Key Points
Auto-HSCT in CR1 provides long-term remission in BPDCN patients.
RIC allo-HSCT and MAC allo-HSCT results are comparable.
Abstract
We sought to clarify the role of high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (auto-HSCT) and allogeneic hematopoietic stem cell transplantation (allo-HSCT) to treat blastic plasmacytoid dendritic cell neoplasm (BPDCN). We retrospectively identified 25 BPDCN patients (allo-HSCT, n = 14; auto-HSCT, n = 11) from registry data of the Japan Society for Hematopoietic Cell Transplantation and analyzed clinicopathologic data and clinical outcomes after transplantation. The median age at HSCT was 58 years (range, 17-67 years). All 11 patients who underwent auto-HSCT were in the first complete remission (CR1). With a median follow-up of 53.5 months, the overall survival rates at 4 years for patients who underwent auto-HSCT and allo-HSCT were 82% and 53% (P = .11), respectively, and progression-free survival rates were 73% and 48% (P = .14), respectively. Auto-HSCT for BPDCN in CR1 appears to provide promising results and deserves further evaluation in the setting of prospective trials.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare malignancy of plasmacytoid dendritic cells, as classified by the World Health Organization,1 and was previously diagnosed as blastic natural killer cell lymphoma or CD4+/CD56+ hematodermic neoplasm.2-5 BPDCN is characterized by distinct clinical, pathologic, and genetic features3,6-8 and is diagnosed based on the expression of immunophenotypic markers such as CD4, CD56, CD123, and TCL1.9,10 BPDCN is a disease of advanced age, and BPDCN patients often initially present with cutaneous lesions.3,8
The optimal treatment strategy for BPDCN has not been established, and the prognosis of BPDCN is extremely poor, with a median overall survival (OS) of ∼1 year.2,3 Commonly used induction treatment of BPDCN varies from cyclophosphamide, doxorubicin, vincristine, and prednisolone to leukemia-like regimens.3 Despite the initial response to chemotherapy with 41% to 94% complete remission (CR) rates,2,3,11-13 almost all cases eventually develop into a fulminant chemoresistant leukemic phase. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) with myeloablative conditioning (MAC) during remission has shown encouraging results for patients with BPDCN.3,12-17 Meanwhile, only a few small case series were described that showed the benefit of high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (auto-HSCT) or reduced-intensity conditioning (RIC) allo-HSCT.12,14,15,18,19 Because BPDCN is a disease of advanced age, clarifying the role of the RIC regimen or auto-HSCT in BPDCN is crucial.
The present study aimed to clarify the role of both allo-HSCT and auto-HSCT in treating BPDCN using registry data from the Japan Society for Hematopoietic Cell Transplantation.
Study design
All patients with a diagnosis of “BPDCN,” “blastic natural killer cell lymphoma,” “CD4+/CD56+ hematodermic neoplasm,” or “dendritic cell leukemia” ≥15 years of age who underwent the first HSCT in 2002 or later were identified in the Transplant Registry Unified Management Program (TRUMP),20 which is the registry data of the Japan Society for Hematopoietic Cell Transplantation . The initial diagnosis was made by hematopathologists in each institution. Baseline information and transplantation characteristics of the selected patients were obtained from TRUMP. Then, we sent out questionnaires to collect additional data regarding clinical presentation, histopathology, and flow cytometry reports and therapies before HSCT and follow-up updates. In addition, a central clinicopathologic review was performed by 7 of the authors to confirm the diagnosis of BPDCN in accordance with the World Health Organization classification.1 The study protocol was approved by the Institutional Review Board at Nagoya Daini Red Cross Hospital and Shimane University where this study was organized and was performed in accordance with the Declaration of Helsinki.
Results and discussion
Of the 109 identified patients in TRUMP, additional data were obtained for 83 patients. After central clinicopathologic review, 58 patients were excluded from analysis because of a different diagnosis or the absence of important clinical data. Finally, the diagnosis of BPDCN was confirmed in 25 patients (allo-HSCT, n = 14; auto-HSCT, n = 11).
The median age at HSCT was 58 years (range, 17-67 years), and male patients were predominant (80%). Involvement of the skin and bone marrow (BM) at diagnosis was observed in 88% and 68% of patients, respectively. No significant difference was found in baseline characteristics between the auto-HSCT group and the allo-HSCT group (Table 1). The induction regimen was as follows: non-Hodgkin lymphoma (NHL)-like (eg, cyclophosphamide, doxorubicin, vincristine, and prednisolone-like or ifosfamide/etoposide based) (n = 11), acute lymphoblastic leukemia (ALL)-like (n = 10), and acute myeloid leukemia (AML)-like (n = 4) regimens. The rate of initial response to induction chemotherapy was high, with a CR rate of 96%.
Patient characteristics
| Characteristic . | All . | Auto-HSCT . | Allo-HSCT . | P value . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Patient number | 25 | 11 | 14 | ||||
| Age at HSCT (years) | |||||||
| Median | 58 | 57 | 58 | .891 | |||
| Range | 17-67 | 19-67 | 17-64 | ||||
| >60 years | 9 | 36 | 3 | 27 | 6 | 43 | .335 |
| Sex, Male | 20 | 80 | 9 | 82 | 11 | 79 | .622 |
| PS, ≥2 at HSCT | 2 | 8 | 1 | 9 | 1 | 7 | .697 |
| Clinical presentation at diagnosis | |||||||
| Skin | 22 | 88 | 10 | 91 | 12 | 86 | .593 |
| Lymph nodes | 12 | 48 | 4 | 36 | 8 | 57 | .265 |
| Peripheral blood | 7 | 28 | 2 | 18 | 5 | 36 | .496 |
| Bone marrow | 17 | 68 | 8 | 73 | 9 | 64 | .496 |
| Induction treatment | |||||||
| NHL-like | 11 | 44 | 7 | 64 | 4 | 29 | .089 |
| ALL-like | 10 | 40 | 4 | 36 | 6 | 43 | .534 |
| AML-like | 4 | 16 | 0 | 0 | 4 | 29 | .079 |
| Disease status at HSCT | |||||||
| CR1 | 21 | 84 | 11 | 100 | 10 | 71 | .079 |
| CR2 | 2 | 8 | 0 | 0 | 2 | 14 | |
| Refractory | 2 | 8 | 0 | 0 | 2 | 14 | |
| Time from diagnosis to HSCT, months | |||||||
| Median | 6 | 6 | 5 | .496 | |||
| Range | 2-22 | 2-7 | 2-22 | ||||
| Year of HSCT, median | 2009 | 2007 | 2010 | ||||
| Range | 2003-2013 | 2003-2011 | 2004-2013 | ||||
| Donor type | |||||||
| Related | 7 | 50 | |||||
| Unrelated | 7 | 50 | |||||
| Stem cell source | |||||||
| Peripheral blood | 5 | 36 | |||||
| Bone marrow | 8 | 57 | |||||
| Cord blood | 1 | 7 | |||||
| Myeloablative conditioning | 8 | 57 | |||||
| TBI+CY | 4 | 29 | |||||
| TBI+CY+CA | 1 | 7 | |||||
| Other TBI based | 1 | 7 | |||||
| BU based | 2 | 14 | |||||
| Reduced-intensity conditioning | 6 | 43 | |||||
| Flu+Bu+TBI | 2 | 14 | |||||
| Flu+Mel+TBI | 1 | 7 | |||||
| Flu+Bu | 1 | 7 | |||||
| Flu+Mel+TBI | 1 | 7 | |||||
| Other Flu based | 1 | 7 | |||||
| High-dose regimen for auto-HSCT | |||||||
| MCEC based | 4 | 36 | |||||
| MEAM based | 2 | 18 | |||||
| TBI bases | 4 | 36 | |||||
| LEED | 1 | 9 | |||||
| Characteristic . | All . | Auto-HSCT . | Allo-HSCT . | P value . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Patient number | 25 | 11 | 14 | ||||
| Age at HSCT (years) | |||||||
| Median | 58 | 57 | 58 | .891 | |||
| Range | 17-67 | 19-67 | 17-64 | ||||
| >60 years | 9 | 36 | 3 | 27 | 6 | 43 | .335 |
| Sex, Male | 20 | 80 | 9 | 82 | 11 | 79 | .622 |
| PS, ≥2 at HSCT | 2 | 8 | 1 | 9 | 1 | 7 | .697 |
| Clinical presentation at diagnosis | |||||||
| Skin | 22 | 88 | 10 | 91 | 12 | 86 | .593 |
| Lymph nodes | 12 | 48 | 4 | 36 | 8 | 57 | .265 |
| Peripheral blood | 7 | 28 | 2 | 18 | 5 | 36 | .496 |
| Bone marrow | 17 | 68 | 8 | 73 | 9 | 64 | .496 |
| Induction treatment | |||||||
| NHL-like | 11 | 44 | 7 | 64 | 4 | 29 | .089 |
| ALL-like | 10 | 40 | 4 | 36 | 6 | 43 | .534 |
| AML-like | 4 | 16 | 0 | 0 | 4 | 29 | .079 |
| Disease status at HSCT | |||||||
| CR1 | 21 | 84 | 11 | 100 | 10 | 71 | .079 |
| CR2 | 2 | 8 | 0 | 0 | 2 | 14 | |
| Refractory | 2 | 8 | 0 | 0 | 2 | 14 | |
| Time from diagnosis to HSCT, months | |||||||
| Median | 6 | 6 | 5 | .496 | |||
| Range | 2-22 | 2-7 | 2-22 | ||||
| Year of HSCT, median | 2009 | 2007 | 2010 | ||||
| Range | 2003-2013 | 2003-2011 | 2004-2013 | ||||
| Donor type | |||||||
| Related | 7 | 50 | |||||
| Unrelated | 7 | 50 | |||||
| Stem cell source | |||||||
| Peripheral blood | 5 | 36 | |||||
| Bone marrow | 8 | 57 | |||||
| Cord blood | 1 | 7 | |||||
| Myeloablative conditioning | 8 | 57 | |||||
| TBI+CY | 4 | 29 | |||||
| TBI+CY+CA | 1 | 7 | |||||
| Other TBI based | 1 | 7 | |||||
| BU based | 2 | 14 | |||||
| Reduced-intensity conditioning | 6 | 43 | |||||
| Flu+Bu+TBI | 2 | 14 | |||||
| Flu+Mel+TBI | 1 | 7 | |||||
| Flu+Bu | 1 | 7 | |||||
| Flu+Mel+TBI | 1 | 7 | |||||
| Other Flu based | 1 | 7 | |||||
| High-dose regimen for auto-HSCT | |||||||
| MCEC based | 4 | 36 | |||||
| MEAM based | 2 | 18 | |||||
| TBI bases | 4 | 36 | |||||
| LEED | 1 | 9 | |||||
BU, busulfan; CA, cytarbine; CY, cyclophosphamide; Flu, fludarabine; LEED, cyclophosphamide, etoposide, melphalan, and dexamethasone; MCEC, ranimustine, carboplatin, etoposide, and cyclophosphamide; MEAM, ranimustine, etoposide, cytarbine, and melphalan; Mel, melphalan; TBI, total body irradiation.
The median time from diagnosis to HSCT was 6 months (range, 2-22 months). Among the patients who underwent allo-HSCT, MAC and RIC regimens were used in 8 (57%) and 6 (43%) patients, respectively. The allogeneic transplant donor was a relative in 7 patients, unrelated bone marrow in 7 patients, and cord blood in 1 patient. Regarding the disease status at transplantation, all 11 patients who underwent auto-HSCT were in CR1, whereas among patients who underwent allo-HSCT, 12 were in CR1 (n = 10; RIC allo-HSCT, n = 5; MAC allo-HSCT, n = 5)/CR2 (n = 2; RIC allo-HSCT, n = 1; MAC allo-HSCT, n = 1), and 2 (MAC allo-HSCT) were not in remission.
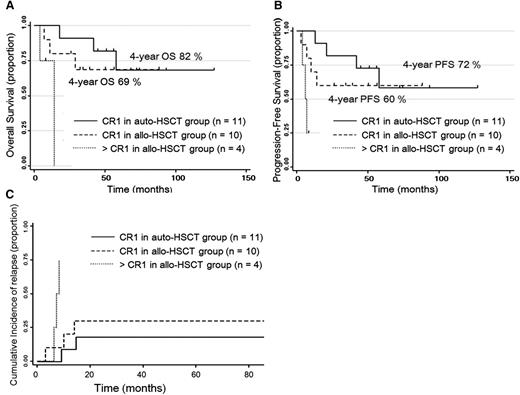
With a median follow-up of 53.5 months in surviving patients, OS and progression-free survival (PFS) at 4 years after HSCT were 65% and 58%, respectively. OS at 4 years for patients who underwent auto-HSCT and allo-HSCT in CR1 was 82% and 69%, respectively (P = .44), and PFS at 4 years was 73% and 60%, respectively (P = .43; Figure 1A-B). The 3 induction regimen groups (NHL-like, ALL-like, and AML-like regimens) had similar OS rates: 62%, 70%, and 67% at 4 years, respectively (P = .86). OS did not differ significantly between MAC and RIC groups (OS at 4 years: 45% vs 60%, respectively; P = .31).
Survival of all patients with BPDCN after auto-HSCT or allo-HSCT according to disease status at HSCT. (A) Overall survival, (B) progression-free survival, and (C) cumulative incidence of relapse after auto-HSCT or allo-HSCT according to disease status at HSCT.
Survival of all patients with BPDCN after auto-HSCT or allo-HSCT according to disease status at HSCT. (A) Overall survival, (B) progression-free survival, and (C) cumulative incidence of relapse after auto-HSCT or allo-HSCT according to disease status at HSCT.
Nonrelapse death within 1 year occurred in 0 and 2 (gastrointestinal bleeding and suicide) patients in the auto-HSCT and allo-HSCT groups, respectively. Cumulative incidence of relapse is shown in Figure 1C. Seven patients (28%) relapsed at a median of 10 months (range, 3-21 months) after HSCT (MAC allo-HSCT, n = 2; RIC allo-HSCT, n = 3; auto-HSCT, n = 2). Notably, no relapse occurred in patients without BM infiltration at diagnosis in the auto-HSCT treatment group. After allo-HSCT, grade 2 to 4 acute graft-versus-host disease was observed in 5 patients (36%). Two patients in the auto-HSCT group died because of a second malignancy at days +1282 and +1768.
With univariate analysis, HSCT beyond CR1 was an adverse prognostic factor for OS and PFS. BM infiltration was not identified as an adverse prognostic factor for survival.
To the best of our knowledge, this is the first report to demonstrate the clinical outcomes of auto-HSCT for BPDCN with a reasonably large number of patients. Auto-HSCT for BPDCN in CR1 appears to provide promising results and deserves further evaluation in the setting of prospective trials.
Several previous small case series were reported that described the outcomes of auto-HSCT for BPDCN.3,18,21 The outcomes, especially beyond CR1, were poor.3 Meanwhile, all patients who underwent auto-HSCT were in CR1 in our study. Taken together, these results suggest that the disease status at auto-HSCT is crucial for a better outcome.
As previous reports have shown,3,15,22 allo-HSCT can also provide long-term survival in patients with BPDCN. Meanwhile, allo-HSCT could not overcome the poor disease status beyond CR1 in the present study. Considering the results of long-term remission without a late relapse in the auto-HSCT group, high-dose chemotherapy may play an important role in curing BPDCN independent of a graft-versus-tumor effect of allo-HSCT. A previous large study also referred to the high-dose effect for BPDCN.15
Although the present study provides novel information about BPDCN, this was a retrospective study; thus, treatment outcomes may have been overestimated or underestimated with possible unrecognized biases. We could not evaluate patients who intended to undergo HSCT but failed during salvage treatment. In addition, determining statistically significant differences in survival analyses and identifying prognostic factors in this study were difficult because of the small number of patients.
In summary, our findings indicated that patients with BPDCN can achieve a durable remission with HSCT regardless of the type of induction regimen. In particular, auto-HSCT in CR1 appears to be a reasonable treatment option and may play an important role in improving the outcomes of BPDCN.
Presented in part at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the physicians and data managers at the centers who contributed valuable data about transplantation for BPDCN to the Japan Society for Hematopoietic Cell Transplantation (JSHCT). The authors thank Yuichi Kato of Yamagata University Hospital, Yasutaka Aoyama of Seichokai Fuchu Hospital, Asao Hirose of Osaka City University Hospital, Masaki Yamaguchi of Ishikawa Prefectural Central Hospital, Yuichi Hasegawa of University of Tsukuba Hospital, Shin Fujisawa of Yokohama City University Medical Center, Yasushi Onishi of Tohoku University Hospital, Toshihiro Iwasaki of Toyohashi Municipal Hospital, Miho Nara of Akita University Hospital, Hiroko Nakamura of Juntendo University Hospital, Yoshihiro Hatta of Nihon University Itabashi Hospital, Hiroatsu Iida of Nagoya Medical Center, Fumihito Tajima of Yonago Medical Center, Tomonori Nakazato of Yokohama Municipal Citizen’s Hospital, Jun Konishi of Okayama Medical Center, Kazutaka Sunami of Okayama Medical Center, and Kazuki Ohashi of Komagome Hospital for providing patient cases and Kana Sakamoto of Cancer Institute Hospital, Japanese Foundation for Cancer Research, for coordinating the pathologic review. The authors also thank all the members of the data management committees of JSHCT for data management.
The views expressed in this report are those of authors and do not indicate the views of the JSHCT.
Authorship
Contribution: T.A., R.S., K.I., and J.S. designed the study; S.K., K.F., J.T., T.K., K.O., T. Ito, Y.K., T.F., and K.T. provided cases; T.A., R.S., K.I., Y.K., S.K., K.T., and J.S. reviewed the clinicopathologic materials; T.A., R.S., K.I., Y.K., S.K., K.T., and J.S. collected data; T.A., R.S, K.I., Y.K., S.K., K.T., and J.S. analyzed and interpreted data; T.A. and R.S. performed the statistical analysis; K.T. and J.S. provided financial support; and T.A., R.S, K.I., Y.K., S.K., K.T., K.F., T. Ichinohe, and J.S. wrote the manuscript. All authors have read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tomohiro Aoki, Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8560, Japan; e-mail: taoki-cib@umin.net.