Key Points
ARNT promotes adult hematopoietic stem cell viability through regulation of BCL-2 and VEGF-A expression.
Fetal liver hematopoietic progenitors experience hypoxia and loss of hypoxia-induced transcription decreases their survival.
Abstract
Hypoxia-inducible factors (HIFs) are master regulators of the transcriptional response to low oxygen and play essential roles in embryonic development, tissue homeostasis, and disease. Recent studies have demonstrated that hematopoietic stem cells (HSCs) within the bone marrow localize to a hypoxic niche and that HIF-1α promotes HSC adaptation to stress. Because the related factor HIF-2α is also expressed in HSCs, the combined role of HIF-1α and HIF-2α in HSC maintenance is unclear. To this end, we have conditionally deleted the HIF-α dimerization partner, the aryl hydrocarbon receptor nuclear translocator (ARNT) in the hematopoietic system to ablate activity of both HIF-1α and HIF-2α and assessed the functional consequence of ARNT deficiency on fetal liver and adult hematopoiesis. We determined that ARNT is essential for adult and fetal HSC viability and homeostasis. Importantly, conditional knockout of both Hif-1α and Hif-2α phenocopied key aspects of these HSC phenotypes, demonstrating that the impact of Arnt deletion is primarily HIF dependent. ARNT-deficient long-term HSCs underwent apoptosis, potentially because of reduced B-cell lymphoma 2 (BCL-2) and vascular endothelial growth factor A (VEGF-A) expression. Our results suggest that HIF activity may regulate HSC homeostasis through these prosurvival factors.
Introduction
Hematopoietic stem cells (HSCs) reside in the bone marrow (BM), where they balance both cell-intrinsic and cell-extrinsic cues to achieve self-renewal and appropriate hematologic differentiation throughout the mammalian lifespan.1 HSCs are regulated by their microenvironment, which consists of endothelial,2 perivascular,2 adipocyte,3 and osteoblast4 support cells; secreted factors; and oxygen (O2) availability.5 Hypoxia has become increasingly recognized as a critical regulator of stem cells, during both embryonic development and adulthood.6 Importantly, HSCs reside in a poorly perfused hypoxic niche,7-10 and recent data suggest that HSC oxygenation levels may be partially regulated by cell-specific mechanisms.10 Although the biological importance of these observations is not entirely clear, hypoxia clearly imposes phenotypic consequences for HSCs. For instance, long-term HSCs (LT-HSCs) are highly quiescent, a cell cycle status often associated with O2- and nutrient-deprived cells. Although many pathways converge on metabolism, the primary transcriptional response to hypoxia is mediated by hypoxia-inducible factors (HIFs).
HIFs are heterodimeric transcription factors composed of a HIF-α subunit (HIF-1α11 and HIF-2α12 ) and their common β subunit, HIF-1β or the aryl hydrocarbon receptor nuclear translocator (ARNT).13 HIF-α/ARNT heterodimers stimulate the transcription of many genes, which promote survival and adaptation to hypoxia by replenishing O2 levels through stimulating angiogenesis, conserving cellular resources and increasing the use of glycolysis for adenosine triphosphate (ATP) production.14 In stem cells, HIFs impose distinct responses compared with differentiated cells.6 Therefore, it is critical to evaluate the role of HIF in each stem cell population that experiences hypoxia, including HSCs.
HIF is a critical regulator of both embryonic and adult hematopoiesis.5,6,15 Embryoid bodies generated in vitro from Arnt-deficient mouse embryonic stem cells form fewer hematopoietic colonies, suggesting that hypoxia promotes formation of early hematopoietic cells.16,17 Consistently, germline Arnt deletion results in diminished numbers of yolk sac hematopoietic progenitors, demonstrating that HIF is critical for hematopoietic development.16 A later stage of embryonic development, definitive hematopoiesis within the aorta-gonad-mesonephros (AGM) region, is also regulated by HIF, because Arnt−/− embryos exhibit fewer AGM hematopoietic progenitors.18 These data suggest that HIF is a core regulatory component of early hematopoiesis. However, it is still unknown whether hypoxia and HIF activity play a role in later embryonic hematopoiesis within the fetal liver (FL).
The HIF pathway regulates adult hematopoiesis in both a cell-autonomous and non–cell-autonomous fashion. Acute postnatal loss of Hif-2α causes anemia as a result of diminished expression of erythropoietin (Epo).19,20 Surprisingly, postnatal Hif-1α deletion did not result in hematopoietic phenotypes in young mice, suggesting independent roles for Hif-1α and Hif-2α in hematopoiesis.19,21 Indeed, when subjected to stressors such as 5-fluorouracil (5-FU) myelosuppression, transplantation, or aging, Hif-1α–deficient HSCs display a loss of quiescence. Pharmacologic activation of HIF signaling also promotes quiescence in vivo, suggesting that HIF may play a role in HSC homeostasis,22 although this has not been fully evaluated. However, because HIF-1α and HIF-2α have both unique and overlapping functions, their combined roles in FL hematopoiesis and normal adult hematopoiesis remain unclear.
In the present study, we used a conditional strategy to delete Arnt in FL and BM HSCs and examined the consequence of Arnt ablation on HSC homeostasis and function. We determined that Arnt-deficient (knockout [KO]) mice had greater numbers of white blood cells (WBCs) in the peripheral blood and changes in the abundance of myeloid and lymphoid cells. ARNT loss caused a reduction in frequency and absolute numbers of LT-HSCs, but an increase in multipotent progenitor cells (MPPs). We also examined O2 levels in the FL and determined that FL hematopoietic progenitors experience physiologic hypoxia. ARNT-deficient fetal livers exhibited reduced numbers of LT-HSCs, indicating that HIF regulates FL hematopoiesis. Of note, adult Arnt KO hematopoietic progenitors displayed enhanced reconstitution of irradiated recipients in primary transplantation studies, but diminished reconstitution of secondary recipients, demonstrating a defect in long-term reconstitution. The defect in both fetal and adult HSC homeostasis was a result of diminished viability and not defects in cell-cycle regulation. This phenotype correlated with a reduction in glycolytic enzyme expression and expression of the prosurvival factors vascular endothelial growth factor A (VEGF-A) and B-cell lymphoma 2 (BCL-2), suggesting that HIF activity promotes HSC survival through multiple mechanisms.
Methods
Mice
Arntfl/fl23 or Hif-1αfl/fl24 and Hif-2αfl/fl19 mice were crossed to Vav-cre25 mice for generation of Vav-cre;Arntfl/fl (KO mice) and Vav-cre;Hif-1αfl/fl;Hif-2αfl/fl (double knockout [DKO] mice); all strains were on a C57BL/6 background. For all experiments, age- and sex-matched 2- to 3-month-old KO mice were compared with littermate control animals with the Vav-cre;Arntfl/wt genotype, whereas DKO mice were compared with littermate control animals with the Vav-cre;Hif-1αfl/wt;Hif-2αfl/wt genotype. Polymerase chain reaction (PCR) genotyping was performed according to previously published protocols.23 Procedures were performed in accordance with the guidelines set forth by the University of Pennsylvania.
Statistical analysis
All data are presented as the mean ± standard error (SE). Statistical significance was determined using the 2-tailed Student t test, with the exception of 5-FU assay, analyzed with the log-rank test.
Results
ARNT loss induces mild changes in hematologic differentiation
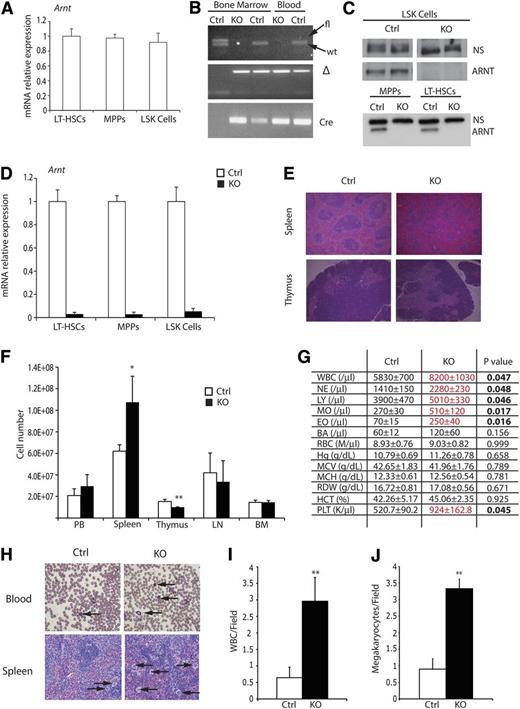
To define the combined roles of HIF-1α and HIF-2α in HSC biology, we conditionally deleted Arnt in HSCs by generating Vav-cre;Arntfl/fl mice (supplemental Figure 1A). VAV-Cre is expressed specifically in FL HSCs from embryonic day 10.5 through adult BM HSCs. Vav-cre;ArntΔ /Δ animals, hereafter referred to as “KO mice,” were born at the expected Mendelian ratios and were otherwise healthy and fertile. Relative Arnt mRNA levels were compared between HSC subsets (including LT-HSCs, multipotent progenitors, and LSK cells) and no significant differences were detected (Figure 1A). The degree of floxed Arnt allele recombination was assessed by PCR analysis of DNA generated from peripheral blood, showing efficient excision in KO mice (Figure 1B). To confirm that recombination occurred in the HSC compartment, fluorescence-activated cell sorting (FACS)-sorted HSCs were subjected to Western blot analysis, revealing loss of ARNT protein in all progenitor populations (Figure 1C). Finally, quantitative PCR (qPCR) analysis was performed and demonstrated efficient Arnt mRNA reduction in all progenitor populations (Figure 1D).
Characterization of Arnt KO phenotype. (A) Quantitative real-time PCR (qRT-PCR) analysis of ARNT expression in murine HSC subsets. (B) PCR analysis of DNA extracted from bone marrow and peripheral blood, from Arnt KO and heterozygous control (Ctrl) animals, demonstrating effective recombination of Arnt in the hematopoietic lineages. (C) Western blot for ARNT performed on FACS-sorted LSK, MPP, and LT-HSC cells shows loss of ARNT protein in all hematopoietic progenitors; a nonspecific band (NS) illustrates equivalent loading of protein. (D) qRT-PCR analysis of sorted HSCs shows effective deletion of Arnt in all progenitor populations. (E) Histologic analysis of spleen and thymus from 8- to 12-week-old KO mice. Note the change in germinal center architecture in KO spleens (original magnification ×50). (F) Absolute cell counts of hematopoietic tissues from control and KO mice. BM counts are from one femur and tibia (n = 6 ctrl, 6 KO). (G) Complete blood counts (CBC) on peripheral blood shows enhanced white blood cell (WBC) numbers in KO mice (n = 6 ctrl, 6 KO). (H) Representative blood smear illustrating WBC (arrows, upper panels) and spleen showing megakaryocytes (arrows, lower panels), which are quantified from at least 6 independent fields per slide in I and J, respectively (n = 7 ctrl, 6 KO; original magnification ×200). PB, peripheral blood; LN, lymph node; BM, bone marrow. *P < .05, **P < .01.
Characterization of Arnt KO phenotype. (A) Quantitative real-time PCR (qRT-PCR) analysis of ARNT expression in murine HSC subsets. (B) PCR analysis of DNA extracted from bone marrow and peripheral blood, from Arnt KO and heterozygous control (Ctrl) animals, demonstrating effective recombination of Arnt in the hematopoietic lineages. (C) Western blot for ARNT performed on FACS-sorted LSK, MPP, and LT-HSC cells shows loss of ARNT protein in all hematopoietic progenitors; a nonspecific band (NS) illustrates equivalent loading of protein. (D) qRT-PCR analysis of sorted HSCs shows effective deletion of Arnt in all progenitor populations. (E) Histologic analysis of spleen and thymus from 8- to 12-week-old KO mice. Note the change in germinal center architecture in KO spleens (original magnification ×50). (F) Absolute cell counts of hematopoietic tissues from control and KO mice. BM counts are from one femur and tibia (n = 6 ctrl, 6 KO). (G) Complete blood counts (CBC) on peripheral blood shows enhanced white blood cell (WBC) numbers in KO mice (n = 6 ctrl, 6 KO). (H) Representative blood smear illustrating WBC (arrows, upper panels) and spleen showing megakaryocytes (arrows, lower panels), which are quantified from at least 6 independent fields per slide in I and J, respectively (n = 7 ctrl, 6 KO; original magnification ×200). PB, peripheral blood; LN, lymph node; BM, bone marrow. *P < .05, **P < .01.
Because HIF is known to regulate both cellular differentiation and survival, the major hematologic organs of control and KO mice were surveyed, demonstrating that KO spleens were slightly larger than littermate controls, whereas their thymi were smaller (supplemental Figure 1B). These findings were confirmed by performing cell counts on all hematologic organs (Figure 1F). Flow cytometric analysis revealed increased numbers of B cells in the BM and spleen, but diminished T cell numbers in the spleen and thymus (supplemental Figure 1C-F). Histologic examination of the spleen demonstrated that KO organs typically displayed a gross loss of germinal center architecture (Figure 1E) and increased numbers of megakaryocytes (Figure 1H,J). These phenotypes are consistent with extramedullary hematopoiesis and suggested a degree of hematopoietic dysfunction in KO animals. To examine this further, we performed complete counts of control and KO peripheral blood; KO samples displayed increased WBC numbers (Figure 1G). WBC counts were also determined by quantifying Wright-Giemsa–stained blood smears (Figure 1H-I), confirming that KO animals exhibit increased numbers of leukocytes. Collectively, these results suggest that ARNT-deficient mice harbor modest defects in hematologic differentiation, specifically in the balance of B- and T-cell lineages and production of megakaryocytes. Interestingly, deficits in red blood cell numbers were not observed in KO mice, confirming that HIF regulates erythropoiesis in a strictly non–cell autonomous manner. This observation is consistent with a previously described cell extrinsic role for HIF-2α in hematopoiesis.26
Arnt KO mice have fewer LT-HSCs
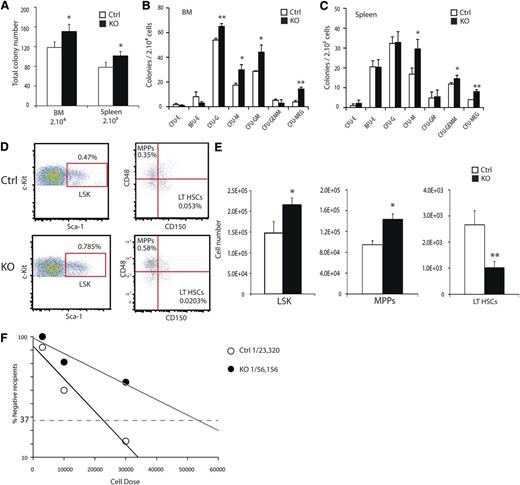
To examine the cell autonomous role for HIF activity during hematopoiesis, we assessed progenitor populations of the BM and spleen by performing methylcellulose colony-forming assays. Total colony-forming activity of both the BM and spleen was increased in KO animals relative to controls (Figure 2A). The distribution of increased colony-forming activity was mainly a result of granulocyte and macrophage progenitors and megakaryocytes (Figure 2B-C). Hematopoietic progenitor populations were quantified using the SLAM markers and flow cytometry.27 In whole bone marrow (WBM) preparations from 8- to 12-week-old KO mice, increased numbers of lineage–, Sca-1+, c-Kit+ (LSK) hematopoietic progenitors relative to littermate controls were detected (Figure 2D-E). Within the LSK population, SLAM markers resolve the lineage-restricted multipotent progenitors (MPPs) from short-term (ST-HSCs) and long-term repopulating HSCs (LT-HSCs). KO mice exhibited both an increase in absolute numbers of MPPs, but a significant decrease in LT-HSCs (Figure 2E). To demonstrate the diminished numbers of functionally defined LT-HSCs in KO mice, we performed limiting dilution reconstitution experiments, and the absolute frequency of LT-HSCs in KO mice was reduced from 1 in 23 320 to 1 in 56 156 BM cells (Figure 2F). We concluded that HIF activity is required for either formation or maintenance of appropriate LT-HSC numbers.
ARNT deletion results in reduced LT-HSC numbers. (A) Methylcellulose colony-forming assays were performed on single-cell preparations from BM and spleen, showing increased frequency of colony-forming cells in KO BM and spleen. Colony counts for specific cell types for BM (B) and spleen (C) (n = 4 ctrl, 4 KO). (D) Representative FACS plots for LSK-SLAM to resolve hematopoietic progenitor cell populations show relative changes in hematopoietic progenitor cell populations of KO mice. Absolute numbers of hematopoietic progenitors are altered in KO mice and quantified in (E) (n = 6 ctrl, 6 KO). Numbers are based on populations present in 1 femur and 1 tibia. (F) Limiting-dilution analysis demonstrates decreased frequency of HSCs in KO mice, with absolute frequencies of 1in 23 320 and 1 in 56 156 for the control and KO mice, respectively, P < .05. (Donor n = 6 ctrl, n = 6 KO. Recipient n = 33 ctrl, n = 32 KO). *P < .05, **P < .01.
ARNT deletion results in reduced LT-HSC numbers. (A) Methylcellulose colony-forming assays were performed on single-cell preparations from BM and spleen, showing increased frequency of colony-forming cells in KO BM and spleen. Colony counts for specific cell types for BM (B) and spleen (C) (n = 4 ctrl, 4 KO). (D) Representative FACS plots for LSK-SLAM to resolve hematopoietic progenitor cell populations show relative changes in hematopoietic progenitor cell populations of KO mice. Absolute numbers of hematopoietic progenitors are altered in KO mice and quantified in (E) (n = 6 ctrl, 6 KO). Numbers are based on populations present in 1 femur and 1 tibia. (F) Limiting-dilution analysis demonstrates decreased frequency of HSCs in KO mice, with absolute frequencies of 1in 23 320 and 1 in 56 156 for the control and KO mice, respectively, P < .05. (Donor n = 6 ctrl, n = 6 KO. Recipient n = 33 ctrl, n = 32 KO). *P < .05, **P < .01.
Functional characterization of Arnt-deficient hematopoietic progenitors
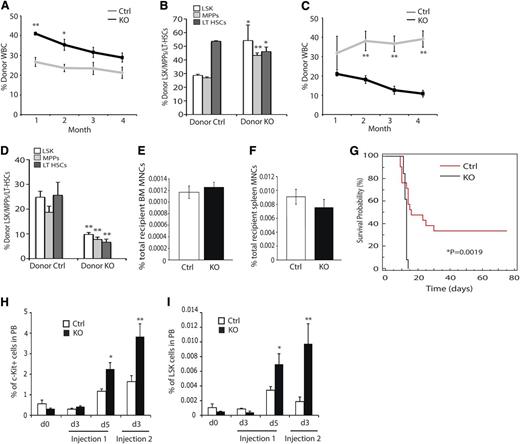
The functionality of ARNT-deficient HSCs was tested by performing bone marrow transplantation (BMT) studies. BM from KO animals displayed enhanced reconstitution of peripheral blood WBCs from the initial examination through 4 months’ post-reconstitution (Figure 3A and supplemental Figure 2A), although reconstitution differences dropped below statistical significance after the second month. Transplanted KO cells also exhibited altered differentiation profiles similar to what was observed in KO mice, because KO cells contributed to the macrophage, granulocyte, and B-cell lineages at higher rates than controls (supplemental Figure 2B). In contrast, contribution to the T-cell compartment occurred at lower rates than controls (supplemental Figure 2B). After 4 months, the relative contribution of donor cells to the HSC compartment was assessed by staining BM preparations for LSK-SLAM, CD45.1 and CD45.2, and flow cytometry. KO animals displayed an increased contribution to the LSK and MPP populations, but a diminished contribution to the LT-HSC population (Figure 3B). Whole BM from the primary recipients was transplanted into irradiated secondary recipient mice; upon secondary transplantation, KO cells exhibited markedly diminished reconstitution capacity compared with controls (Figure 3C and supplemental Figure 2C). Examination of the HSC compartment at 4 months’ postsecondary reconstitution revealed diminished reconstitution of all HSC populations (Figure 3D). This phenotype was observed across all major lineages examined (supplemental Figure 2C) and demonstrates that Arnt KO HSCs have reduced long-term reconstitution capacity. Because HIF regulates expression of Cxcr4, an essential receptor for HSC homing, we evaluated the homing capacity of ARNT-deficient progenitor cells.28,29 Importantly, no defect in KO cell homing to either the BM or spleen (Figure 3E-F) was observed, suggesting that this does not contribute to the phenotypes detected in our BMT studies.
Impact of ARNT deletion on HSC function. (A) Competitive reconstitution analysis demonstrates enhanced reconstitution of irradiated recipients by KO BM (n = 4 ctrl donors, n = 33 recipients, n = 4 KO donors, n = 33 recipients). (B) Four months after reconstitution, we evaluated reconstitution of hematopoietic progenitors in recipient animals. (C) Secondary reconstitution reveals defects in long-term reconstitution in KO HSCs (n = 19 ctrl recipients, n = 15 KO recipients). (D) Four months after secondary transplantation, we assessed donor contribution to the hematopoietic progenitors and found diminished reconstitution of all progenitors. (E-F) Homing to the bone marrow (E) and spleen (F) is unaffected by loss of ARNT (n = 5 ctrl, n = 5 KO). (G) 5-FU myelosuppression assay shows diminished survival of KO animals (black) relative to control animals (red) (n = 13 ctrl, n = 13 KO). (H-I) KO animals exhibited enhanced mobilization of both c-Kit+ progenitors (H) and LSK cells (I) in the peripheral blood after 5-FU treatment (n = 10 ctrl, n = 10 KO). *P < .05, **P < .01.
Impact of ARNT deletion on HSC function. (A) Competitive reconstitution analysis demonstrates enhanced reconstitution of irradiated recipients by KO BM (n = 4 ctrl donors, n = 33 recipients, n = 4 KO donors, n = 33 recipients). (B) Four months after reconstitution, we evaluated reconstitution of hematopoietic progenitors in recipient animals. (C) Secondary reconstitution reveals defects in long-term reconstitution in KO HSCs (n = 19 ctrl recipients, n = 15 KO recipients). (D) Four months after secondary transplantation, we assessed donor contribution to the hematopoietic progenitors and found diminished reconstitution of all progenitors. (E-F) Homing to the bone marrow (E) and spleen (F) is unaffected by loss of ARNT (n = 5 ctrl, n = 5 KO). (G) 5-FU myelosuppression assay shows diminished survival of KO animals (black) relative to control animals (red) (n = 13 ctrl, n = 13 KO). (H-I) KO animals exhibited enhanced mobilization of both c-Kit+ progenitors (H) and LSK cells (I) in the peripheral blood after 5-FU treatment (n = 10 ctrl, n = 10 KO). *P < .05, **P < .01.
BMT is a test of HSC functional stem cell properties but also subjects them to stress conditions, because they must rapidly proliferate to reconstitute the recipient BM. The myelosuppressant 5-FU induces a similar stress in HSCs and can be used to evaluate HSC exhaustion. KO and control mice were subjected to 5-FU treatment, and KO mice displayed diminished survival compared with control animals (Figure 3G), suggesting that KO HSCs became exhausted earlier than controls. Consistent with this, we monitored the peripheral blood of these animals and found increased mobilization of c-Kit+ and LSK progenitor cells in the periphery, a phenotype consistent with HSC hyperproliferation (Figure 3H-I).
KO LT-HSCs exhibit diminished viability
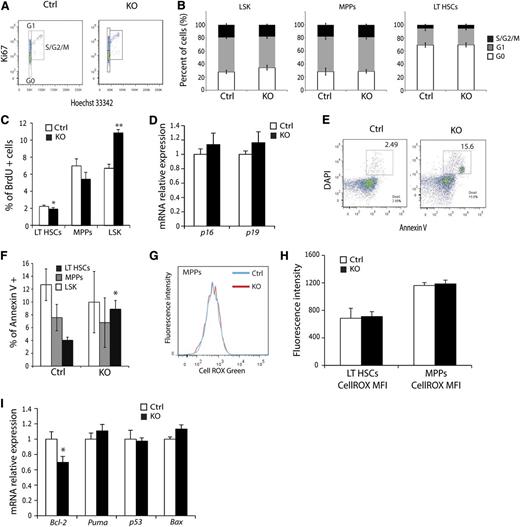
The observation that KO mice exhibit fewer HSCs suggests that HSC homeostasis is perturbed by loss of ARNT activity. To evaluate this mechanistically, cell cycle status of KO HSCs was examined using Ki67 to resolve the G0 from G1 phase of the cell cycle. HSCs analyzed by flow cytometry demonstrated no significant difference in the cell cycle profile of KO LSK, MPPs, or LT-HSCs relative to controls (Figure 4A-B). However, cell cycle analysis via BrdU incorporation revealed enhanced proliferation of LSK cells, with a subtle but significant decrease in LT-HSC proliferation (Figure 4C). A previous study implicated HIF-mediated induction of p16 and p19 in the stress response,30 suggesting that this mechanism may underlie the observed homeostatic defect in LT-HSC numbers. However, quantitative real-time PCR (qRT-PCR) studies of sorted LT-HSCs detected no difference in their expression (Figure 4D). When HSC viability was assessed, KO mice displayed significantly more apoptotic LT-HSCs than control mice (Figure 4E-F). Interestingly, this phenotype was specific to LT-HSCs, because MPPs showed no changes in Annexin V positivity. To test whether increased apoptosis was caused by inadequate vascularization of the hematologic organs, we stained BM, spleen, and thymus sections for the endothelial cell marker CD31 (supplemental Figure 3A). KO mice exhibited a normal number and distribution of blood vessels in all 3 organs (supplemental Figure 3B-C), indicating that enhanced HSC apoptosis was not caused by diminished vasculature. Loss of HIF activity has been previously associated with increased levels of reactive oxygen species (ROS) that trigger apoptosis through activation of the unfolded protein response pathway.31 However, examination of ROS demonstrated no difference in any HSC subtype between control and KO cells (Figure 4G-H). Subsequent qRT-PCR evaluation of known regulators of apoptosis, such as BCL-2, Puma, p53, and Bax, uncovered a significant and specific reduction in Bcl-2 expression in Arnt KO LT-HSCs (Figure 4I), indicating that these cells are more prone to cell death as a result of decreased abundance of this anti-apoptotic factor, rather than from changes in redox homeostasis, per se.
ARNT KO LT-HSCs exhibit diminished viability. (A) Representative cell-cycle FACS plot of LT-HSCs from control and KO animals. (B) No significant differences in cell cycle were observed in any hematopoietic progenitors from KO animals (n = 6 ctrl, n = 6 KO). (C) Quantification of BrdU proliferation assay in HSC subsets. (D) qRT-PCR analysis of p16 and p19 in FACS-sorted LT-HSCs. (E) Representative Annexin V FACS plot of LT-HSC shows enhanced apoptosis in KO LT-HSCs. (F) LT-HSCs, but not MPPs or LSK cells, display enhanced apoptosis (n = 6 ctrl, n = 6 KO). (G) Histogram of representative ROS analysis and (H) quantification of mean fluorescence intensity for LT-HSCs and MPPs. No differences were observed. (I) qRT-PCR analysis of FACS-sorted LT-HSCs for cell survival factors. *P < .05, **P < .01.
ARNT KO LT-HSCs exhibit diminished viability. (A) Representative cell-cycle FACS plot of LT-HSCs from control and KO animals. (B) No significant differences in cell cycle were observed in any hematopoietic progenitors from KO animals (n = 6 ctrl, n = 6 KO). (C) Quantification of BrdU proliferation assay in HSC subsets. (D) qRT-PCR analysis of p16 and p19 in FACS-sorted LT-HSCs. (E) Representative Annexin V FACS plot of LT-HSC shows enhanced apoptosis in KO LT-HSCs. (F) LT-HSCs, but not MPPs or LSK cells, display enhanced apoptosis (n = 6 ctrl, n = 6 KO). (G) Histogram of representative ROS analysis and (H) quantification of mean fluorescence intensity for LT-HSCs and MPPs. No differences were observed. (I) qRT-PCR analysis of FACS-sorted LT-HSCs for cell survival factors. *P < .05, **P < .01.
Hypoxia is a component of the FL HSC microenvironment
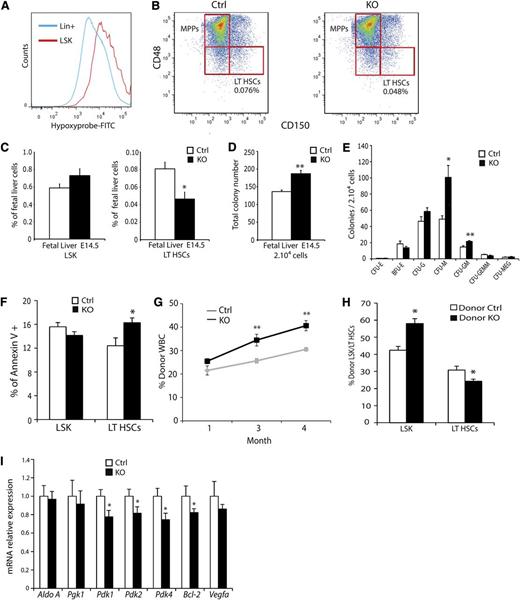
Although a role for hypoxia in early embryonic and adult hematopoiesis is well established,7,26,30 it is currently unclear whether hypoxia is a component of the FL HSC microenvironment and whether HIFs are functionally important at this stage. To assess the O2 status of the FL, E14.5 pregnant mice were injected with the compound Hypoxyprobe, which forms stable adducts with proteins at O2 concentrations of 1% or less, detectable by immunoreactivity.32 FL cell preparations analyzed by flow cytometry indicated that LSK hematopoietic progenitors exhibited increased Hypoxyprobe reactivity compared with differentiated lineage+ cells (Figure 5A). These results suggest that FL HSCs, just as adult BM HSCs, experience physiologic hypoxia. Because VAV-Cre is expressed in FL hematopoietic progenitors, we assessed their relative abundance using SLAM markers (Figure 5B).25,33 At E14.5, KO mice displayed a trend toward more LSK cells but had statistically significantly fewer LT-HSCs (Figure 5C). Methylcellulose colony-forming assays of E14.5 FLs indicated that KO FLs produced increased colony numbers compared with littermate controls (Figure 5D), primarily because of increased numbers of myeloid progenitors (Figure 5E). Similar results were observed in E18.5 FLs (supplemental Figure 4A-B and data not shown). To examine the deficit in fetal LT-HSCs further, we quantified cell viability and found enhanced apoptosis of LT-HSCs, but not LSK cells (Figure 5F). Transplantation studies with FL cells revealed both enhanced repopulation of peripheral blood cells (Figure 5G) and reduced numbers of LT-HSCs at 4 months posttransplant (Figure 5H) for KO cells, similar to results obtained for adult BMT experiments. Finally, we analyzed the expression of multiple HIF target genes, including members of the family of pyruvate dehydrogenase kinases (PDKs) by qRT-PCR in sorted control and KO LT-HSCs. Of note, Pdk1, Pdk2, and Pdk4 expression was significantly reduced in E14.5 KO LT-HSCs (Figure 5I). Furthermore, Bcl-2 expression was also decreased as noted for adult LT-HSCs, suggesting that the HIF-BCL-2 axis also promotes fetal LT-HSC survival. We conclude that the FL HSC microenvironment is O2-limited and that HIF is also an important regulator of this stage of hematopoiesis.
Fetal liver hematopoietic progenitors experience hypoxia. (A) Hypoxyprobe staining of E14.5 FL preparations. LSK cells (red) display enhanced staining relative to Lin+ differentiated cells (blue). (B) Representative LSK-SLAM FACS plots from E14.5 FLs. (C) Quantifications showing that KO animals have fewer LT-HSCs compared with controls and a trend toward more LSK cells (n = 9 ctrl, n = 4 KO). (D) Methylcellulose colony-forming assays on E14.5 FL cells show enhanced colony-forming activity in KO mice. (E) Distribution of colony subtypes from colony-forming assay (n = 9 ctrl, n = 4 KO). (F) Annexin V staining of control and Arnt KO E14.5 FL preparations. (G-H) Enhanced reconstitution of irradiated recipient peripheral blood by KO FL progenitors (G) but diminished contribution to the LT-HSC compartment (H). (I) qRT-PCR analysis for metabolic and survival factors of FACS-sorted LT-HSCs from control and Arnt KO E14.5 FL. *P < .05, **P < .01.
Fetal liver hematopoietic progenitors experience hypoxia. (A) Hypoxyprobe staining of E14.5 FL preparations. LSK cells (red) display enhanced staining relative to Lin+ differentiated cells (blue). (B) Representative LSK-SLAM FACS plots from E14.5 FLs. (C) Quantifications showing that KO animals have fewer LT-HSCs compared with controls and a trend toward more LSK cells (n = 9 ctrl, n = 4 KO). (D) Methylcellulose colony-forming assays on E14.5 FL cells show enhanced colony-forming activity in KO mice. (E) Distribution of colony subtypes from colony-forming assay (n = 9 ctrl, n = 4 KO). (F) Annexin V staining of control and Arnt KO E14.5 FL preparations. (G-H) Enhanced reconstitution of irradiated recipient peripheral blood by KO FL progenitors (G) but diminished contribution to the LT-HSC compartment (H). (I) qRT-PCR analysis for metabolic and survival factors of FACS-sorted LT-HSCs from control and Arnt KO E14.5 FL. *P < .05, **P < .01.
ARNT deficiency induces small transcriptional changes of metabolic genes but does not result in a “metabolic crisis” in HSCs
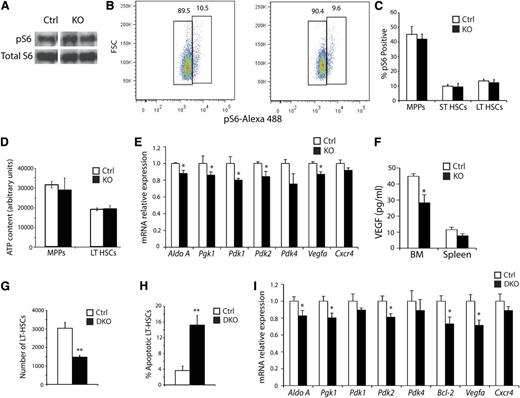
Because HIF-1α inhibits the mechanistic target of the rapamycin pathway (mTOR), which itself is a critical regulator of HSC biology,34,35 we performed Western blots on FACS-sorted LSK cells for phosphorylated ribosomal protein S6 (pS6), a readout of mTOR activity (Figure 6A).36 No changes in mTOR pathway activity were observed in KO cells, although signal from MPPs could mask changes in the LT-HSCs. To clarify this, BM preparations were stained for LSK-SLAM and pS6, and no changes in the numbers of pS6-positive LT-HSCs were detected (Figure 6B-C). Several studies have demonstrated that glycolysis is the primary means of ATP generation in HSCs,10,37 suggesting that diminished energy availability may result in apoptosis. To test this, ATP levels were measured in sorted LT-HSCs and MPPs. However, no significant changes in ATP levels were noted (Figure 6D), indicating that defective cellular energetics do not cause apoptosis in KO LT-HSCs. Because the regulation of glycolysis by HIF-1α, PDK2, and PDK4 activity affects HSC quiescence through ROS,30 we evaluated the expression of multiple glycolytic genes and PDKs in KO HSCs. A small but statistically significant decrease in the expression of aldolase A, pyruvate glycerol kinase 1 (Pgk1), Pdk1, Pdk2, and Pdk4 was observed in KO HSCs (Figure 6E). However, Hif-1α KO HSCs display no homeostatic phenotype,27,30 and no differences in ROS or ATP levels were observed in Arnt KO HSCs. Therefore, neither redox imbalance nor decreased bioenergetics can account for diminished LT-HSC survival. Interestingly, although Cxcr4 mRNA levels were not affected, expression of the prosurvival cytokine Vegfa was reduced in KO LT-HSCs based on qRT-PCR assays (Figure 6E), whereas whole BM displayed decreased VEGF-A protein by enzyme-linked immunosorbent assay (Figure 6F). These findings suggest that VEGF signaling may influence the KO hematopoietic phenotypes.
Analysis of the molecular consequence of ARNT deficiency; simultaneous deletion of Hif-1α and Hif-2α phenocopy of the Arnt KO phenotype. (A) Representative Western blot analysis for Phospho-S6 and total S6 as a loading control on FACS-sorted LSK cells (n = 3 ctrl, n = 3 KO). (B-C) Representative FACS plots of Phospho-S6 staining in control and KO LT-HSCs (B), quantified for all hematopoietic progenitors in (C), showing no difference in Phospho-S6 levels (n = 6 ctrl, n = 6 KO). (D) ATP assay performed on FACS-sorted MPPs and LT-HSCs (n = 6 ctrl, n = 6 KO). (E) qRT-PCR analysis of Arnt KO LT-HSCs (n = 5 ctrl, n = 6 KO). (F) VEGF enzyme-linked immunosorbent assay performed on BM and spleen extracts (n = 6 ctrl, n = 6 KO). (G) Vav-Cre;Hif-1αfl/fl/Hif-2αfl/fl DKO mice have fewer LT-HSCs than control mice (n = 3 ctrl, n = 3 DKO). (H) Annexin V staining revealed enhanced apoptosis in DKO LT-HSCs (n = 6 ctrl, n = 6 KO). (I) qRT-PCR analysis of sorted DKO LT-HSCs (n = 3 ctrl, n = 4 KO). *P < .05, **P < .01.
Analysis of the molecular consequence of ARNT deficiency; simultaneous deletion of Hif-1α and Hif-2α phenocopy of the Arnt KO phenotype. (A) Representative Western blot analysis for Phospho-S6 and total S6 as a loading control on FACS-sorted LSK cells (n = 3 ctrl, n = 3 KO). (B-C) Representative FACS plots of Phospho-S6 staining in control and KO LT-HSCs (B), quantified for all hematopoietic progenitors in (C), showing no difference in Phospho-S6 levels (n = 6 ctrl, n = 6 KO). (D) ATP assay performed on FACS-sorted MPPs and LT-HSCs (n = 6 ctrl, n = 6 KO). (E) qRT-PCR analysis of Arnt KO LT-HSCs (n = 5 ctrl, n = 6 KO). (F) VEGF enzyme-linked immunosorbent assay performed on BM and spleen extracts (n = 6 ctrl, n = 6 KO). (G) Vav-Cre;Hif-1αfl/fl/Hif-2αfl/fl DKO mice have fewer LT-HSCs than control mice (n = 3 ctrl, n = 3 DKO). (H) Annexin V staining revealed enhanced apoptosis in DKO LT-HSCs (n = 6 ctrl, n = 6 KO). (I) qRT-PCR analysis of sorted DKO LT-HSCs (n = 3 ctrl, n = 4 KO). *P < .05, **P < .01.
Loss of both HIF-1α and HIF-2α phenocopies the decrease in LT-HSC numbers observed in Arnt KO mice
Although ARNT is the obligate transcriptional cofactor for HIF-1α and HIF-2α, it is also required for aryl hydrocarbon receptor (AHR) activity, raising the possibility that some of the observed Arnt KO phenotypes are caused by defects in AHR function. Ahr mutant mice display some hematopoietic anomalies similar to those observed for Arnt KO animals, including splenomegaly and increased WBC counts.38 However, Ahr-deficient mice exhibited greater numbers of LT-HSCs, suggesting that the effects we observed in Arnt KO mice are caused by a loss of HIF activity instead. In addition, expression of several AHR target genes, such as Ugt1a1, Cyp1a1, or Cyp1b1, was unaffected in KO LT-HSCs (supplemental Figure 5A-C and data not shown). Nonetheless, to clarify the possible AHR role in Arnt KO mice hematopoietic deficiencies, Vav-cre;Hif1-α/Hif-2α DKO animals were generated (supplemental Figure 6A). qRT-PCR and Western blotting analyses revealed efficient conditional deletion of Hif1-α and Hif-2α (supplemental Figure 6B-D). Similar to the Arnt KO animals, DKO animals displayed both a relative and an absolute reduction in LT-HSC numbers (Figure 6G). DKO LT-HSCs also underwent apoptosis at elevated rates relative to littermate controls and at a similar level to KO animals (Figure 6H and supplemental Figure 6E). Furthermore, methylcellulose colony-forming assays revealed that DKO BM cells produced increased colony numbers compared with controls (supplemental Figure 6F), because of increased numbers of granulocyte and macrophage progenitors (supplemental Figure 6G), similar to the results obtained for Arnt KO mice. Transcriptional changes in DKO LT-HSCs were comparable with those found in Arnt KO LT-HSCs (Figure 6I), including for Bcl-2. Importantly, analysis of Hif-1α KO and Hif-2α KO mice found no defects in LT-HSC numbers (supplemental Figure 6H), suggesting functional redundancy between HIF-1α and HIF-2α in this context. These results demonstrate that the defect in HSC maintenance we observed in Arnt KO mice is primarily caused by a loss of HIF activity and not by AHR.
Discussion
The bone marrow and FL microenvironments provide essential factors for the maintenance and expansion of HSCs. However, reduced O2 availability in the HSC niche also imposes a stress upon HSCs, and although it is now generally accepted that hypoxia is a feature of the HSC microenvironment,9,10 the role of the HIF response in HSC homeostasis is incompletely understood. To this end, we conditionally deleted Arnt in HSCs and found that it is a critical regulator of HSC viability. We also demonstrate that HIF-mediated signaling may promote LT-HSC viability through the prosurvival factor BCL-2. BCL-2 has previously been shown to be induced by hypoxia39 as a direct transcriptional target of HIF.40,41 These results reveal a new role for HIF in HSC maintenance and suggest that HIF directly controls HSC survival.
The phenotype of KO and DKO HSCs contrasts with those described for Hif-1α–deficient HSCs, which lacked homeostatic or viability phenotypes but exhibited a loss of quiescence when subjected to stress.21 This could be explained by a compensatory upregulation of Hif-2α in Hif-1α KO HSCs, as previously reported.42 Furthermore, our results depict a functional redundancy between HIF-1α and HIF-2α in this system, and that viability is reduced only when a complete loss of HIF activity is achieved. We did not observe decreased HSC numbers in either Hif-1α or Hif-2α single-KO mice (supplemental Figure 6H), consistent with this hypothesis. Of note, our results conflict with a previous study43 that found no defect in homeostasis or reconstitution after ablation of both Hif-1α and Hif-2α. Further studies will be needed to evaluate this discrepancy.
Both cell-extrinsic and cell-intrinsic cytokines are important regulators of HSC survival. Specifically, cell-intrinsic VEGF-A expression is critical for HSC viability, because conditional deletion of Vegfa in HSCs results in dramatic HSC apoptosis and BM failure.44 Hemizygous conditional Vegfa KO mice also display a diminished life span, suggesting that HSCs are highly sensitive to Vegfa dosage.44 Because HIF-1α is an essential regulator of an autocrine loop of VEGF expression,45 it is possible that altered VEGF-A levels in KO mice result in elevated rates of HSC apoptosis. Mice with the hypoxia response element deleted from the Vegfa gene lack HIF-mediated Vegfa expression and display a number of hematologic phenotypes, including reduced numbers of functional reconstituting hematopoietic progenitors.46 Because HSC apoptosis was not evaluated in this model, it is unclear whether enhanced apoptosis underlies this phenotype. Nonetheless, this result is consistent with the findings of our study and provides further support for a role of the HIF-α–mediated VEGF signaling in HSC biology.
HIF-mediated stimulation of glycolysis is an important adaptive response to hypoxia that generates ATP when oxidative phosphorylation becomes ineffective. For example, loss of HIF-1α in hypoxic macrophages causes a significant decrease in cellular ATP levels.47 Because we found diminished expression of glycolytic genes in KO mice, this suggests that an inability to maintain adequate cellular ATP may contribute to HSC apoptosis noted in KO mice. However, we did not find changes in ATP or ROS levels of KO HSCs. This may be caused by compensatory upregulation of autophagy48 or, alternatively, the levels of glycolytic enzymes in KO mice may be sufficient to maintain cellular ATP.
We have shown that FL hematopoietic progenitors experience physiologic hypoxia and that HIF activity plays an important role in this stage of hematopoietic development. A recent study also observed hypoxia in the FL,15 and ours is the first description of a role for HIF signaling in the survival of FL HSCs. Given the previously described role for hypoxia in yolk sac,16 AGM,18 and BM HSCs,21 we propose that hypoxia- and O2-sensitive factors represent universal regulators of hematopoietic progenitor cells. The presence of hypoxia in the FL was particularly surprising considering its highly vascular nature. However, many factors contribute to the oxygenation status of a cell and tissue, including localized blood flow and O2 uptake by neighboring cells, which may create steep, localized O2 gradients within an otherwise vascular tissue.
Because ARNT has at least 2 other binding partners, AHR and aryl hydrocarbon receptor repressor (aka, AHRR), some of the phenotypes described in KO mice may not be exclusively caused by diminished HIF-α activity. The primary findings of our study (ie, reduced numbers and enhanced apoptosis of adult KO LT-HSCs) are recapitulated by the simultaneous deletion of both Hif-1α and Hif-2α, suggesting that these phenotypes are HIF-dependent. Nonetheless, other ARNT binding partners may contribute to the phenotypes observed, either alone or in combination with HIF. Moreover, the VAV-dependent conditional mouse model results in deletion around gestational day 10.5, raising the possibility that cell-autonomous and/or cell–non-autonomous compensatory changes may have altered the adult phenotypes detected here. In conclusion, our data demonstrate that ARNT is a critical factor for adult and FL HSC survival and maintenance, potentially through the HIF-α–mediated regulation of prosurvival factors such as BCL-2 and VEGF-A.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the University of Pennsylvania Flow Cytometry Facility and Hongwei Yu for histology.
Authorship
Contribution: B.L.K. and N.S. designed and performed the research, analyzed data, and wrote the manuscript; T.S.E.-M., D.N.G., J.E.S., M.G., D.S.L., M.S.N., and J.S. performed research and analyzed data; and M.C.S. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicolas Skuli, INSERM U1037, Institut Claudius Regaud, 20-24 Rue du Pont St Pierre, 31052 Toulouse, France; e-mail: nicolas.skuli@inserm.fr; M. Celeste Simon, Abramson Family Cancer Research Institute, University of Pennsylvania Perelman School of Medicine, 456 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104-6160; e-mail: celeste2@mail.med.upenn.edu.
References
Author notes
N.S. and M.C.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal