Key Points
Patients with LCH, risk organs, refractory to standard VBL-steroid regimen have a poor survival, ∼30%.
In a phase 2 study, with 5 years’ median follow-up, cladribine and Ara-C was shown to improve the survival up to 85% for this group.
Abstract
An international phase 2 study combining cladribine and cytarabine (Ara-C) was initiated for patients with refractory, risk-organ–positive Langerhans cell histiocytosis (LCH) in 2005. The protocol, comprising at least two 5-day courses of Ara-C (1 g/m2 per day) plus cladribine (9 mg/m2 per day) followed by maintenance therapy, was administered to 27 patients (median age at diagnosis, 0.7 years; median follow-up, 5.3 years). At inclusion, all patients were refractory after at least 1 course of vinblastine (VBL) plus corticosteroid, all had liver and spleen involvement, and 25 patients had hematologic cytopenia. After 2 courses, disease status was nonactive (n = 2), better (n = 23), or stable (n = 2), with an overall response rate of 92%. Median disease activity scores decreased from 12 at the start of therapy to 3 after 2 courses (P < .0001). During maintenance therapy, 4 patients experienced reactivation in risk organs. There were 4 deaths; 2 were related to therapy toxicity and 2 were related to reactivation. All patients experienced severe toxicity, with World Health Organization grade 4 hematologic toxicity and 6 documented severe infections. The overall 5-year survival rate was 85% (95% confidence interval, 65.2%-94.2%). Thus, the combination of cladribine/Ara-C is effective therapy for refractory multisystem LCH but is associated with high toxicity.
Introduction
The clinical presentation and outcome of Langerhans cell histiocytosis (LCH) is extremely variable, ranging from a single isolated, spontaneously remitting bone lesion to multisystem disease with life-threatening organ dysfunction. Since the early 1990s, cooperative international approaches to this rare disease have been organized under the aegis of the Histiocyte Society.1-3 These trials showed that the use of vinblastine (VBL) plus a steroid is effective in the majority of patients with multisystem LCH. The studies also identified many prognostic factors and gave us a better understanding of the natural history of the disease. Single system and multisystem LCH are distinguished according to the number of involved organs. In patients with multisystem LCH, those with spleen, liver, and hematologic dysfunction are considered to have “risk organs” because the involvement of such organs may confer a life-threatening prognosis.4 In addition to extension of the disease, the short-term response after an initial course of VBL and a steroid is a very powerful prognostic factor. Several studies have reported that poor response to the initial standard chemotherapy defines a small group of patients with a <30% survival rate 2 years after diagnosis.1,5,6 Poor response is defined by progression in risk organs and by resistance or failure if the patient presents a risk organ which remains unresponsive to the therapy. Such patients comprise the majority of early deaths.6,7 A pilot study of 10 patients showed that the combination of cladribine and cytosine-arabinoside (cytarabine [Ara-C])8 was promising as salvage therapy for refractory, risk-organ–positive LCH. The present study reports the results of a phase 2 study (LCH-S-2005) that included 27 patients and 5-year median follow-up.
Patients and methods
Inclusion and exclusion criteria
All patients included in this study had a definitive pathological diagnosis of LCH with involvement of at least 1 risk organ and had failed standard therapy. Failure of initial therapy was characterized by disease progression in 1 or more risk organs, except for isolated lung involvement, after at least 6 weekly doses of VBL and 28 days of prednisolone at a minimum dose of 40 mg/m2, with or without the addition of a third drug. The patient was considered resistant to the therapy if there was no improvement in one or more risk organs, except for isolated lung involvement, after the initial therapy.
Failure can be observed at the onset of the disease or during the course of the disease in a patient who initially responds and then experiences reactivation in one or more risk organ(s) defined elsewhere.4 The exclusion criteria were isolated sclerosing cholangitis without evidence of active LCH, isolated lung involvement at any age, or lung disease as the only risk organ involvement.
Ethics and regulatory approval
The study protocol was approved by the ethics committee (Comité Consultatif pour les Personnes Soumis à une Recherche Biomédicale) at the Centre Hospitalier Universitaire (CHU) de Montpellier, France on July 15, 2004, and amended protocols were approved on November 8, 2005 and on January 10, 2006. The protocol was later approved in the Netherlands, Italy, Sweden, and Denmark according to their national regulations. Because all patients were under the legal age of consent, their legal guardians signed a consent form that was approved by the research ethics board. In The Netherlands, the treatment was considered standard of care by the LCH Protocol Committee of the Dutch Childhood Oncology Group (DCOG) after the publication of the pilot study.8 According to national regulations, the protocol was therefore not considered a phase 2 study. For all patients, approval was obtained for the transfer of treatment data.
Intervention
The initial 2 courses consisted of cladribine/Ara-C. Ara-C was administered at a dose of 500 mg/m2 twice daily for 5 days as a 2-hour IV infusion. For newborns and children up to 3 months old, the dose of Ara-C was 33 mg/kg per day. Cladribine was started the second day of the Ara-C course and was administered at a dose of 9 mg/m2 per day daily for 5 days as a 2-hour IV infusion. Cladribine and Ara-C should not be administrated in the same time. For children who weighed <10 kg, the dose of cladribine was 0.3 mg/kg per day. The second course was started the fifth week after therapy initiation regardless of hematologic values in the absence of life-threatening infections or any other adverse events. The response was evaluated 5 to 6 weeks after the second cycle (ie, weeks 9-10 after the start of the first cycle unless delayed). In case of poor response after 2 courses, a third course was recommended. In case of poor response after a third course, the treating physician was allowed to decide whether to start a fourth course or even more courses. As soon as a good response was observed, patients changed to maintenance therapy, which consisted of 2 courses of cladribine 5 mg/m2 per day for 3 days IV, followed by VBL 6 mg/m2 every 2 weeks for 6 months combined with prednisolone 40 mg/m2 per day orally for 5 days every second week, 6-mercaptopurine (6MP) 50 mg/m2 orally daily, and methotrexate (MTX) 20 mg/m2 orally weekly. Oral 6MP and MTX were continued for 12 additional months. The overall scheme of the protocol is presented in Figure 1.
Study flowchart showing decision points after the first 2 therapeutic courses. The cumulative dose of cladribine in this protocol was 120 mg/m2 if the patient had a good response to the initial course.
Study flowchart showing decision points after the first 2 therapeutic courses. The cumulative dose of cladribine in this protocol was 120 mg/m2 if the patient had a good response to the initial course.
Statistical analysis
This open-label, prospective, nonrandomized, phase 2 study was organized following a 2-step Simon plan.9 The primary end point was the overall response rate (ORR) after 2 courses of therapy, evaluated 9 to 10 weeks after initiation of the first therapeutic course. The ORR was considered favorable if the patient’s status was “active disease, better” or “nonactive disease” based on the standard criteria for response in risk organs used in previous LCH clinical trials (nonactive disease, or active disease, better/stable/worse)1,2 (see supplemental Tables 1-2, available on the Blood Web site). In the literature, the best ORR of historical controls for this patient group was ∼25%.5,6 Therefore, an ORR of <25% was considered unacceptable and an ORR of 50% was considered a positive response to the protocol. The α error rate (accepting poor treatment) was 0.09, and the β error rate (rejecting a promising treatment) was 0.08. A total of 13 patients were planned for the first Simon step; 17 were planned for the second step. Secondary end points were the number of courses of cladribine/Ara-C, the time to achieve nonactive disease, the type of subsequent and/or maintenance therapy used, early and late toxicity, early and late morbidity and mortality, and the applicability of the disease activity score (supplemental Table 3)10 at diagnosis and during the course of the disease to evaluate disease activity. An intent-to-treat analysis was performed.
Results
Demographic data
Twenty-seven patients (20 male, 7 female) from 6 countries were enrolled between April 2005 and November 2010. The median age at diagnosis was 0.73 years (range, 0.1-3.3 years). At diagnosis, patients were classified as multisystem risk-organ–positive (n = 19), multisystem risk-organ–negative (n = 4), or as single system (always skin disease) that progressed to multisystem risk-organ disease (n = 4). Patients were included at the time of the first occurrence of the disease (n = 17) or during reactivation of the disease (n = 10). At the start of the study, all patients fulfilled the study criteria by having multisystem risk-organ–positive LCH that was refractory to at least 1 course of VBL and a steroid. Only 2 patients had no hematologic dysfunction at inclusion; both had spleen and liver involvement in addition to systemic symptoms and bone and lymph node involvement. At the start of the protocol, the median disease activity score (DAS) that was evaluable in all patients was 12. The median time between initial diagnosis and the start of the protocol was 0.37 years; this interval was shorter in cases of primary failure (0.3 years) than in cases of secondary failure (1 year). Initial therapy consisted of induction therapy with VBL and steroids in all 27 cases (1 course in 18, 2 courses in 9), and 1 patient also received cladribine monotherapy (cumulative dose, 145 mg/m2) before inclusion in the protocol. The median follow-up of the 23 survivors was 5.37 years (range 3 to 9.3 years). Detailed information about the patients’ status prior to study inclusion is summarized in Table 1.
Patient medical history and disease status prior to the initiation of therapy (N = 27)
| UPN . | Age at diagnosis, y . | Sex . | Organ involvement at diagnosis . | Initial therapy . | No. of reactivations . | Therapy of reactivation before inclusion . | Duration from diagnosis to inclusion, mo . | Disease extension at start . |
|---|---|---|---|---|---|---|---|---|
| 1506648 | 1.67 | M | Bone, skin, ENT, hematologic | VBL + steroid 2 inductions, maintenance 6 mo | 2 | VBL + steroid 1 induction | 9.7 | MS RO+ lung, hematologic, liver, spleen, bone |
| 1506863 | 0.17 | M | Bone, skin, hematologic, liver, spleen, lung, gingiva | VBL + steroid 2 inductions | 1 | 8.6 | MS RO+ bone, skin, hematologic, liver, spleen, lung, gingiva | |
| 1506802 | 3.34 | F | Bone, skin, pituitary, ENT | VBL + steroid 2 inductions | 1 | 6.4 | MS RO+ bone, skin, pituitary, ENT, liver, spleen, hematologic | |
| 1506961 | 0.5 | M | Bone, skin, ENT (external auditory canal), lung, hematologic, liver, nodes, thymus, | VBL + steroid 2 inductions | 1 | 3.4 | MS RO+ bone, lung, hematologic, liver, skin, nodes, thymus, external auditory canal | |
| 1506962 | 0.42 | M | Liver, skin, hematologic, alimentary canal | VBL + steroid 1 induction | 1 | 4.1 | MS RO+ liver, skin, hematologic, gut | |
| 1507009 | 1 | M | Bone, skin, ENT, liver, hematologic, | VBL + steroid 2 inductions | 1 | 3.2 | MS RO+ liver, hematologic, skin, bone, ENT | |
| 1506970 | 0.67 | F | Bone, skin, ENT (external auditory canal) | VBL + steroid 1 induction | 3 | VBL + steroid 1 induction | 12.9 | MS RO+ hematologic, GI, ENT, genital mucosa, liver, spleen |
| 1507092 | 0.67 | M | Bone, skin, liver, spleen, lung, nodes, hematologic, GI | VBL + steroid 1 induction + | 1 | 1.2 | MS RO+ skin, liver, spleen, lung, bone, nodes, hematologic, GI | |
| 1507202 | 0.67 | M | Bone, skin, hematologic | VBL + steroid 1 induction | 1 | 2.3 | MS RO+ skin, bone, hematologic, liver, spleen | |
| 1507020 | 1.42 | M | Bone, skin, liver, hematologic, spleen | VBL + steroid 2 inductions | 2 | VBL + steroid 1 induction | 16.5 | MS RO+ liver, spleen, lung, hematologic, GI |
| 1507220 | 0.67 | M | Bone, skin | VBL + steroid 1 induction + | 2 | VBL + steroid 1 induction | 12.9 | MS RO+ hematologic, bone, gingival mucosa, GI |
| 1507379 | 1.5 | M | Skin | Topical mustard | 2 | VBL + steroid 1 induction | 13.0 | MS RO+ bone, skin, ENT, liver, hematologic, GI (hypoalbuminemia), external auditory canal |
| 1507361 | 2.5 | M | Bone, hematologic, nodes, lung, fever | VBL + steroid 1 induction | 2 | VBL + steroid 1 induction | 14.5 | MS RO+ anemia, bone, hypoalbuminemia, fever, GI, lung |
| 1507236 | 0.58 | F | Skin, ENT, nodes, spleen, hematologic, liver | VBL + steroid 2 inductions | 1 | 3.9 | MS RO+ liver, hematologic, skin, spleen, gut | |
| 1507241 | 1.67 | M | Bone, skin, hematologic, liver, spleen, thyroid, lung, GI | VBL + steroid 1 induction | 1 | 1.0 | MS RO+ bone, skin, hematologic, liver, spleen, thyroid, lung, gut | |
| 1507473 | 2.0 | F | Bone, skin, liver, spleen | VBL + steroid | 1 | 8.9 | MS RO+ bone, skin, spleen gut, lung | |
| 1507255 | 0.58 | M | Bone, skin, spleen, hematologic, liver | VBL + steroid | 1 | 1.9 | MS RO+ spleen, hematologic, liver, skin, bone | |
| 3000050 | 0.08 | F | Hematologic, skin, gut, liver, spleen | VBL + steroid | 1 | 2.4 | MS RO+ hematologic, skin, gut, liver, spleen | |
| 3000051 | 1.16 | M | Bone, skin, hematologic, liver, lung, GI, nodes | VBL + steroid then VP16 steroid CSA | 1 | 3.7 | MS RO+ skin, hematologic, liver, lung, gut | |
| 3000052 | 0.6 | M | Skin, node | No therapy | 3 | VBL + steroid 2 inductions | 26.0 | MS RO+ liver, spleen, hematologic, lung, fever |
| 3000053 | 1.5 | F | Bone, skin, ENT (inner ear), nodes | VBL + steroid 1 induction then maintenance | 2 | Cladribine 5 courses, CD 75 mg/m2 | 12.0 | MS RO+ bone, CNS, ENT, nodes, hematologic |
| 3000054 | 0.7 | M | Skin | No therapy | 2 | VBL + steroid 1 induction | 4.1 | MS RO+ skin, fever, liver, spleen, hematologic, bone |
| 3000055 | 1.66 | F | Bone, CNS tumor, ENT, nodes, hematologic, liver, spleen | VBL + steroid 2 courses | 1 | 4.7 | MS RO+ bone, CNS tumor, ENT, nodes, hematologic, liver, spleen | |
| 3000057 | 0.66 | M | Skin, nodes, hematologic, liver, GI | VBL + steroid 1 induction | 1 | 4.6 | MS RO+ hematologic, liver, skin, GI, spleen | |
| 3000058 | 1.25 | F | Skin, liver, hematologic, nodes, spleen | VBL + steroid 1 induction | 1 | 2.7 | MS RO+ skin, liver, hematologic, nodes, spleen, lung | |
| 3000059 | 0.003 | M | Skin | Surgery | 2 | VBL + steroid 1 induction | 3.1 | MS RO+ skin, liver, spleen, hematologic, bone, lung, nodes |
| 3000069 | 1.5 | M | Bone, soft tissue, ENT | Topical mustard | 1 | VBL + steroid 1 induction maintenance | 12.1 | MS RO+ bone, soft tissue, ENT, spleen, liver, skin, hematologic |
| UPN . | Age at diagnosis, y . | Sex . | Organ involvement at diagnosis . | Initial therapy . | No. of reactivations . | Therapy of reactivation before inclusion . | Duration from diagnosis to inclusion, mo . | Disease extension at start . |
|---|---|---|---|---|---|---|---|---|
| 1506648 | 1.67 | M | Bone, skin, ENT, hematologic | VBL + steroid 2 inductions, maintenance 6 mo | 2 | VBL + steroid 1 induction | 9.7 | MS RO+ lung, hematologic, liver, spleen, bone |
| 1506863 | 0.17 | M | Bone, skin, hematologic, liver, spleen, lung, gingiva | VBL + steroid 2 inductions | 1 | 8.6 | MS RO+ bone, skin, hematologic, liver, spleen, lung, gingiva | |
| 1506802 | 3.34 | F | Bone, skin, pituitary, ENT | VBL + steroid 2 inductions | 1 | 6.4 | MS RO+ bone, skin, pituitary, ENT, liver, spleen, hematologic | |
| 1506961 | 0.5 | M | Bone, skin, ENT (external auditory canal), lung, hematologic, liver, nodes, thymus, | VBL + steroid 2 inductions | 1 | 3.4 | MS RO+ bone, lung, hematologic, liver, skin, nodes, thymus, external auditory canal | |
| 1506962 | 0.42 | M | Liver, skin, hematologic, alimentary canal | VBL + steroid 1 induction | 1 | 4.1 | MS RO+ liver, skin, hematologic, gut | |
| 1507009 | 1 | M | Bone, skin, ENT, liver, hematologic, | VBL + steroid 2 inductions | 1 | 3.2 | MS RO+ liver, hematologic, skin, bone, ENT | |
| 1506970 | 0.67 | F | Bone, skin, ENT (external auditory canal) | VBL + steroid 1 induction | 3 | VBL + steroid 1 induction | 12.9 | MS RO+ hematologic, GI, ENT, genital mucosa, liver, spleen |
| 1507092 | 0.67 | M | Bone, skin, liver, spleen, lung, nodes, hematologic, GI | VBL + steroid 1 induction + | 1 | 1.2 | MS RO+ skin, liver, spleen, lung, bone, nodes, hematologic, GI | |
| 1507202 | 0.67 | M | Bone, skin, hematologic | VBL + steroid 1 induction | 1 | 2.3 | MS RO+ skin, bone, hematologic, liver, spleen | |
| 1507020 | 1.42 | M | Bone, skin, liver, hematologic, spleen | VBL + steroid 2 inductions | 2 | VBL + steroid 1 induction | 16.5 | MS RO+ liver, spleen, lung, hematologic, GI |
| 1507220 | 0.67 | M | Bone, skin | VBL + steroid 1 induction + | 2 | VBL + steroid 1 induction | 12.9 | MS RO+ hematologic, bone, gingival mucosa, GI |
| 1507379 | 1.5 | M | Skin | Topical mustard | 2 | VBL + steroid 1 induction | 13.0 | MS RO+ bone, skin, ENT, liver, hematologic, GI (hypoalbuminemia), external auditory canal |
| 1507361 | 2.5 | M | Bone, hematologic, nodes, lung, fever | VBL + steroid 1 induction | 2 | VBL + steroid 1 induction | 14.5 | MS RO+ anemia, bone, hypoalbuminemia, fever, GI, lung |
| 1507236 | 0.58 | F | Skin, ENT, nodes, spleen, hematologic, liver | VBL + steroid 2 inductions | 1 | 3.9 | MS RO+ liver, hematologic, skin, spleen, gut | |
| 1507241 | 1.67 | M | Bone, skin, hematologic, liver, spleen, thyroid, lung, GI | VBL + steroid 1 induction | 1 | 1.0 | MS RO+ bone, skin, hematologic, liver, spleen, thyroid, lung, gut | |
| 1507473 | 2.0 | F | Bone, skin, liver, spleen | VBL + steroid | 1 | 8.9 | MS RO+ bone, skin, spleen gut, lung | |
| 1507255 | 0.58 | M | Bone, skin, spleen, hematologic, liver | VBL + steroid | 1 | 1.9 | MS RO+ spleen, hematologic, liver, skin, bone | |
| 3000050 | 0.08 | F | Hematologic, skin, gut, liver, spleen | VBL + steroid | 1 | 2.4 | MS RO+ hematologic, skin, gut, liver, spleen | |
| 3000051 | 1.16 | M | Bone, skin, hematologic, liver, lung, GI, nodes | VBL + steroid then VP16 steroid CSA | 1 | 3.7 | MS RO+ skin, hematologic, liver, lung, gut | |
| 3000052 | 0.6 | M | Skin, node | No therapy | 3 | VBL + steroid 2 inductions | 26.0 | MS RO+ liver, spleen, hematologic, lung, fever |
| 3000053 | 1.5 | F | Bone, skin, ENT (inner ear), nodes | VBL + steroid 1 induction then maintenance | 2 | Cladribine 5 courses, CD 75 mg/m2 | 12.0 | MS RO+ bone, CNS, ENT, nodes, hematologic |
| 3000054 | 0.7 | M | Skin | No therapy | 2 | VBL + steroid 1 induction | 4.1 | MS RO+ skin, fever, liver, spleen, hematologic, bone |
| 3000055 | 1.66 | F | Bone, CNS tumor, ENT, nodes, hematologic, liver, spleen | VBL + steroid 2 courses | 1 | 4.7 | MS RO+ bone, CNS tumor, ENT, nodes, hematologic, liver, spleen | |
| 3000057 | 0.66 | M | Skin, nodes, hematologic, liver, GI | VBL + steroid 1 induction | 1 | 4.6 | MS RO+ hematologic, liver, skin, GI, spleen | |
| 3000058 | 1.25 | F | Skin, liver, hematologic, nodes, spleen | VBL + steroid 1 induction | 1 | 2.7 | MS RO+ skin, liver, hematologic, nodes, spleen, lung | |
| 3000059 | 0.003 | M | Skin | Surgery | 2 | VBL + steroid 1 induction | 3.1 | MS RO+ skin, liver, spleen, hematologic, bone, lung, nodes |
| 3000069 | 1.5 | M | Bone, soft tissue, ENT | Topical mustard | 1 | VBL + steroid 1 induction maintenance | 12.1 | MS RO+ bone, soft tissue, ENT, spleen, liver, skin, hematologic |
CD, cumulative dose; CNS, central nervous system; CSA, cyclosporine A; ENT, ear, nose, and throat; F, female; GI, gastrointestinal; M, male; MS RO+, multisystem risk-organ positive; UPN, unique patient number.
Therapy
All patients received at least 2 courses of cladribine/Ara-C. One case (UPN 0057) experienced a significant change to the protocol: the first course consisted of 3 days of therapy instead of 5 days. The total number of courses was 2 in 10 patients, 3 in 12 patients, 4 in 3 patients, and 5 in 2 patients. Maintenance therapy followed the protocol in 17 cases, and the median cumulative dose of cladribine was 198 mg/m2, ranging from 90 mg/m2 to 380 mg/m2. Changes were made in the maintenance plan in the case of reactivation before the end of maintenance therapy in 5 cases and because of physician choice in 2 cases. The maintenance plan was not evaluated in 2 patients who died before maintenance therapy. For 1 patient (UPN 6883), the family declined any maintenance therapy after 2 courses of induction therapy. Detailed information about the therapy received by each patient, as well as outcome events, is provided in Table 2.
Individual patients’ therapy and outcomes (N = 27)
| UPN . | No. of cladribine + Ara-C courses . | HS status and DAS at D1 cladribine + Ara-C . | Response and DAS after 2 courses . | Toxicity . | Time to NAD, d . | TT duration, y . | Cladribine cumulative dose, mg/m2 . | Reactivation /organ at reactivation . | Therapy of reactivation . | Duration of follow-up, y . | Vital status (cause of death) . | Sequelae . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1506648 | 2 | ADW 12 | ADB 8 | Hem: WHO 4 | 136 | 1.8 | 120 | None | 9.2 | L | ND MRI exophthalmia | |
| 1506863 | 2 | ADW 20 | ADB 15 | Hem: WHO 4 | MD | 0.2 | 90 | None | 9.2 | L | ||
| 1506802 | 2 | ADW 7 | ADB 2 | Hem: WHO 4 | 78 | 1.4 | 120 | None | 8.2 | L | DI | |
| 1506961 | 3 | ADW 13 | ADB 9 | Hem: WHO 4; PNP Aspergillus fumigatus | 181 | 2.0 | 215 | None | 7.9 | L | Development delay; not LCH related | |
| 1506962 | 2 | ADW 15 | ADB 8 | Hem: WHO 4 | 144 | 1.4 | 135 | None | 7.4 | L | ||
| 1507009 | 2 | ADW 8 | ADB 2 | Hem: WHO 4, skin rash, myalgia | 1.7 | 120 | None | 6.6 | L | |||
| 1506970 | 3 | ADW 9 | ADB 3 | Hem: WHO 4 long-term: condyloma | 128 | 3.3 | 195 | Skin | VBL + steroid for 18 mo | 6.8 | L | |
| 1507092 | 3 | ADW 19 | ADB 13 | Hem: WHO 4 gut: WHO 3 septicemia candida ICU | NA | NA | 165 | Liver, lung | Cladribine | 0.8 | D Reactivation | Sclerosing cholangitis |
| 1507202 | 3 | ADW 10 | ADB 2 | Hem: WHO 4 | NA | 1.7 | 380 | Liver, skin, spleen | Cladribine Ara-C HSCT | 1.7 | D (PNP D12 HSCT) | |
| 1507020 | 2 | ADW 15 | ADB 14 | Hem: WHO 4 gut: WHO 3 | NA | 2.8 | 198 | Liver, skin, spleen | Cladribine + Ara-C HSCT then VBL + steroid | 5.1 | L | |
| 1507220 | 2 | ADW 12 | ADS 4 | Hem: WHO 4 | MD | 1.6 | 210 | None | 3.2 | L | Mandible malformation | |
| 1507379 | 2 | ADW 8 | ADB 2 | Hem: WHO 4 sepsis Staphylococcus aureus | 59 | 1.9 | 120 | None | 4.6 | L | ||
| 1507361 | 2 | ADW 6 | NAD 0 | Hem: WHO 4 gut: WHO 3 septicemia, Clostridium difficile aspergillosis, ICU | 76 | 1.9 | 135 | None | 3.1 | L | ||
| 1507236 | 3 | ADS 12 | ADB 3 | Hem: WHO 4 tubulopathy HT, mucosis (G3) | 84 | 2.0 | 215 | None | 4.9 | L | ||
| 1507241 | 3 | ADW 16 | ADB 4 | Hem: WHO 4 ICU | MD | MD | 215 | None | 4.4 | L | Thyroid insufficiency | |
| 1507473 | 3 | ADW 9 | ADB 4 | Hem: WHO 4 | 144 | 1.9 | 165 | None | 4.1 | L | Sclerosing cholangitis OLT 3.8 y | |
| 1507255 | 2 | ADW 10 | ADB 3 | Hem: WHO 4 ICU | 64 | 3.8 | 315 | Liver, skin, spleen | Cladribine, Ara-C | 4.9 | L | |
| 3000050 | 3 | ADW 11 | ADB 7 | Hem: WHO 4 gut: WHO 3 | 172 | 2.0 | 225 | None | 5.9 | L | No | |
| 3000051 | 3 | ADW 13 | ADB 7 | Hem: WHO 4 | 82 | 4.9 | 225 | None | 5.4 | L | ||
| 3000052 | 3 | ADW 14 | ADB 3 | Hem: WHO 4 | 122 | 2.1 | 190 | None | 5.5 | L | ||
| 3000053 | 3 | ADW 6 | ADB 3 | Hem: WHO 4 gut: WHO 3 | NA | NA | 135 | None | 0.2 | D VZV D21 course 3 | ||
| 3000054 | 5 | ADW 19 | ADS 18 | Hem: WHO 4 | NA | NA | 245 | None | 0.7 | D pancytopenia Viral infection | ||
| 3000055 | 3 | ADW 6 | ADB 2 | Hem: WHO 4 | 364 | 2.0 | 185 | None | 3.4 | L | ND MRI | |
| 3000057 | 5 | ADW 14 | ADB 16 | Hem: WHO 4 gut: WHO 3 septicemia, ICU | 1971 | 5.4 | 269 | Skin | Skin 6MP MTX per os | 6.9 | L | |
| 3000058 | 4 | ADW 16 | NAD 0 | Hem: WHO 4 | 65 | 1.9 | 235 | None | 6,7 | L | ||
| 3000059 | 4 | ADW 9 | ADB 2 | Hem: WHO 4 pulmonary cavities (Aspergillus ?) | MD | 2.0 | 220 | None | 3,5 | L | Short stature no GHD | |
| 3000069 | 4 | ADW 14 | ADB 3 | Hem: WHO 4 | MD | 1.9 | 210 | None | 5,1 | L | Unilateral deafness, short stature (−2.5 SD)l | |
| UPN . | No. of cladribine + Ara-C courses . | HS status and DAS at D1 cladribine + Ara-C . | Response and DAS after 2 courses . | Toxicity . | Time to NAD, d . | TT duration, y . | Cladribine cumulative dose, mg/m2 . | Reactivation /organ at reactivation . | Therapy of reactivation . | Duration of follow-up, y . | Vital status (cause of death) . | Sequelae . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1506648 | 2 | ADW 12 | ADB 8 | Hem: WHO 4 | 136 | 1.8 | 120 | None | 9.2 | L | ND MRI exophthalmia | |
| 1506863 | 2 | ADW 20 | ADB 15 | Hem: WHO 4 | MD | 0.2 | 90 | None | 9.2 | L | ||
| 1506802 | 2 | ADW 7 | ADB 2 | Hem: WHO 4 | 78 | 1.4 | 120 | None | 8.2 | L | DI | |
| 1506961 | 3 | ADW 13 | ADB 9 | Hem: WHO 4; PNP Aspergillus fumigatus | 181 | 2.0 | 215 | None | 7.9 | L | Development delay; not LCH related | |
| 1506962 | 2 | ADW 15 | ADB 8 | Hem: WHO 4 | 144 | 1.4 | 135 | None | 7.4 | L | ||
| 1507009 | 2 | ADW 8 | ADB 2 | Hem: WHO 4, skin rash, myalgia | 1.7 | 120 | None | 6.6 | L | |||
| 1506970 | 3 | ADW 9 | ADB 3 | Hem: WHO 4 long-term: condyloma | 128 | 3.3 | 195 | Skin | VBL + steroid for 18 mo | 6.8 | L | |
| 1507092 | 3 | ADW 19 | ADB 13 | Hem: WHO 4 gut: WHO 3 septicemia candida ICU | NA | NA | 165 | Liver, lung | Cladribine | 0.8 | D Reactivation | Sclerosing cholangitis |
| 1507202 | 3 | ADW 10 | ADB 2 | Hem: WHO 4 | NA | 1.7 | 380 | Liver, skin, spleen | Cladribine Ara-C HSCT | 1.7 | D (PNP D12 HSCT) | |
| 1507020 | 2 | ADW 15 | ADB 14 | Hem: WHO 4 gut: WHO 3 | NA | 2.8 | 198 | Liver, skin, spleen | Cladribine + Ara-C HSCT then VBL + steroid | 5.1 | L | |
| 1507220 | 2 | ADW 12 | ADS 4 | Hem: WHO 4 | MD | 1.6 | 210 | None | 3.2 | L | Mandible malformation | |
| 1507379 | 2 | ADW 8 | ADB 2 | Hem: WHO 4 sepsis Staphylococcus aureus | 59 | 1.9 | 120 | None | 4.6 | L | ||
| 1507361 | 2 | ADW 6 | NAD 0 | Hem: WHO 4 gut: WHO 3 septicemia, Clostridium difficile aspergillosis, ICU | 76 | 1.9 | 135 | None | 3.1 | L | ||
| 1507236 | 3 | ADS 12 | ADB 3 | Hem: WHO 4 tubulopathy HT, mucosis (G3) | 84 | 2.0 | 215 | None | 4.9 | L | ||
| 1507241 | 3 | ADW 16 | ADB 4 | Hem: WHO 4 ICU | MD | MD | 215 | None | 4.4 | L | Thyroid insufficiency | |
| 1507473 | 3 | ADW 9 | ADB 4 | Hem: WHO 4 | 144 | 1.9 | 165 | None | 4.1 | L | Sclerosing cholangitis OLT 3.8 y | |
| 1507255 | 2 | ADW 10 | ADB 3 | Hem: WHO 4 ICU | 64 | 3.8 | 315 | Liver, skin, spleen | Cladribine, Ara-C | 4.9 | L | |
| 3000050 | 3 | ADW 11 | ADB 7 | Hem: WHO 4 gut: WHO 3 | 172 | 2.0 | 225 | None | 5.9 | L | No | |
| 3000051 | 3 | ADW 13 | ADB 7 | Hem: WHO 4 | 82 | 4.9 | 225 | None | 5.4 | L | ||
| 3000052 | 3 | ADW 14 | ADB 3 | Hem: WHO 4 | 122 | 2.1 | 190 | None | 5.5 | L | ||
| 3000053 | 3 | ADW 6 | ADB 3 | Hem: WHO 4 gut: WHO 3 | NA | NA | 135 | None | 0.2 | D VZV D21 course 3 | ||
| 3000054 | 5 | ADW 19 | ADS 18 | Hem: WHO 4 | NA | NA | 245 | None | 0.7 | D pancytopenia Viral infection | ||
| 3000055 | 3 | ADW 6 | ADB 2 | Hem: WHO 4 | 364 | 2.0 | 185 | None | 3.4 | L | ND MRI | |
| 3000057 | 5 | ADW 14 | ADB 16 | Hem: WHO 4 gut: WHO 3 septicemia, ICU | 1971 | 5.4 | 269 | Skin | Skin 6MP MTX per os | 6.9 | L | |
| 3000058 | 4 | ADW 16 | NAD 0 | Hem: WHO 4 | 65 | 1.9 | 235 | None | 6,7 | L | ||
| 3000059 | 4 | ADW 9 | ADB 2 | Hem: WHO 4 pulmonary cavities (Aspergillus ?) | MD | 2.0 | 220 | None | 3,5 | L | Short stature no GHD | |
| 3000069 | 4 | ADW 14 | ADB 3 | Hem: WHO 4 | MD | 1.9 | 210 | None | 5,1 | L | Unilateral deafness, short stature (−2.5 SD)l | |
ADB, active disease, better; ADS, active disease, stable; ADW, active disease, worse; D, dead; DAS, disease activity score10 ; DI, diabetes insipidus; GHD, growth hormone deficiency; Hem, hematological; HS, hematopoeitic stem cell transplantation; HT, hypertension; ICU, intensive care unit; L, living; MD, missing data; NA, not achieved; NAD, nonactive disease; ND MRI, neuro degenerative MRI features; OLT, orthotopic liver transplantation; PNP, pneumonitis; UPN, unique patient number; VZV, varicella zoster virus; WHO, World Health Organization (scale for side effects).
Primary end point: response after 2 courses
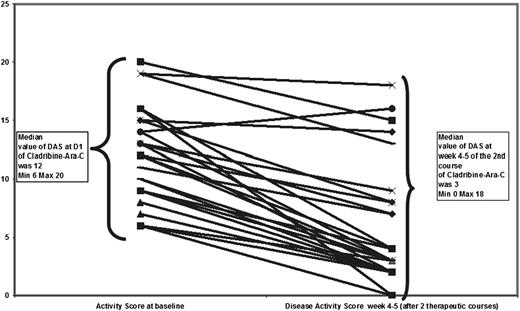
After 2 courses of therapy, 2 patients were evaluated as having “nonactive disease,” 2 as having “active disease, stable,” and 23 as having “active disease, better.” The ORR was 92%. The median DAS decreased from 12 (range, 6-20) at the start of therapy to 3 (range, 0-18; Wilcoxon test for matched pairs, P < .0001; Figure 2) 28 to 35 days after the completion of 2 courses.
The response after 2 courses of cladribine/Ara-C. At inclusion, the disease activity was active disease (AD) worse in 23 patients and AD stable in 4 patients. The disease score was similar in AD worse and AD stable patients.12 After 2 courses, disease activity was nonactive disease, AD stable in 2 patients, and AD better in 23 patients. The median score dropped from 12 at the start of therapy to 3 after 2 courses.
The response after 2 courses of cladribine/Ara-C. At inclusion, the disease activity was active disease (AD) worse in 23 patients and AD stable in 4 patients. The disease score was similar in AD worse and AD stable patients.12 After 2 courses, disease activity was nonactive disease, AD stable in 2 patients, and AD better in 23 patients. The median score dropped from 12 at the start of therapy to 3 after 2 courses.
Secondary end point: time to achieve complete remission
Twenty-three patients (85%) achieved nonactive disease status. For the 21 patients with sufficient information, nonactive disease status was achieved after a median delay of 128 days (range, 52-1971 days). Four patients did not achieve nonactive disease status. One patient could not be assessed due to a lethal viral infection on day 21 of course 3. In 1 patient, persistent pancytopenia and fever were interpreted as a disease-related event; this patient died after 5 courses of cladribine/Ara-C. Despite a clear decrease in disease activity, the condition of 2 other patients was never considered fully controlled.
Disease activity score
The DAS (supplemental Table 3) was applicable to all cases and appeared more accurate (both for describing disease status and for monitoring the outcome) than the Histiocyte Society classification, which splits the response into nonactive disease/active disease-better or -stable or -worse. The initial responses to the first 2 courses are shown in Figure 2.
Reactivation
Reactivation of the disease was observed in 6 cases with a median delay of 0.9 years. In 2 cases, the reactivation was limited to the skin, and conventional therapy with 6MP and/or VBL was efficiently resumed or prolonged. In 4 cases, the reactivation involved risk organs. One such case (UPN 7092) was treated with cladribine alone, and the patient died soon after the initiation of therapy; 3 cases were treated with cladribine/Ara-C, and disease control achieved in these cases. Of the 3 cladribine/Ara-C–treated cases, 1 patient (UPN 7255) achieved complete remission with maintenance therapy without reactivation. The other 2 patients underwent genoidentical hematopoietic stem cell transplantation (HSCT) with a reduced-intensity conditioning regimen with fludarabine. One HSCT patient (UPN 7202) died of pneumonia 12 days after the transplant. The other (UPN 7220) experienced reactivation after transplantation but achieved disease control without additional therapy and has been disease-free and treatment-free for 5 years.
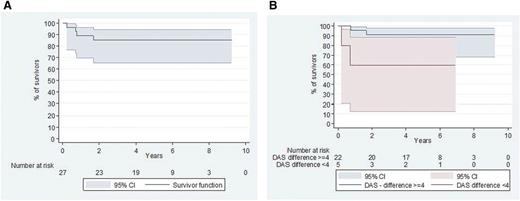
Five-year survival and causes of death
The 5-year survival rate was 85% (95% confidence interval [CI], 65.2%-94.2%) (Figure 3A). Four fatalities were observed at a median of 0.74 years (range, 0.21-1.6 years) after starting therapy. The cause of death was considered therapy-related in 2 cases: 1 after an invasive varicella zoster virus infection and 1 in the context of prolonged pancytopenia 9 months (5 therapeutic courses) after therapy was initiated. An autopsy of the latter failed to find any LCH, and the death was attributed to chemotherapy-induced pancytopenia complicated by an unknown viral infection. Two deaths were considered consequent to a lack of therapeutic efficacy: 1 patient died of pneumonitis 12 days after a transplant (which was administered in response to a severe reactivation), and the second died of severe risk-organ dysfunction soon after reactivation.
Survival since the start of the protocol (D1 cladribine/Ara-C). (A) Overall survival. (B) Survival according to DAS10 evolution. Good responders (DAS decrease >4 points) appeared to have a better outcome than poor responders (DAS decrease ≤4 points) (P < .04).
Survival since the start of the protocol (D1 cladribine/Ara-C). (A) Overall survival. (B) Survival according to DAS10 evolution. Good responders (DAS decrease >4 points) appeared to have a better outcome than poor responders (DAS decrease ≤4 points) (P < .04).
Toxicity
The principal acute toxicity was hematologic: all patients experienced profound pancytopenia (World Health Organization [WHO] grade 4) complicated by fever (>38°C). For the first course of therapy, the median duration of recovery to an absolute neutrophil count (ANC) of >500/mL was 23 days (range, 13-62 days). All patients received transfusions of packed red cells (median number of transfusions, 5.5; range, 1-17) and platelets (median number of transfusions, 6; range, 1-20). Five patients (18%) presented with WHO grade 3-4 enteritis with massive diarrhea. Documented septicemia was observed in 3 patients (11%); 3 patients (11%) were considered to have invasive pulmonary aspergillosis; and myalgia was observed in 1 patient in addition to tubulopathy. Five patients (18%) were admitted to the intensive care unit during the first 2 therapeutic courses. The median duration of the initial hospitalization (measured from the beginning of course 1 until discharge) was 65 days (range, 48-1098 days).
Sequelae
Six of the 23 surviving patients experienced sequelae to the disease that were not influenced by therapy. One patient with initial severe liver involvement later experienced severe sclerosing cholangitis complicated by liver failure and underwent a successful liver transplant 3 years and 8 months after the completion of therapy. Two patients presented with endocrinopathy: 1 with diabetes insipidus and 1 with hypothyroidism. One patient developed unilateral deafness, and 1 patient developed mandibular abnormalities related to initial bone destruction. Finally, 1 patient presented with a developmental delay.
Discussion
During the last 30 years, reports have indicated that several new drugs and procedures are useful as salvage treatment in LCH patients. However, all of these drugs have had limited impact on the survival of patients with risk-organ–positive disease that is refractory to standard therapy. These include many chemotherapeutic agents, as well as cyclosporine A,11 interferon α,12 and anti-tumor necrosis factor α (TNF α).13 Although HSCT is effective in refractory patients, its utility is limited by the rapid progression of disease and, notably, high treatment-related mortality in these very ill young patients.14,15 As a single agent, cladribine is effective in refractory LCH, albeit mainly in the absence of risk-organ involvement.16 Indeed, the LCH S 98 protocol included 83 refractory LCH cases. Among the 46 risk-organ–positive cases, the ORR did not exceed 22%, and the overall survival rate was 48%.16 Two small, retrospective studies that included 17 patients appeared to show that clofarabine is a very effective drug in refractory histiocytosis17,18 ; however, these studies encompassed various clinical situations, such as multifocal bone lesions and isolated lung involvement. Furthermore, these studies included only 7 patients with risk-organ lesions refractory to first-line therapy, 2 of whom died and 1 of whom later achieved disease control through cladribine/Ara-C treatment. The efficacy of clofarabine, another purine analog that is chemically closely related to cladribine, remains to be demonstrated in a prospective study of risk-organ refractory patients.
Our stringent eligibility criteria meant that we only treated patients with severe LCH, which historically has been characterized by a long-term survival rate of only 20% to 30%.1,5,6 Compared with previously published results in this restricted group of patients, the ORR and the survival rate for treatment with the combination of cladribine/Ara-C was significantly improved, with an ORR of 92% and a long-term (>5 years) overall survival of 85%. This phase 2 study thus demonstrated that cladribine/Ara-C is effective therapy for the highest-risk LCH patients, that is, those with refractory hematologic dysfunction. It confirmed the overall results of the pilot study of 10 patients,8 which reported a survival rate of 70%. There are very few studies of the efficacy of this therapeutic schedule, and only about 15 cases have been reported. A literature review identified only one case report in the United States,19 one survey of 5 cases without risk organs,19,20 a small survey from China (written in Chinese),21 and a retrospective survey of 9 cases in Argentina.22
An important finding in this study involves the DAS (supplemental Table 3). Due to the varied clinical manifestations of LCH, a comprehensive LCH activity scoring system was used to determine the initial severity of the disease and to objectively assess the efficacy of therapy.10 This study demonstrated that the DAS can be measured easily in a multinational setting. Adoption of a unified scoring system (such as the DAS) offers a more reliable way to compare the results of different anti-LCH therapies and may be a way to reliably apply the Histiocyte Society criteria (supplemental Tables 1-2), which are not defined precisely and which may lack precision.10 Indeed, the severity of the disease was not accurately described by the status active disease-worse or active disease-stable. For example, any disease progression (even minor progression) resulted in active disease-worse, whereas a very severely ill patient could remain “stable” with more severe disease than a patient with the status of active disease-worse. It may also help to define the concept of disease progression as an increase in DAS whereas resistance of the disease corresponds to the stability of the DAS, if this score is high before any therapy.
Despite the global efficacy of the cladribine/Ara-C combination, 2 very important limitations must be emphasized. The most important is that the treatment has substantial toxicity. This toxicity was observed in the initial report, which used the cladribine Ara-C combination to treat acute myeloid leukemia (AML) patients.23 After the first course of treatment, all patients experienced pancytopenia (median duration, 23 days). All had at least a fever, and 30% had severe infections in addition to other severe comorbidities, such as gastrointestinal toxicity. Some of the observed toxicity may be related to the extremely poor condition of these infants at the start of therapy, which is related to the disease itself. Notably, the 2 children who died in our study as a result of protocol-related toxicity had received either pretherapeutic cladribine or more than 2 courses of cladribine/Ara-C; the cumulative doses of cladribine were 250 and 225 mg/m2, respectively. Because cladribine is known to cause profound lymphopenia and potentially prolonged cumulative pancytopenia,24 we suggest that cladribine should be limited to a cumulative dose of 200 mg/m2 and treatment limited to a maximum of 3 courses of cladribine/Ara-C. In addition, this intense level of therapy should not be used at centers that do not have very good supportive care available and should be used only at centers trained in providing AML chemotherapy regimens and/or hematopoietic transplantation.
The second limitation of this therapeutic approach is the very slow response. The median delay to achieving complete control of the disease was 125 days. An important part of the learning curve in using this therapy in LCH is that unlike leukemia patients, LCH patients who responded to therapy but who have not achieved a complete response after 2 to 3 courses may achieve a complete response with the continuation of less-toxic therapy. Accordingly, we recommend that care be taken at this time to avoid potential toxicity on the one hand but also, on the other hand, to avoid prematurely deciding that the therapy is not working and possibly discontinuing treatment that might help longer-term.
These 2 limitations suggest that new therapeutic approaches are needed to help these children. Following the discovery in 2010 of the high prevalence of somatic mutations in the B-RAF oncogene in the tumoral tissues of LCH patients,25 the key role of such an activating mutation was confirmed using a conditional mouse model.26 Beyond a better understanding of the pathophysiology, the identification of a B-RAF mutation in LCH and in Erdheim-Chester disease offers the possibility of using B-RAF inhibitors as targeted therapy in adults27,28 and also in infants29 if a therapeutic trial demonstrates the benefit and the long-term safety of such therapy for young children. However, despite this promising perspective, the combination of cladribine/Ara-C can currently be considered the most effective salvage therapy for high-risk LCH.
In conclusion, here we report the results of a phase 2 study of patients with high-risk, risk-organ–positive LCH that was refractory to standard therapy. This study demonstrated that cladribine/Ara-C is an efficient regimen that resulted in an ORR of 92% and an overall survival rate of 85%. Complete response was usually achieved very slowly, and all patients experience hematologic toxicity.
Preliminary data from this study were presented orally at the 30th meeting of the Histiocyte Society, Washington, DC, 22 October 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their participation in this study, and the treating physicians and nurses who contributed to the studies, particularly Guy Leverger, Caroline Thomas, Alain Robert, Karima Yakouben, Véronique Minard, and Annelies Mavinkurve-Groothuis. This study was based on research performed at the Centre de Reference des Histiocytoses (www.histiocytose.org).
This work was supported by a grant from the French Ministry of Health (LCH-S-2005, PHRCN 2003). This project received unlimited financial support throughout the study period from the Association Histiocytose France and was also supported by a grant from the association Recherche Maladies Hématologiques de l’Enfant (RMHE).
Authorship
Contribution: J.D. and F.B. designed the study, and it was approved by the Salvage Therapy Group of the Histiocyte Society, which included J.D., M.A., S.W., J.I.H., C.V.D.B., and all members of the Salvage Group committee; J.D., F.B., M.v.N., R.M., C.P., V.G., C.A.A., N.C., E.J., A.L., J.I.H., and C.V.D.B. treated the patients; J.D., M.B., O.B., and C.V.D.B. collected and checked the data; J.D. performed statistical analyses and wrote the first draft of the manuscript; and all authors contributed to the writing and revision of the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
Bob Arceci died on June 8, 2015.
A complete list of the members of the Salvage Group of the Histiocyte Society appears in “Appendix.”
Correspondence: Jean Donadieu, Service d’Hémato-Oncologie Pédiatrique, Centre de Référence des Histiocytoses, Hopital Trousseau, Paris, France; e-mail: jean.donadieu@trs.aphp.fr.
Appendix: study group members
Salvage Group of the Histiocyte Society: Robert Arceci, Baltimore, MD; Jorge Braier, Hospital Garrahan, Buenos Aires, Argentina; Maarten Egeler, The Hospital for Sick Children, Toronto, ON, Canada; Nicole Grois, St. Anna Kinderspital, Vienna, Austria; Ken McClain, Houston, TX; Milen Minkov, St. Anna Kinderspital, Vienna, Austria; Carlos Rodriguez-Galindo, Dana-Farber Institute, Boston, MA; Kimo Stine, Little Rock, AR; Takamato, Kyoto, Japan; Stefaan Van Gool, Leuven, Belgium; Kevin Windebank, Newcastle, United Kingdom; Jim Whitlock, Sick Children Hospital, Toronto, ON, Canada.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal