 and 6.
Echinomycin treatment did not reduce normal hematopoietic progenitor cells and HSCs. Wild-type CD45.1+ mice received (1) sublethal irradiation alone (top lane); (2) sublethal irradiation and 2 cycles of echinomycin therapy starting same day as started in group 3 (middle lane), or (3) sublethal irradiation, intravenous injection of tertiary relapsed CD45.2+ AML blasts (1 × 106/mouse), and 2 cycles of echinomycin therapy starting day 8 after infusion of AML (bottom). Mice were then observed for 150 or 165 days and killed, and harvested BM cells were stained as defined in the Materials and methods to assess frequency of CD45.1+ (A) LSK, MP, CMP, GMP, and MEP and (B) LSK, MP, LT-HSCs, MPC, MPP, and ST-HSCs. The data were reproduced in 2 independent experiments. An additional example of A is shown in supplemental Figure 3; an additional example of B is shown in supplemental Figures 5 and 6.
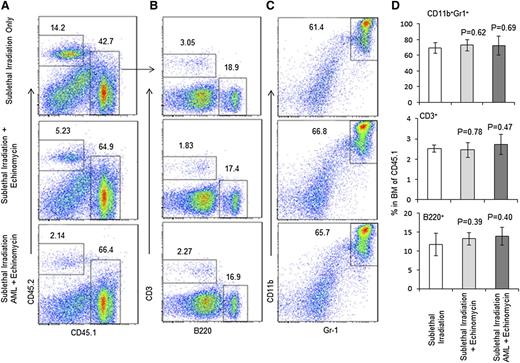
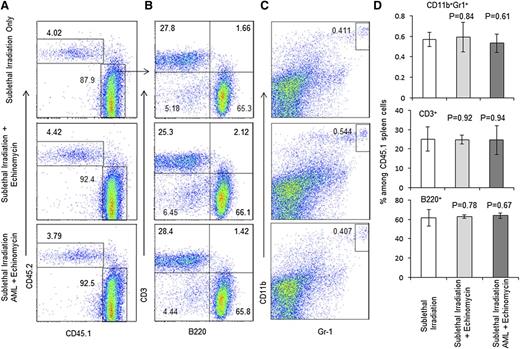
Second, we mixed the donor BM cells from the abovementioned 3 groups of mice (killed on day 165) at a 1:1 ratio with CD45.2+ wild-type BM cells and transplanted them into the lethally irradiated CD45.2+ host to measure long-term stem cell activity by reconstitution of donor-type cells in the recipients. As shown in Figure 4A and supplemental Figure 7, based on the frequency of CD45.1+ donor cells, the HSCs from echinomycin-treated mice are at least as effective as those used from untreated mice. Within the donor cell compartment, the composition of the major leukocyte subsets, including T, B, and myeloid cells, are unaffected by echinomycin (Figure 4B-D). The frequencies of T, B, and myeloid cells in the spleen are also normal (Figure 5A-D). The normal development of all lineages in the mixed chimera mice also revealed that BM from the remission mice in the third group did not develop leukemia in the new host, which indicates that the leukemia stem cells have been eliminated by echinomycin. As expected, BM from mice who received sublethal irradiation and AML without echinomycin treatment, even when admixed 1:1 with CD45.2+ wild-type BM cells and then transplanted into the lethally irradiated CD45.2+ host, shows rapid development of leukemia in all recipients (supplemental Figure 8).
Echinomycin treatment does not affect long-term HSC activity as revealed by competitive BMT. Three groups of CD45.1+ mice prepared in an identical fashion to those described in the legend of Fig. 2 were killed on day 165. Their BM cells were mixed with recipient wild-type CD45.2+ BM cells in a 1:1 ratio and transplanted into the lethally irradiated CD45.2+ host. Twenty-nine weeks later, the recipient mice were killed to assess the hematopoiesis of the CD45.1+ HSCs. (A) Long-term hematopoiesis based on frequency of CD45.1+ cells. (B) Frequency of B and T cells among CD45.1+ BM cells. (C) Frequency of granulocytes among CD45.1+ BM cells. (D) Summary data of A-C. Data shown represent the means ± SD for 5 mice. These data have been reproduced in 2 independent experiments.
Echinomycin treatment does not affect long-term HSC activity as revealed by competitive BMT. Three groups of CD45.1+ mice prepared in an identical fashion to those described in the legend of Fig. 2 were killed on day 165. Their BM cells were mixed with recipient wild-type CD45.2+ BM cells in a 1:1 ratio and transplanted into the lethally irradiated CD45.2+ host. Twenty-nine weeks later, the recipient mice were killed to assess the hematopoiesis of the CD45.1+ HSCs. (A) Long-term hematopoiesis based on frequency of CD45.1+ cells. (B) Frequency of B and T cells among CD45.1+ BM cells. (C) Frequency of granulocytes among CD45.1+ BM cells. (D) Summary data of A-C. Data shown represent the means ± SD for 5 mice. These data have been reproduced in 2 independent experiments.
Echinomycin treatment does not affect long-term HSC activity as revealed by composition of spleen cells. Spleen cells from the CD45.2+ recipients of competitive BMT were analyzed for composition of CD45.1+ donor-type leukocytes. (A) Frequency of CD45.1+ cells. (B) Frequency of B and T cells. (C) Frequency of granulocytes. (D) Summary data of A-C. Data shown represent the mean and standard error of the mean for 5 mice. These data have been repeated in 2 independent experiments.
Echinomycin treatment does not affect long-term HSC activity as revealed by composition of spleen cells. Spleen cells from the CD45.2+ recipients of competitive BMT were analyzed for composition of CD45.1+ donor-type leukocytes. (A) Frequency of CD45.1+ cells. (B) Frequency of B and T cells. (C) Frequency of granulocytes. (D) Summary data of A-C. Data shown represent the mean and standard error of the mean for 5 mice. These data have been repeated in 2 independent experiments.
To determine whether echinomycin treatment affects self-renewal of normal HSCs, we gated on the donor-derived cells and analyzed the composition of HSCs and progenitor populations. As shown in Figure 6A-B, regardless of whether the host had been transplanted with AML, the echinomycin treatment had no effect on the number of MPs, LT-HSCs, ST-HSCs, and MPPs. These data demonstrate the HSC self-renewal and differentiation are unaffected by echinomycin.
Echinomycin treatment does not affect self-renewal of HSCs. BM cells from the recipients of competitive BMT were analyzed for composition of hematopoietic progenitors and stem cells at 29 weeks after transplantation. (A) Representative fluorescence-activated cell sorter profiles. Lin−CD45.1+ cells were analyzed for frequency of CD45.1+ hematopoietic progenitors and stem cells, including LSK, MP, MPP, LT-HSCs, and ST-HSCs. (B) Summary data of A. Data shown represent the means ± SD for 5 mice. These data have been repeated in 2 independent experiments.
Echinomycin treatment does not affect self-renewal of HSCs. BM cells from the recipients of competitive BMT were analyzed for composition of hematopoietic progenitors and stem cells at 29 weeks after transplantation. (A) Representative fluorescence-activated cell sorter profiles. Lin−CD45.1+ cells were analyzed for frequency of CD45.1+ hematopoietic progenitors and stem cells, including LSK, MP, MPP, LT-HSCs, and ST-HSCs. (B) Summary data of A. Data shown represent the means ± SD for 5 mice. These data have been repeated in 2 independent experiments.
Discussion
Our data presented herein demonstrate that echinomycin selectively targets AML LICs and confers significant therapeutic effect in mice with syngeneic relapsed AML. These data are relevant not only to the concept of AML LICs, but more importantly, to therapeutic development of echinomycin as a potential drug for treatment of relapsed AML.
First, unlike the previous studies on the therapeutic effect in xenogeneic AML models,4,13,14,17,18 the current study involves a syngeneic mouse model that has targeted knock-in of 2 well-established mutations in human AML cases, namely the MLL-PTD and the FLT3-ITD.24 The use of a syngeneic system is considered important in the concept of cancer stem cells, as some have suggested that use of a xenogeneic system may create barriers that are unrelated to cancer stem cell biology.20,21 In addition, use of syngeneic leukemia models avoids the caveats of mismatches in cytokines and receptors between the donor leukemia and the host. Notably, the mutations that caused the relapsed AML in the mouse are associated with a poor prognosis and among the most frequent in human relapsed AML with normal cytogenetics.26 These results provide a compelling case for clinical development of echinomycin for relapsed AML.
Second, although our previous studies have shown that echinomycin confers a therapeutic effect to both ALL and AML in the mouse model,18 this is the first report to our knowledge that the drug can confer therapeutic effect in relapsed leukemia. Successful therapeutic options for relapsed AML are restricted to allogeneic BMT, which is limited by the age of the recipient, availability of donors, cost, and graft-versus-host disease. Conversesly, higher-intensity chemotherapy alone has generally been ineffective secondary to chemo-resistant disease.5 Our data presented herein provide the first direct evidence that targeting AML LICs with echinomycin may result in a new therapeutic option for relapsed human AML. In conjunction with our previous data that show therapeutic effect of echinomycin in a xenogeneic model using samples from newly diagnosed AML patients,18 our data suggest that echinomycin may be valuable for both primary and relapsed AML.
Third, unlike conventional chemotherapy that tends to enrich for cancer stem cells,3,4,32-35 echinomycin shows preferential reduction of CFU activity in the relapsed AML model. Thus, our in vitro studies showed that the drug has a lower IC50 when measured against a quantitative CFU assay (a surrogate for LICs) than when measured against a MTT assay that quantifies viability of bulk AML blasts.
Our extensive analysis showed that, at the therapeutic doses that induce a long-term complete remission in relapsed AML, echinomycin does not affect normal hematopoiesis in the host. Thus, the numbers of normal HSCs and hematopoietic progenitor cell populations found in BM of mice exposed only to sublethal irradiation are indistinguishable from those in mice treated with both sublethal irradiation and echinomycin, without or with engraftment and subsequent cure of relapsed AML. Likewise, in the stringent competitive BMT assays, the echinomycin-treated groups are as efficient as the vehicle-treated control mice. The mice engrafted with relapsed leukemia that were not treated cannot be tested because they give rise to leukemia that overwhelms host hematopoiesis. Although our previous studies have shown that echinomycin is ∼100-fold more effective in inhibiting de novo ALL and AML LICs than the hematopoietic progenitor cells in the in vitro CFU assay,17 the current body of work shows selective targeting of AML LICs over normal HSCs for the first time. This concept provides a foundation for therapeutic development of echinomycin for treatment of relapsed AML.
Paradoxically, although targeted mutation of HIF1α alone diminishes hematopoiesis in BMT, combinatorial deletion of HIF1α in conjunction with either Cited2 or Von Hippel-Lindau rescues the defect in hematopoiesis caused by either gene alone.36,37 These data suggest that, although overaccumulation of HIF1α may be toxic for HSCs, complete absence of HIF1α also affects hematopoiesis. How the HSC avoids inhibition by echinomycin remains to be fully explained. Nevertheless, the fact that echinomycin had no measurable effect on normal hematopoiesis suggest that a therapeutic window can be exploited to harness the more stringent requirement for HIF1α activity in maintaining LICs as opposed to the that of normal HSCs.
Echinomycin has manageable toxicities in humans as documented in multiple prior clinical trials. The drug development was halted as it showed minimal therapeutic effect for terminal patients with solid tumors, although it has not been tested in patients with hematologic malignancies.38-45 In fact, dose escalation studies indicated that grade I toxicity was observed starting after doses of 180 μg/m2, with no toxicity found at doses of 60 and 120 μg/m2.46 By showing that a low dose of echinomycin (30 μg/m2) eliminates AML LICs without a significant effect on the HSCs, our data, along with our recent data on primary AML samples,17 provide a foundation for further clinical development of the drug for treatment of both primary and relapsed AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Cancer Institute grants R01CA58033, R01CA171972, R21CA164469 CA89341, and CA140158.
The sponsor has no role in experimental design, data interpretation, and preparation of this manuscript.
Authorship
Contribution: Y.W., Yan Liu, and F.T. performed the experiments; K.M.B. provided untreated and relapsed AML cells for the study; R.S. and G.M. provided input in manuscript preparation; and Yang Liu, Y.W., P.Z., and M.A.C. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Caligiuri, The Ohio State University Comprehensive Cancer Center, The James Cancer Hospital & Solove Research Center, 300 W 10th Ave, Suite 519, Columbus, OH 43210; e-mail: michael.caligiuri@osumc.edu; Pan Zheng, Center for Cancer and Immunology Research, Children's National Medical Center, 111 Michigan Ave NW, Washington, DC 20010; e-mail: pzheng@cnmc.org; and Yang Liu, Center for Cancer and Immunology Research, Children's National Medical Center, 111 Michigan Ave NW, Washington, DC 20010; e-mail: yaliu@cnmc.org.

![Figure 2. Echinomycin induces long-lasting remission in syngeneic hosts transplanted with relapsed MllPTD/WTFlt3ITD/WT AML. (A) Relapsed and untreated AML cells were equally sensitive to echinomycin. The untreated and relapsed AML cells were thawed and allowed to recover overnight in vitro. After treatment with vehicle control or different concentrations of echinomycin for 48 hours, the drugs were washed away, and the cells were placed in a CFU assay or a MTT assay to determine viability. The drug doses are 0, 50, 150, 450, and 1350 pM. Data shown are means ± SD of colony numbers plated in triplicate or assessment of overall viability by MTT assay in triplicate. The data are representative of 2 independent experiments. (B-E) Therapeutic efficacy of a low dose of echinomycin in vivo for relapsed AML. (B) Diagram of experimental design. One million CD45.2+ relapsed AML cells were injected intravenously into each of 20 sublethally irradiated CD45.1+ recipients. On day 8, when the CD45.2+ AML cells were detectable in all recipient mice, 2 5-dose cycles of echinomycin treatments were initiated. Arrows indicate dosing and arrowheads indicate date of flow cytometry of blood cells. (C) Flow cytometry of PBL for CD45.2+ leukemia cells in the blood of vehicle-treated (top 2 rows) or echinomycin-treated (bottom 2 rows) mice at 23 days after transplantation. Each histogram represents 1 mouse, and data show reduction in the number of leukemic cells in the blood following 1 cycle of echinomycin treatment. (D) Complete remission in a significant fraction of Echinomycin-treated mice shown in [c] harboring relapsed AML. The lower panel shows percent of leukemic cells in the 10 vehicle-treated mice throughout their life span and the point at which death was recorded in all 10, while the upper panel shows percent of leukemic cells in the 10 Echinomycin-treated mice over a 12-week period. Of the 10 Echinomycin-treated mice harboring relapsed AML, 4 had a complete remission at 12 weeks (as defined by lack of leukemia cell in the PBLs) and never relapsed over the 300 days of observation or serial transplantation. No mice in the vehicle-treated group experienced remission. Similar results were observed in another independent experiment involving 5 mice per group. (E) Echinomycin treatment significantly increased the life span of mice with transplanted relapsed AML. Data shown are Kaplan-Meier survival curves of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/7/10.1182_blood-2013-12-544221/5/m_1127f2.jpeg?Expires=1771014614&Signature=IPKFmQ4OVrm36IJkJCC9XyMyCyWmnRRyeCC-eO90XLww63lIabTKV6rIJeXoLh7lbr4TW-4qW3N7VRvzctjSNbos~Vxa83IZV1Qh1twXERw-XiQP9TLVp9U9gobTCCJ-fRj8HQQc25vHKRQT3FLLTjirHd380b5nnqxU9swhitbc691muZWWlqQ0rMlkz9NdaBvopF8Oi2n7jpJMYlm5reUd7IQ7ve-EHt7nN-svAGC3c021HI2DFFv6oo47mNc7hx~LoX~vtX~NWPakk-2~kjbDjzKNJiHW~Y~d9of4k6vkF~zhrbRbu5CygceCxq7D1cv3YD-0CGzUMdHALO7DcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal