Key Points
Small changes in MCL-1 levels have severe consequences in the context of hematopoietic recovery from stress.
Modulation of MCL-1 and PUMA is likely to reduce chemotherapy-associated toxicity and enhance blood stem cell transplantation in the clinic.
Abstract
Understanding the critical factors that govern recovery of the hematopoietic system from stress, such as during anticancer therapy and bone marrow transplantation, is of clinical significance. We investigated the importance of the prosurvival proteins myeloid cell leukemia-1 (MCL-1) and B-cell lymphoma–extra large (BCL-XL) in stem/progenitor cell survival and fitness during hematopoietic recovery from stress. Loss of a single Mcl-1 allele, which reduced MCL-1 protein levels, severely compromised hematopoietic recovery from myeloablative challenge and following bone marrow transplantation, whereas BCL-XL was dispensable in both contexts. We identified inhibition of proapoptotic p53 upregulated modulator of apoptosis (PUMA) as the key role of MCL-1 in both settings, with Mcl-1+/−;Puma−/− mice completely protected from the deleterious effects of loss of 1 Mcl-1 allele. These results reveal the molecular mechanisms that govern cell survival during hematopoietic recovery from stress.
Introduction
Cancer therapy, traumatic blood loss, and acute infection can all result in the depletion of mature blood cells, leading to immunodeficiency, anemia, and other life-threatening complications. The hematopoietic stem and progenitor cell compartment responds rapidly to such stress by increasing blood cell production through a process known as “emergency hematopoiesis.” Once the mature blood cell pools have been replenished, hematopoiesis returns to homeostasis.1,2
Apoptosis is a form of programmed cell death that plays a prominent role in the hematopoietic system. Insufficient apoptosis causes an increase in hematopoietic cells, which can be a forerunner of leukemia or lymphoma, whereas excessive apoptosis causes immunodeficiency, anemia, and thrombocytopenia.3 The B-cell lymphoma 2 (BCL-2) protein family members are critical regulators of apoptosis. The prosurvival BCL-2–like members (eg, BCL-2, B-cell lymphoma–extra large [BCL-XL], myeloid cell leukemia-1 [MCL-1]) are required for cell survival. The multi-Bcl-2 homology (BH) domain proapoptotic members BCL-2–associated X-protein (BAX) and BCL-2 homologous antagonist/killer (BAK) unleash the demolition phase of apoptosis, and the proapoptotic BH3-only proteins (eg, BCL-2 interacting mediator of cell death [BIM], p53 upregulated modulator of apoptosis [PUMA]) are critical for initiation of apoptosis signaling.4,5 Apoptosis is initiated when BH3-only proteins are transcriptionally or posttranscriptionally upregulated to activate BAX/BAK, either through direct interaction or indirectly by unleashing them from their restraint by the prosurvival BCL-2–like proteins.4,5 Members of the BCL-2 family regulate apoptosis in a cell type– and apoptotic stimulus–specific manner. For example, PUMA is required for DNA damage–induced apoptosis,6-8 whereas BIM is critical for apoptosis following cytokine withdrawal.9 Prosurvival BCL-XL is essential for survival of erythroid progenitors10 whereas MCL-1 maintains numerous cell types, including many hematopoietic cell subsets.11-15
Little is known about the roles of the different BCL-2 family members in the control of the survival of stem/progenitor cells during emergency hematopoiesis, most notably whether changes in the level of these proteins may influence chemotherapy-associated toxicity or the likelihood of successful bone marrow transplantation. These are important issues because inhibitors of prosurvival BCL-2 family members, the BH3 mimetics navitoclax/ABT-263 and ABT-199, are showing promise in clinical trials of certain lymphomas and leukemias5 and these drugs may in future be used in combination with DNA damage–inducing chemotherapeutics. There are currently no BH3 mimetic drugs available that inhibit MCL-1. Hence, we examined the impact of lower levels of MCL-1 protein (loss of a single allele of Mcl-1) on hematopoietic recovery and progenitor cell survival following treatment with 5-fluorouracil (5-FU) or γ-irradiation and during bone marrow transplantation.
First, our results show that stem cells with low MCL-1 levels are severely impaired in their ability to reconstitute the hematopoietic system following transplantation. Second, MCL-1 deficiency impaired hematopoietic recovery after exposure to DNA damage–inducing cancer therapy. Third, we identify inhibition of PUMA as the key function of MCL-1 during recovery of the hematopoietic system from stress. These discoveries are important for the development of combination cancer therapies involving inhibitors of MCL-1 and DNA damage–inducing chemotherapeutics.
Materials and methods
Generation of bone marrow chimeras
Bone marrow cells were harvested from both femora of mice (all C57BL/6-Ly5.2 background) at the age of 10 to 12 weeks, and single-cell suspensions were prepared. Bone marrow cells from such test mice of the genotypes of interest and from competitor, wild-type (C57BL/6-Ly5.1), or green fluorescent protein (GFP) transgenic mice, were mixed 1-to-1 in mouse tonicity adjusted saline (phosphate-buffered saline). From such cell mixtures, a total of 6 × 106 cells per mouse were injected IV into 3 lethally irradiated female congenic C57BL/6-Ly5.1 recipient mice (2 × 5.5 Gy, 3 hours between doses) 2 hours after the second dose of γ-irradiation. Eight weeks after transplantation, retro-orbital bleeds were taken for hematologic and flow cytometric analyses to confirm successful hematopoietic reconstitution. Lin−SCA-1+c-KIT+ (LSK) bone marrow homing experiments were performed by injection of 1 × 107 bone marrow cells (1-to-1 mix of wild-type [GFP+] and Mcl-1+/− cells) labeled with Cell Trace Violet (Life Technologies) into lethally irradiated C57BL/6-Ly5.1 recipient mice. The proportions of wild-type and Mcl-1+/− LSK cells were determined preinjection and 15 hours after transplantation, using cell tracking velocimetry labeling to discriminate transplanted cells from recipient cells.
Treatment with 5-FU or γ-irradiation
Mice (10-12 weeks old, male and female) were injected once intraperitoneally with either 150 mg/kg 5-FU or vehicle (phosphate-buffered saline), or were subjected to 8 Gy γ-irradiation. Mandible bleeds were taken for a hemogram before treatment commenced. Further mandible bleeds were taken on days 4, 7, 10, 14, and 21 to monitor recuperation of the hematopoietic system. Blood composition was analyzed using the ADVIA blood analyzer and flow cytometric analysis. For the purpose of analyzing leukocyte numbers, erythroid cells were removed using red blood cell removal buffer. The experiments were concluded on day 21 by sacrificing the animals and harvesting organ samples for histologic analysis. Mice that presented with signs of failure of the hematopoietic system, such as weight loss and anemia before day 21 (as judged by an experienced animal technician, blinded to the treatment and genotype of the mice), were sacrificed and organs taken for histologic analysis.
Flow cytometric analysis and cell sorting
Thymus, spleen, and bone marrow cells were harvested from reconstituted mice and single-cell suspensions prepared. Cells were counted using the CasyCell Counter (Schaefe System GmbH). Retro-orbital bleeds were taken for a hemogram (ADVIA). The numbers of LSK hematopoietic stem/progenitor cells from bone marrow samples of reconstituted mice were determined by flow cytometric (fluorescence-activated cell sorter [FACS]) analysis after staining for hematopoietic subset-specific surface markers (B220 [RA3-6B2], CD4 [YTA3.2.1], CD8 [YTS169], Gr-1 [RB6-8C5], Mac-1 [MI/70], Ter119 [TER-119]) plus antibodies to c-KIT (2B8; BioLegend) and Sca-1 (E13.161.7). Antibodies recognizing the congenic markers Ly5.1 (A201; competitor cells) and Ly5.2 (5.450.15.2; test cells) were used to determine the chimerism of the bone marrow–reconstituted recipient mice. Samples were analyzed in a LSR-II flow cytometer (BD Biosciences). Dead cells were identified by staining with propidium iodide (PI) (2 µg/mL; Sigma-Aldrich) and excluded from analysis. Erythroid cells were removed from retro-orbital bleeds using red blood cell removal buffer for flow cytometric analysis. Cells of each organ sample (1 × 106) were incubated as reported16 with fluorochrome-conjugated monoclonal antibodies against surface markers specific for T cells (CD4 [YTA3.2.1], CD8 [YTS169], and Thy-1 [T24.3.21]), granulocytes/myeloid cells (Gr-1 [RB6-8C5] and Mac-1 [MI/70]), B cells (immunoglobulin M [IgM; 5.1], IgD [11-26C], and B220 [RA3-6B2]), and the congenic markers Ly5.1 and Ly5.2 for 30 minutes at 4°C in 24G2 hybridoma supernatant (antibody against FcγR) plus 2% rat serum (both to block nonspecific antibody binding), washed twice with buffered saline supplemented with 5% fetal bovine serum. Samples were supplemented with PI (2 µg/mL) and cells analyzed in a FACSCalibur flow cytometer (BD Biosciences). Dead cells (PI+) were excluded from analysis.
MCL-1 intracellular staining was performed using the BD Cytofix/Cytoperm kit (BD Biosciences) with anti-MCL-1 (rat; 19C4-1517 ) directly conjugated to AlexaFluor 647 (Life Technologies). LSK cells were sorted in a BD Influx (BD Biosciences) using the antibody staining protocol described in the previous paragraph. The cells were cultured in medium supplemented with stem cell factor (100 ng/mL), interleukin-6 (10 ng/mL), thrombopoietin (50 ng/mL), and Flt3 (10 ng/mL) overnight and then γ-irradiated (4 Gy) or maintained in medium alone. Cell viability was monitored by flow cytometric analysis over 24 hours by staining with Annexin V and PI. Annexin V−PI− cells were deemed viable.
Statistical analysis
In all cases, sample and mouse numbers were chosen based on previous experience with mice deficient for various BCL-2 family members. All data points were included in final analysis, without exclusions. No randomization methods were performed for sample treatment allocations. All animal survival experiments were conducted in a blinded manner with “sick” mice identified by an experienced mouse technician blinded to the treatment (see Figures 1, 2, 4) or to the genotype, in the case of acute Mcl-1 deletion experiments (see Figure 5).
MCL-1 is essential for recovery of the hematopoietic system from 5-FU challenge. (A) Experimental strategy. (B) Mcl-1+/−, Bcl-x+/−, and wild-type (control) mice were treated with 1 dose of 5-FU and survival was monitored over a 21-day period (median survival, Mcl-1+/− mice: 10 days; log-rank [Mantle-Cox] test, P < .0001). (C) RBC numbers in Mcl-1+/−, Bcl-x+/−, and wild-type mice at 10 days or when sick (Mcl-1+/−) after treatment with 5-FU. (D) Histologic analysis of the bone marrow of the mice from panels A and B at the same time point (representative images; ×40 objective, 250 µm scale bar). (E) RBC levels in the blood of 5-FU–treated Mcl-1+/−, Bcl-x+/−, and wild-type mice on the days indicated. n = 15 (wild type), 5 (Bcl-x+/−), 10 (Mcl-1+/−) mice; pooled results from 9 independent experiments. Data represent mean ± SEM. * and ** denote P < .05 or < .01, respectively, for the indicated comparisons (Student t test).
MCL-1 is essential for recovery of the hematopoietic system from 5-FU challenge. (A) Experimental strategy. (B) Mcl-1+/−, Bcl-x+/−, and wild-type (control) mice were treated with 1 dose of 5-FU and survival was monitored over a 21-day period (median survival, Mcl-1+/− mice: 10 days; log-rank [Mantle-Cox] test, P < .0001). (C) RBC numbers in Mcl-1+/−, Bcl-x+/−, and wild-type mice at 10 days or when sick (Mcl-1+/−) after treatment with 5-FU. (D) Histologic analysis of the bone marrow of the mice from panels A and B at the same time point (representative images; ×40 objective, 250 µm scale bar). (E) RBC levels in the blood of 5-FU–treated Mcl-1+/−, Bcl-x+/−, and wild-type mice on the days indicated. n = 15 (wild type), 5 (Bcl-x+/−), 10 (Mcl-1+/−) mice; pooled results from 9 independent experiments. Data represent mean ± SEM. * and ** denote P < .05 or < .01, respectively, for the indicated comparisons (Student t test).
MCL-1 is essential for recovery of the hematopoietic system from sublethal γ-irradiation. (A) Mice (wild-type or Mcl-1+/−) were subjected to 8 Gy γ-irradiation and their survival monitored (median survival of Mcl-1+/− mice: 11 days; log-rank [Mantle-Cox] test, P < .0001); n = 14 (wild-type), 10 (Mcl-1+/−) mice. Pooled results from 2 independent experiments are shown. (B) Histologic analysis of the bone marrow of wild-type and Mcl-1+/− mice was performed 12 days after γ-irradiation. Representative hematoxylin and eosin (H&E)-stained bone marrow sections (×20 objective, 500 µm scale bar).
MCL-1 is essential for recovery of the hematopoietic system from sublethal γ-irradiation. (A) Mice (wild-type or Mcl-1+/−) were subjected to 8 Gy γ-irradiation and their survival monitored (median survival of Mcl-1+/− mice: 11 days; log-rank [Mantle-Cox] test, P < .0001); n = 14 (wild-type), 10 (Mcl-1+/−) mice. Pooled results from 2 independent experiments are shown. (B) Histologic analysis of the bone marrow of wild-type and Mcl-1+/− mice was performed 12 days after γ-irradiation. Representative hematoxylin and eosin (H&E)-stained bone marrow sections (×20 objective, 500 µm scale bar).
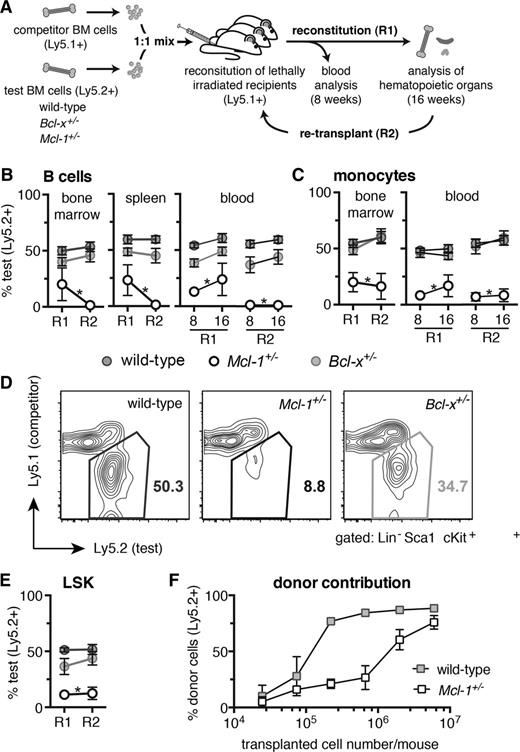
MCL-1 is essential for efficient hematopoietic reconstitution of lethally irradiated mice. (A) Competitive reconstitution strategy. Test (wild-type, Bcl-x+/−, or Mcl-1+/−, all C57BL/6-Ly5.2) and competitor (wild-type, C57BL/6-Ly5.1) bone marrow cells were mixed 1-to-1 and each injected into 3 lethally irradiated C57BL/6-Ly5.1 recipient mice (R1). At 16 weeks, the bone marrow cells from the 3 competitively reconstituted recipient mice were pooled and injected into a further 3 lethally irradiated C57BL/6-Ly5.1 recipient mice (R2). Analysis for R1 and R2 was performed after 8 and 16 weeks. (B-E) Flow cytometric analysis of the contribution of test bone marrow cells to repopulate the (B) lymphoid, (C) myeloid, and (D-E) hematopoietic stem/progenitor (LSK) cell compartments in transplant recipients. (F) Lethally irradiated C57BL/6-Ly5.1 recipient mice were reconstituted with titrated numbers of either Mcl-1+/− or wild-type (both C57BL/6-Ly5.2) bone marrow cells and the proportions of donor-derived leukocytes determined after 8 weeks by flow cytometric analysis. Staining with antibodies against B220 and Mac-1 was used to identify B cells and monocytes, respectively. (B-E) n = 15 (wild-type), 4 (Bcl-x+/−, Mcl-1+/−) biological samples (each circle represents the mean of 3 replicate mice). Pooled results from 7 independent experiments or (F) for each cell number: 2.5 × 104, 7.4 × 104, 2.2 × 105, 6.7 × 105, 2 × 106, 6 × 106; n = 2, 3, 2, 3, 6, 3, respectively (wild-type), and n = 3, 3, 4, 4, 8, 4, respectively (Mcl-1+/−). Data represent mean ± SEM. *P < .05 Student t test with the color indicating the genotype compared with wild type. Representative FACS plots are shown.
MCL-1 is essential for efficient hematopoietic reconstitution of lethally irradiated mice. (A) Competitive reconstitution strategy. Test (wild-type, Bcl-x+/−, or Mcl-1+/−, all C57BL/6-Ly5.2) and competitor (wild-type, C57BL/6-Ly5.1) bone marrow cells were mixed 1-to-1 and each injected into 3 lethally irradiated C57BL/6-Ly5.1 recipient mice (R1). At 16 weeks, the bone marrow cells from the 3 competitively reconstituted recipient mice were pooled and injected into a further 3 lethally irradiated C57BL/6-Ly5.1 recipient mice (R2). Analysis for R1 and R2 was performed after 8 and 16 weeks. (B-E) Flow cytometric analysis of the contribution of test bone marrow cells to repopulate the (B) lymphoid, (C) myeloid, and (D-E) hematopoietic stem/progenitor (LSK) cell compartments in transplant recipients. (F) Lethally irradiated C57BL/6-Ly5.1 recipient mice were reconstituted with titrated numbers of either Mcl-1+/− or wild-type (both C57BL/6-Ly5.2) bone marrow cells and the proportions of donor-derived leukocytes determined after 8 weeks by flow cytometric analysis. Staining with antibodies against B220 and Mac-1 was used to identify B cells and monocytes, respectively. (B-E) n = 15 (wild-type), 4 (Bcl-x+/−, Mcl-1+/−) biological samples (each circle represents the mean of 3 replicate mice). Pooled results from 7 independent experiments or (F) for each cell number: 2.5 × 104, 7.4 × 104, 2.2 × 105, 6.7 × 105, 2 × 106, 6 × 106; n = 2, 3, 2, 3, 6, 3, respectively (wild-type), and n = 3, 3, 4, 4, 8, 4, respectively (Mcl-1+/−). Data represent mean ± SEM. *P < .05 Student t test with the color indicating the genotype compared with wild type. Representative FACS plots are shown.
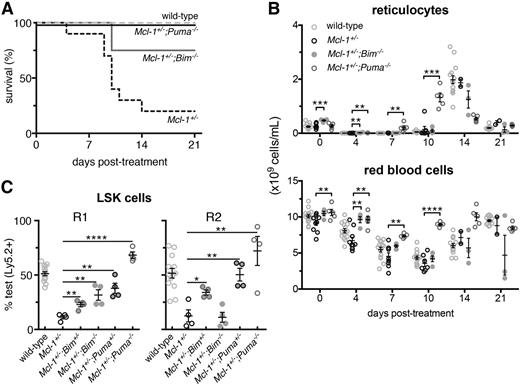
Complete rescue of the Mcl-1+/− hematopoietic repopulation defects by PUMA deficiency. (A) Survival of Mcl-1+/−;Puma−/−, Mcl-1+/−;Bim−/−, Mcl-1+/−, and wild-type mice after treatment with 1 dose of 5-FU; wild-type and Mcl-1+/− animal survival curves are reproduced here from Figure 1 to aid comparison. (B) Numbers of reticulocytes and RBCs in the blood of the mice described in panel A; values from mice reconstituted with wild-type bone marrow cells are reproduced here from Figure 1 to aid comparison. (C) Bone marrow (test) cells from wild-type, Mcl-1+/−, Mcl-1+/−;Bim+/−, Mcl-1+/−;Bim−/−, Mcl-1+/−;Puma+/−, and Mcl-1+/−;Puma−/− mice (all C57BL/6-Ly5.2) were mixed 1-to-1 with competitor C57BL/6-Ly5.1 bone marrow cells and injected into lethally irradiated C57BL/6-Ly5.1 recipients (R1). After 16 weeks, the percentages of test vs competitor-derived LSK stem/progenitor cells in the bone marrow of R1 reconstituted mice were determined by flow cytometric analysis. R2 was achieved as described in Figure 3A. After 16 weeks, percentages of test- vs competitor-derived LSK stem/progenitor cells in the bone marrow of R2 reconstituted mice were determined by flow cytometric analysis. Data from animals reconstituted with wild-type and Mcl-1+/− bone marrow cells are reproduced from Figure 3E to aid comparison with cells from the other genotypes. (A-B) n = 4 mice. Pooled results from 2 independent experiments are shown. (C) n = 4 biological samples (each circle represents the mean of 3 replicate mice). Pooled results from 9 independent experiments are shown. Data represent mean ± SEM. *, **, ***, **** denote significant differences where P < .05, < .01, < .001, < .0001 between the indicated groups (Student t test).
Complete rescue of the Mcl-1+/− hematopoietic repopulation defects by PUMA deficiency. (A) Survival of Mcl-1+/−;Puma−/−, Mcl-1+/−;Bim−/−, Mcl-1+/−, and wild-type mice after treatment with 1 dose of 5-FU; wild-type and Mcl-1+/− animal survival curves are reproduced here from Figure 1 to aid comparison. (B) Numbers of reticulocytes and RBCs in the blood of the mice described in panel A; values from mice reconstituted with wild-type bone marrow cells are reproduced here from Figure 1 to aid comparison. (C) Bone marrow (test) cells from wild-type, Mcl-1+/−, Mcl-1+/−;Bim+/−, Mcl-1+/−;Bim−/−, Mcl-1+/−;Puma+/−, and Mcl-1+/−;Puma−/− mice (all C57BL/6-Ly5.2) were mixed 1-to-1 with competitor C57BL/6-Ly5.1 bone marrow cells and injected into lethally irradiated C57BL/6-Ly5.1 recipients (R1). After 16 weeks, the percentages of test vs competitor-derived LSK stem/progenitor cells in the bone marrow of R1 reconstituted mice were determined by flow cytometric analysis. R2 was achieved as described in Figure 3A. After 16 weeks, percentages of test- vs competitor-derived LSK stem/progenitor cells in the bone marrow of R2 reconstituted mice were determined by flow cytometric analysis. Data from animals reconstituted with wild-type and Mcl-1+/− bone marrow cells are reproduced from Figure 3E to aid comparison with cells from the other genotypes. (A-B) n = 4 mice. Pooled results from 2 independent experiments are shown. (C) n = 4 biological samples (each circle represents the mean of 3 replicate mice). Pooled results from 9 independent experiments are shown. Data represent mean ± SEM. *, **, ***, **** denote significant differences where P < .05, < .01, < .001, < .0001 between the indicated groups (Student t test).
Loss of PUMA confers resistance to bone marrow failure elicited by acute loss of both alleles of Mcl-1. (A) Lethally irradiated C57BL/6-Ly5.1 mice were reconstituted with the hematopoietic system of the following genotypes: Mcl-1fl/fl;Puma−/−, Mcl-1fl/fl;Puma+/−, Mcl-1fl/fl;Bim−/−, Mcl-1fl/fl, Puma−/− (all RosaCreERKi/+), and wild-type (CreER only). Survival of these reconstituted animals was monitored after treatment with tamoxifen to activate the latent CreER recombinase to cause deletion of the floxed Mcl-1 alleles in the hematopoietic cells. (B-C) Lethally irradiated C57BL/6-Ly5.1 recipient mice were reconstituted with Mcl-1fl/fl;Puma−/−, Mcl-1fl/fl;Puma+/−, Mcl-1fl/fl;Bim−/−, Mcl-1fl/fl, Puma−/− (all RosaCreERKi/+), and wild-type (CreER only) test bone marrow cells mixed 1-to-1 with competitor wild-type (GFP+) bone marrow cells. (B) After 8 weeks, a baseline measurement of test vs competitor bone marrow–derived contribution to leukocytes in the blood was conducted by flow cytometric analysis; tamoxifen was administered to induce Mcl-1fl deletion. The contributions of the test- vs competitor-derived leukocytes in the blood were determined by flow cytometric analysis after 30 days. (C) After 30 days, the contributions of the test- vs competitor-derived LSK population was determined in the bone marrow of reconstituted mice by flow cytometric analysis. (A) Mice numbers (n) indicated in brackets. (B-C) Mice numbers are as follows (pre-, post-TAM): Mcl-1fl/fl;Puma−/− (14,14), Mcl-1fl/fl;Puma+/− (12,9), Mcl-1fl/fl;Bim−/− (6,6), Mcl-1fl/fl (12,9), Puma−/− (18,18), CreER only (6,3). Data represent mean ± SEM. **, ***, **** denote significant differences where P < .01, < .001, < .0001 between the indicated groups (Student t test).
Loss of PUMA confers resistance to bone marrow failure elicited by acute loss of both alleles of Mcl-1. (A) Lethally irradiated C57BL/6-Ly5.1 mice were reconstituted with the hematopoietic system of the following genotypes: Mcl-1fl/fl;Puma−/−, Mcl-1fl/fl;Puma+/−, Mcl-1fl/fl;Bim−/−, Mcl-1fl/fl, Puma−/− (all RosaCreERKi/+), and wild-type (CreER only). Survival of these reconstituted animals was monitored after treatment with tamoxifen to activate the latent CreER recombinase to cause deletion of the floxed Mcl-1 alleles in the hematopoietic cells. (B-C) Lethally irradiated C57BL/6-Ly5.1 recipient mice were reconstituted with Mcl-1fl/fl;Puma−/−, Mcl-1fl/fl;Puma+/−, Mcl-1fl/fl;Bim−/−, Mcl-1fl/fl, Puma−/− (all RosaCreERKi/+), and wild-type (CreER only) test bone marrow cells mixed 1-to-1 with competitor wild-type (GFP+) bone marrow cells. (B) After 8 weeks, a baseline measurement of test vs competitor bone marrow–derived contribution to leukocytes in the blood was conducted by flow cytometric analysis; tamoxifen was administered to induce Mcl-1fl deletion. The contributions of the test- vs competitor-derived leukocytes in the blood were determined by flow cytometric analysis after 30 days. (C) After 30 days, the contributions of the test- vs competitor-derived LSK population was determined in the bone marrow of reconstituted mice by flow cytometric analysis. (A) Mice numbers (n) indicated in brackets. (B-C) Mice numbers are as follows (pre-, post-TAM): Mcl-1fl/fl;Puma−/− (14,14), Mcl-1fl/fl;Puma+/− (12,9), Mcl-1fl/fl;Bim−/− (6,6), Mcl-1fl/fl (12,9), Puma−/− (18,18), CreER only (6,3). Data represent mean ± SEM. **, ***, **** denote significant differences where P < .01, < .001, < .0001 between the indicated groups (Student t test).
Kaplan-Meier mouse survival curves were generated in GraphPad Prism (GraphPad Software Inc) for the 5-FU treatment, γ-irradiation, and acute Mcl-1 deletion experiments. Mouse cohorts were compared using the log-rank Mantel-Cox test. P values (unpaired, equal variance, 2-sided) of <.05 were considered significant.
Blood and organ cell counts were plotted and analyzed with GraphPad Prism using the 2-tailed Student t test comparing 2 groups with each other. We found no evidence for deviation of the data points from a normal distribution nor for differences in variance between groups under analysis. Error bars represent standard error of mean (±SEM).
Results
MCL-1 but not BCL-XL is required for hematopoietic recovery following treatment with 5-FU
Hematopoietic recovery from stress was induced in Mcl-1+/−, Bcl-x+/−, and wild-type mice by treatment with 5-FU, a DNA-damaging chemotherapeutic that has the side-effect of depleting cycling immature progenitors, lymphocytes, and other mature blood cells in clinical settings (Figure 1A).18 After an initial dramatic reduction in red bloods cells (RBCs), white blood cells, and platelets, the 5-FU–treated wild-type and Bcl-x+/− mice exhibited robust recovery (Figure 1; supplemental Figure 1, see supplemental Data available at the Blood Web site). In contrast, most Mcl-1+/− mice failed to recover from 5-FU–induced cytopenia (Figure 1B; median survival: Mcl-1+/− = 10 days, Mcl-1+/− vs wild-type: P < .0001), exhibiting persistent anemia (Figure 1C; Mcl-1+/− vs wild-type: P = .0036). Histologic analysis of the bone marrow of sick Mcl-1+/− mice revealed profound hematopoietic hypocellularity (Figure 1D). By 21 days, all wild-type and Bcl-x+/− mice but only a minor fraction of Mcl-1+/− mice (2 of 10) had recovered from the 5-FU–induced cytopenia (Figure 1E; supplemental Figure 1).
Flow cytometric analysis revealed comparable reductions in myeloid, B- and T-cell numbers in the peripheral blood of 5-FU–treated mice of all genotypes (supplemental Figure 2). Leukocyte subset analysis of the few surviving 5-FU–treated Mcl-1+/− mice (2 of 10) revealed that their ability to regenerate B and T lymphocytes as well as myeloid cells (Mac-1+Gr-1lo monocytes and Mac-1+Gr-1hi granulocytes) on day 7 was comparable to that seen in wild-type mice (supplemental Figure 2), explaining why they did not die. Although loss of only 1 allele of Mcl-1 does not noticeably perturb hematopoiesis at steady state (supplemental Figure 3), these results demonstrate that MCL-1 is dose-limiting for recovery from 5-FU–induced myeloablation.
MCL-1 is required for hematopoietic recovery following γ-irradiation
We next examined whether loss of 1 allele of Mcl-1 would also compromise hematopoietic recovery after γ-irradiation, another genotoxic agent. For this, we used an independently generated strain of Mcl-1+/− mice13 to confirm and extend our findings. Upon exposure to a sublethal dose (8 Gy) of γ-irradiation, most (75%) wild-type animals survived long-term, whereas all Mcl-1+/− mice succumbed between 9 and 13 days (Figure 2A; P < .0001). Severe sickness was attributed to hematopoietic insufficiency, exemplified by more severe bone marrow hypocellularity in Mcl-1+/− mice compared with the wild-type controls (Figure 2B). These results confirm that MCL-1 is critical for the recovery of the hematopoietic system after DNA damage–induced cytopenia.
Analysis by flow cytometry of bone marrow LSK stem/progenitor cells revealed reduced expression of MCL-1 at the protein level in Mcl-1+/− mice relative to wild-type mice, whereas LSK numbers were found to be normal (supplemental Figure 4A-B). Interestingly, isolated Mcl-1+/− and wild-type LSK cells exhibited similar survival in vitro following γ-irradiation (supplemental Figure 4C). This suggests that the inability of the Mcl-1+/− mice to recover from myelotoxic challenge is not due to hypersensitivity of their stem cells to DNA damage but must rather be due to increased apoptosis of progenitor cells in response to the stresses imposed by mobilization and consequently increased proliferation during emergency hematopoiesis.
Mcl-1 haploinsufficiency impairs hematopoietic reconstitution after bone marrow transplantation
We next compared Mcl-1+/−, Bcl-x+/−, and wild-type bone marrow hematopoietic stem cells (HSCs) in a competitive setting over 2 rounds of transplantation: primary reconstitution (R1) and secondary reconstitution (R2) (Figure 3A). Lethally irradiated (2 × 5.5 Gy) C57BL/6-Ly5.1 recipient mice were transplanted with either Mcl-1+/−, Bcl-x+/−, or wild-type (all C57BL/6-Ly5.2) “test” bone marrow cells mixed 1-to-1 with wild-type (C57BL/6-Ly5.1) “competitor” bone marrow cells (3 replicate mice per sample). After 8 weeks, blood from the reconstituted mice was analyzed to determine the relative contributions of the test (Ly5.2+) and competitor (Ly5.1+) cells in the lymphoid and myeloid compartments. After 16 weeks, mice were sacrificed to examine test vs competitor contribution within the cellular subsets of the blood, thymus, spleen, and bone marrow (R1). At this time point, bone marrow samples from the 3 replicate mice for each sample were pooled and then injected (this time without addition of wild-type “competitor” bone marrow cells) into a further 3 lethally irradiated recipient mice. Blood from such secondary transplant recipients (R2) was analyzed at 8 and 16 weeks and hematopoietic tissues harvested at 16 weeks.
In both rounds of reconstitution (R1 and R2), Mcl-1+/− bone marrow stem/progenitor cells were substantially outcompeted by wild-type competitor cells, resulting in an almost complete absence of Mcl-1+/−–derived B, T, and myeloid cells in the peripheral blood, bone marrow, spleen, and thymus (Figure 3B-C; supplemental Figure 5). In contrast, Bcl-x+/− test bone marrow cells were as efficient as their wild-type counterparts in repopulating all hematopoietic organs and cell subsets examined, during both rounds of reconstitution (Figure 3B-C; supplemental Figure 5).
The death of leukocytes derived from the Mcl-1+/− bone marrow cells could be due to impaired stem/progenitor cell activity or could simply be a consequence of poor survival of mature T, B, and myeloid cells. To resolve this, we investigated whether loss of 1 Mcl-1 allele would compromise contribution within the LSK stem/progenitor cell compartment of mice reconstituted with a 1-to-1 mix of Mcl-1+/− and wild-type bone marrow cells. The Mcl-1+/− LSK cells were almost completely outcompeted by the wild-type (Ly5.1+) competitor LSK cells in both the R1 and R2 reconstitution (Figure 3D-E). Consistent with this finding, a far greater number of Mcl-1+/− bone marrow cells than wild-type control cells were required (even in the absence of competitor cells) to achieve robust reconstitution of lethally irradiated mice (Figure 3F). Interestingly, Mcl-1+/− stem/progenitor (LSK) cells were able to home to the bone marrow as efficiently as wild-type LSK cells (supplemental Figure 6). These results demonstrate that loss of 1 Mcl-1 allele severely impairs the ability of hematopoietic stem/progenitor cells to achieve reconstitution of the hematopoietic system in transplant recipients.
BIM or PUMA deficiency rescues the hematopoietic repopulation defect caused by Mcl-1 haploinsufficiency but only loss of PUMA provides durable rescue in serial transplantation
We have shown that both alleles of Mcl-1 are required for normal recovery of the hematopoietic system after a cytotoxic insult (5-FU: Figure 1; γ-irradiation: Figure 2) and for efficient hematopoietic reconstitution in the context of bone marrow transplantation (competitive: Figure 3B-E; and even noncompetitive: Figure 3F). Given that BIM and PUMA can both antagonize MCL-119 and are critical for stress-induced apoptosis of hematopoietic stem/progenitor cells,20-24 we hypothesized that deficiency in either of these BH3-only proteins would rescue the defects in emergency hematopoiesis caused by loss of 1 allele of Mcl-1.
In contrast to Mcl-1+/− mice, the majority of the Mcl-1+/−;Bim−/− and Mcl-1+/−;Puma−/− compound mutant mice survived 5-FU treatment of the entire duration of the experiment (Figure 4A; P = .0169, .0831 for Mcl-1+/−;Puma−/− and Mcl-1+/−;Bim−/− vs wild-type, respectively, log-rank [Mantel-Cox] test). Blood analysis confirmed robust recovery from 5-FU in the Mcl-1+/−;Puma−/− mice and to a lesser extent also in the Mcl-1+/−;Bim−/− mice (Figure 4B). Because BH3-only proteins, particularly PUMA, are critical for DNA damage–induced apoptosis in all leukocyte subsets tested,6-8 the enhanced survival of the Mcl-1+/−;Bim−/− and Mcl-1+/−;Puma−/− mice could simply be due to resistance of the mature blood cells to 5-FU rather than more robust stem/progenitor cell repopulating activity.
To discriminate between these alternatives, we tested whether loss of BIM or PUMA would rescue the defective hematopoietic repopulating ability of Mcl-1+/− bone marrow stem/progenitor cells in the context of competitive bone marrow transplantation using the strategy described in Figure 3A. As controls, we performed reconstitutions using bone marrow cells from wild-type, Bcl-x+/−;Bim+/− as well as Bcl-x+/−;Bim−/− mice and from all the relevant single knockout strains. Consistent with the established proapoptotic roles of BIM and PUMA,6-9 recipients reconstituted with 1-to-1 mixtures of bone marrow cells from wild-type (competitor) plus Bim+/−, Bim−/−, Puma+/−, or Puma−/− (test cells) mice contained a higher proportion of test-derived lymphoid, myeloid, and stem/progenitor cells of BIM- or PUMA-deficient origin in the blood and other hematopoietic tissues relative to the competitor cells (supplemental Figures 7-8). Bone marrow cells from Bcl-x+/−;Bim+/− and Bcl-x+/−;Bim−/− mice behaved comparably to those from the Bim+/− and Bim−/− mice, respectively (supplemental Figures 7-8).
The loss of a single allele of Puma completely rescued the defect in hematopoietic repopulation caused by the Mcl-1 haploinsufficiency, reflected by the enhanced contribution of Mcl-1+/−;Puma+/− derived cells to the LSK population in the bone marrow and to the mature lymphoid and myeloid populations in the blood (Figure 4C; supplemental Figure 9). Loss of both alleles of Bim or Puma was able to completely rescue the defects caused by haploinsufficiency of Mcl-1 during the first round of reconstitution (R1) (Figure 4C; supplemental Figure 9). However, only complete loss of Puma was able to restore the serial repopulating capacity of the Mcl-1+/− bone marrow cells over 2 rounds of transplantation (R1 and R2) (Figure 4C; supplemental Figure 9). These results show that the critical function of MCL-1 in recovery of the hematopoietic system from stress is to restrain proapoptotic PUMA and (to a lesser extent) BIM.
Complete loss of PUMA alleviates but does not abrogate the defect in survival of HSCs caused by acute deletion of both alleles of Mcl-1
Because loss of PUMA, and to a lesser extent loss of BIM, could overcome the deleterious impact of haploinsufficiency of Mcl-1 during competitive bone marrow reconstitution, we wondered whether their loss could rescue the bone marrow failure elicited by acute deletion of both alleles of Mcl-1.12 We therefore generated Mcl-1fl/fl;Puma−/−;RosaCreERKi/+, Mcl-1fl/fl;Puma+/−;RosaCreERKi/+, Mcl-1fl/fl;Bim−/−;RosaCreERKi/+, Mcl-1fl/fl;RosaCreERKi/+, and Puma−/−;RosaCreERKi/+ mice, in which tamoxifen treatment allows highly efficient deletion of “floxed” Mcl-1.25,26 To circumvent nonhematopoietic effects of MCL-1 deletion, we generated chimeric mice in which only the hematopoietic system was of the genotype of interest whereas all other tissues were wild type.
Eight weeks following reconstitution, Mcl-1 deletion was induced in hematopoietic cells by administration of tamoxifen. As expected, the majority of mice reconstituted with Mcl-1fl/fl;RosaCreERKi/+ bone marrow cells became severely ill over the 10 days following tamoxifen administration, presenting with symptoms consistent with hematopoietic failure (Figure 5A, median survival: 8 days; Mcl-1fl/fl;RosaCreERKi/+ vs RosaCreERKi/+, P = .0044). Tamoxifen-treated animals transplanted with test bone marrow taken from RosaCreERKi/+ or Puma−/−;RosaCreERKi/+ mice remained healthy throughout the duration of the experiment (Figure 5A). These findings concur with the aforementioned study using a transcriptionally inducible Cre transgene (Mxi-Cre12 ) and confirm that MCL-1 is essential for HSC survival in vivo.
Remarkably, Mcl-1fl/fl;Puma−/−;RosaCreERKi/+ reconstituted mice following tamoxifen treatment (Figure 5A; Mcl-1fl/fl;Puma−/−;RosaCreERKi/+ vs Mcl-1fl/fl;RosaCreERKi/+, P = .0066) survived significantly longer than mice reconstituted with Mcl-1fl/fl; RosaCreERKi/+ bone marrow. Although we also observed prolonged survival for Mcl-1fl/fl;Bim−/−;RosaCreERKi/+ reconstituted mice after tamoxifen treatment, this difference was not significant (P = .0863).
These findings were confirmed and extended through analysis of mice co-reconstituted with a 1-to-1 mixture of Mcl-1fl/fl;RosaCreERKi/+, Mcl-1fl/fl;Puma+/−;RosaCreERKi/+, Mcl-1fl/fl;Puma−/−;RosaCreERKi/+, or Mcl-1fl/fl;Bim−/−;RosaCreERKi/+ (all test cells) and wild-type (GFP+; competitor) bone marrow cells. When analyzed 1 month after tamoxifen treatment, LSK cells that had undergone acute deletion of Mcl-1 were substantially outcompeted by their coinjected wild-type (GFP+) counterparts resulting in very low levels of test-derived cells in the blood. Complete loss of PUMA or BIM could attenuate the catastrophic hematopoietic failure caused by acute (inducible) loss of MCL-1, but after recovery from acute myeloablation the surviving wild-type recipient stem progenitor cells preferentially contributed to sustained hematopoiesis (Figure 5B-C), indicating that they had better survival than the Mcl-1−/−;Puma−/− progenitors.
Discussion
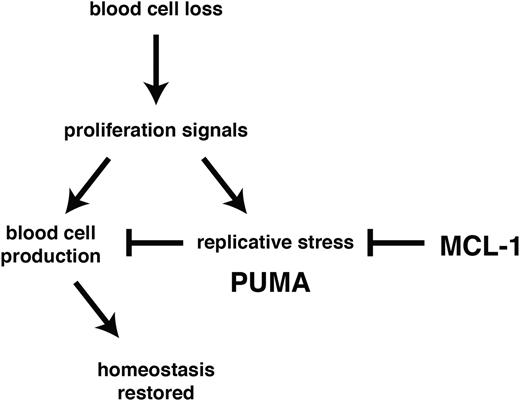
Using experimental models of hematopoietic recovery that mimic DNA damage inducing cancer therapy and bone marrow transplantation, we investigated the role of BCL-2 family members in the control of stem/progenitor cell survival. In particular, we determined their importance during the rebound phase following myelotoxic challenge with clinically relevant anticancer therapeutics (5-FU treatment or γ-irradiation) and in the burst of donor-derived stem/progenitor cell proliferation required to achieve stable reconstitution in myeloablated transplant recipients. We found that MCL-1 was essential to countermand stress-induced activation of PUMA (and to a lesser extent BIM) with consequent apoptosis induction (Figure 6).
MCL-1 and PUMA govern cell survival during recovery of the hematopoietic system from stress. Following acute cell loss or during bone marrow transplantation, recuperation of the hematopoietic system requires prosurvival MCL-1 in stem/progenitor cells to prevail over stress-induced activation of proapoptotic PUMA.
MCL-1 and PUMA govern cell survival during recovery of the hematopoietic system from stress. Following acute cell loss or during bone marrow transplantation, recuperation of the hematopoietic system requires prosurvival MCL-1 in stem/progenitor cells to prevail over stress-induced activation of proapoptotic PUMA.
Our data show that under conditions of hematopoietic stress HSC survival is exquisitely dependent on the levels of MCL-1, with loss of even a single Mcl-1 allele causing severe defects in the recovery of the hematopoietic system. Remarkably, we found that concomitant loss of PUMA allowed Mcl-1+/− stem/progenitor cells to survive and function normally during recovery of the hematopoietic system. Loss of PUMA also provided HSCs with marked (albeit temporary) protection from apoptosis induced by acute deletion of both Mcl-1 alleles (in the absence of other stress). These results demonstrate that antagonism between PUMA and MCL-1 constitutes the major axis of control of HSC survival.
These findings have important clinical implications for the treatment of cancer and stem cell transplantation in patients. Stem cell transplantation is the only curative therapy available for many patients with hematologic malignancies. Its use is, however, often restricted due to the risks associated with prolonged periods of neutropenia,27 thrombocytopenia,28 and immunodeficiency. Developing strategies to enhance stem cell engraftment and thereby shorten the period of bone marrow aplasia is therefore of great clinical importance. Our data provide new avenues for investigation in hematopoietic transplantation medicine: short-term antagonism of PUMA or impairment of MCL-1 degradation may expedite recovery from neutropenia, thrombocytopenia, and immunodeficiency following stem cell transplantation although caution will be required, especially in the context of treatment of MCL-1–dependent cancers.
Our data reveal that even small changes in MCL-1 levels can have severe consequences in the context of emergency hematopoiesis. Accordingly, therapeutic strategies designed to inhibit MCL-1 (directly with BH3 mimetics or indirectly by drugs that affect its stability) in the context of DNA damage–inducing cancer therapeutics may have significant side effects on hematopoietic stem/progenitor cells in patients. Similar concerns are also relevant to the risk of infection during periods of cancer therapy–associated lymphopenia and neutropenia. Treatment with MCL-1 antagonists is likely to impair survival and hence mobilization of lymphocytes and neutrophils to fight nosocomial (and other) infections. These considerations are important when defining dosing schedules for MCL-1 inhibitory drugs in patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs A. Ng for providing expert advice on serial competitive reconstitutions; B. Croker for advice regarding 5-FU experiments; J. M. Adams, P. M. Colman, A. Villunger, S. Cory, P. Bouillet, and L. Hennighausen for support and/or gifts of mice; B. Aubrey and D. Gray for reviewing the manuscript; D. Fairlie, E. Lee, and D. Gray for reagents; K. Humphries, K. Walker, E. Lanera, J. Mansheim, T. Camilleri, G. Siciliano, S. O’Connor, and L. Reid for expert animal care; B. Helbert, H. Ierino, K. Mackwell, and C. Young for genotyping; J. Corbin and J. McManus for automated blood analysis; E. Tsui, V. Babo, K. Weston, Y. Hoang, and S. Hasanein for histology; and D. Quilici and T. Nikolaou for γ-irradiation services.
This work was supported by grants and fellowships from the Cancer Council of Victoria (S.G. and A.R.D.D., Sydney Parker Smith Postdoctoral Research Fellowship), Lady Tata Postdoctoral Fellowship (S.G.), the National Health and Medical Research Council (NHMRC; program grant no. 1016701, NHMRC Senior Principal Research Fellow [SPRF] Fellowship 1020363 [A.S.]), and the Leukemia & Lymphoma Society (Specialized Center of Research [SCOR] grant no. 7001-13), Melbourne International Research Scholarship (University of Melbourne [S.G.]), Melbourne International Fee Remission Scholarship (University of Melbourne [S.G.]), Australian Postgraduate Award (A.R.D.D.) and Cancer Therapeutics Cooperative Research Centre (CRC) Top-up Scholarship (S.G. and A.R.D.D.), National Institutes of Health National Heart, Lung, and Blood Institute (R01HL102175, J.T.O.), American Lebanese Syrian Associate Charities (ALSAC; J.T.O.), and a Cancer Center Support Grant P30CA021765 (J.T.O.). This work was made possible by operational infrastructure grants through the Australian Government Independent Medical Research Institutes Infrastructure Support Scheme (IRIISS) and the Victorian State Government Operational Infrastructure Support (OIS).
Authorship
Contribution: A.R.D.D., S.G., J.T.O., and A.S. designed and conceived the study and experiments and prepared the manuscript; and A.R.D.D., S.G., and J.T.O. conducted and analyzed the experiments.
Conflict-of-interest disclosure: A.R.D.D., S.G., and A.S. are employed by The Walter and Eliza Hall Institute, which receives milestone payments from Genentech and AbbVie for the development of ABT-199 for cancer therapy. J.T.O. declares no competing financial interests.
Correspondence: Andreas Strasser, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, 3052 Victoria, Australia; e-mail: strasser@wehi.edu.au.
References
Author notes
S.G. and A.S. share senior authorship.

![Figure 1. MCL-1 is essential for recovery of the hematopoietic system from 5-FU challenge. (A) Experimental strategy. (B) Mcl-1+/−, Bcl-x+/−, and wild-type (control) mice were treated with 1 dose of 5-FU and survival was monitored over a 21-day period (median survival, Mcl-1+/− mice: 10 days; log-rank [Mantle-Cox] test, P < .0001). (C) RBC numbers in Mcl-1+/−, Bcl-x+/−, and wild-type mice at 10 days or when sick (Mcl-1+/−) after treatment with 5-FU. (D) Histologic analysis of the bone marrow of the mice from panels A and B at the same time point (representative images; ×40 objective, 250 µm scale bar). (E) RBC levels in the blood of 5-FU–treated Mcl-1+/−, Bcl-x+/−, and wild-type mice on the days indicated. n = 15 (wild type), 5 (Bcl-x+/−), 10 (Mcl-1+/−) mice; pooled results from 9 independent experiments. Data represent mean ± SEM. * and ** denote P < .05 or < .01, respectively, for the indicated comparisons (Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/21/10.1182_blood-2015-01-621250/4/m_3273f1.jpeg?Expires=1769802219&Signature=wBAXY51D6emMkFv1ahwJSWQLBAbqR7sV7JkbCw4dMMGlgZu0t9EwoyPHgAE7xMFqV~2Oj2pTPxd~B1xnpzTNlB3JUe8J~0K1LE4VWbnakNUgqogOiCPQnGIzX4zv5FMkZ43BzqDR9nXqNQkusku8yOhFZ6xwOL2bKnQh9TwtPl6NFnuFKwFyBtCn98p-EPi9N0jm~ULx700vcuipG8CCfd6EfDH3D3ZvIVD~XvPV-nhWm9SSIHqTPPZPyKMx9-zZAoER6Tw6XWU0f7xwPVAJD34sgqZlZhfj96MfncxCnuuMoaNNGk-9qf6ghovEk5A461FnqVIwJJNW7tJgBW8F-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. MCL-1 is essential for recovery of the hematopoietic system from sublethal γ-irradiation. (A) Mice (wild-type or Mcl-1+/−) were subjected to 8 Gy γ-irradiation and their survival monitored (median survival of Mcl-1+/− mice: 11 days; log-rank [Mantle-Cox] test, P < .0001); n = 14 (wild-type), 10 (Mcl-1+/−) mice. Pooled results from 2 independent experiments are shown. (B) Histologic analysis of the bone marrow of wild-type and Mcl-1+/− mice was performed 12 days after γ-irradiation. Representative hematoxylin and eosin (H&E)-stained bone marrow sections (×20 objective, 500 µm scale bar).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/21/10.1182_blood-2015-01-621250/4/m_3273f2.jpeg?Expires=1769802219&Signature=ymMrWfQZBh8wYL1sT--RobwzjTZbryzT1B8IJgmpE27jZiXkPTfbdk6EIDy6pgGPfRwJZ6DGPnMPaxHPzPzqQmjId38oE6nUyV6NsM1XNknLJSdHvKVwqSJd89LVaSB3LCKp2mqaeWK5WS8aGT61xH7CXLErDPnp-4M4ugfEEzmOmw2Xn5U2dUEeI3ZmyAokAFpQQ4gmbWyfCt2Vf6cWs8x-TYnbKWiyy1vngNnKapMyFHGlckfC0sF0Ig4LkvcFYOTJ4sU9m-2X1XQ-QsQr95EVU5bVrZ9uHFvMMFCRvmzZdKlAZg2QdYj00-irwJCb4Vf531VHpfHeKBsvFCQZcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal