Abstract
Introduction:
T-cell acute lymphoblastic leukemia (T-ALL) represents approximately 20-25% of all cases of ALL diagnoses per year. Given the rarity of T-ALL, there are limited data to guide the treatment of these patients, particularly as it relates to allogeneic stem cell transplantation (allo-SCT). Early thymic-precursor (ETP) ALL is a rare variant of T-ALL which expresses both myeloid and lymphoid markers, and has been associated with increased relapse and worse overall survival in some series. Minimal residual disease (MRD) is established as a prognostic marker for increased relapse in B-ALL, but its effect on T-ALL, particularly as it relates to allo-SCT, is less clear. We conducted a multi-center analysis of patients with T-ALL to determine the effect of T-ALL subtype and MRD on transplant outcomes to better guide decisions regarding therapy for this aggressive disease.
Methods:
Data from MD Anderson Cancer Center (MDACC), the National University Cancer Institute of Singapore, and the Oregon Health & Science University were reviewed. All patients with a diagnosis of T-ALL who received a first allo-SCT after January 1, 2000 were included in the analysis. Flow cytometry data were reviewed centrally at MDACC to determine the T-ALL subtype. ETP was defined according to Coustan-Smith et al. (Lancet Oncol 2009;10:147-56). T-ALL subtype was determined using a similar system based upon Ludwig (Leuk Lymph 1994: 13:Suppl 1: 71-76) and Gassmann et al (Br J Haem 1997:97:372-82). MRD was evaluated by flow cytometry within one month prior to allo-SCT on bone marrow biopsy, and was detected with a sensitivity of 10-3. Overall survival (OS) and progression free survival (PFS) were estimated using the Kaplan-Meier method. Disease progression, acute GVHD (aGVHD), and chronic GVHD (cGVHD) were determined utilizing the cumulative incidence (CI) method to account for competing risks. Prognostic factors for OS were assessed using Cox Proportional Hazards regression analysis on univariate and multivariate analysis.
Results:
103 patients received first allo-SCT, with a median age of 31 years (range 2-72 years), and median follow-up time of 2.2 years (3.4-7.3 years). Of these, 91 had an identifiable subtype. The 3-year OS, PFS, CI NRM and CI progression was 34%, 31%, 11%, and 58% for the whole cohort, respectively. The CI 100-day NRM was 5%, while the incidence of grade II-IV aGVHD was 48% and CI cGVHD was 29% at 3 years. There was no difference in OS or NRM amongst T-ALL subtypes, including ETP (p=0.8), (Table 1). Furthermore, there was no difference in the rate progression of ETP (HR 1.5 (0.6-3.3, p=0.4).
Amongst patients transplanted after 2007, when flow cytometry for MRD was widely utilized, 66 patients had available data: 41 MRD negative, 17 MRD positive, and 8 with no CR defined by >5% marrow blasts. On univariate analysis for OS, there was no difference according to institution, age, disease status at transplant (CR1 vs CR2+), white blood count, presence of extra-medullary disease at diagnosis, presence of CNS involvement, stem cell source, TBI vs no TBI, cytogenetic risk (SWOG classification), or complex cytogenetics. No CR at transplant (HR 3.9, p=0.003) and MRD positivity (HR 2.5, p=0.015) were associated with worse OS.
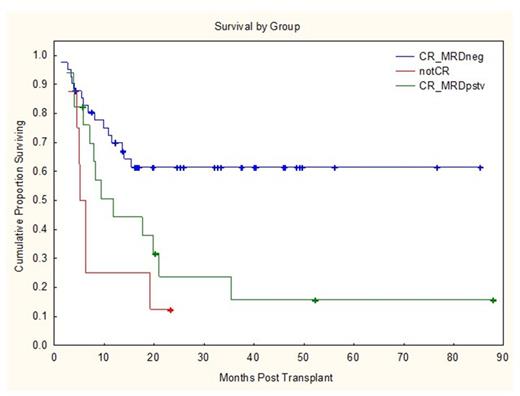
With a median follow-up time of 2 years, the OS rates for patients who were MRD negative, MRD positive, and no CR were 62%, 24%, and 12%, respectively (Figure 1), with CI of progression of 30%, 69%, and 87% respectively (Figure 2). On multivariate analysis, MRD positivity was strongly predictive of OS (HR 2.3, p=0.03) and PFS (HR 2.1, p=0.04).
Conclusions:
There is no difference in outcomes of patients with T-ALL according to disease subtype. In particular, patients with ETP-ALL who underwent allo-SCT had similar outcomes to other T-ALL subtypes, suggesting that allo-SCT may overcome this poor prognostic primary disease feature. MRD at transplant is the strongest predictor of disease progression and lower survival in patients with T-ALL, with outcomes comparable to those transplanted with active disease. Novel approaches pre-transplant and post-transplant are necessary to mitigate the poor prognostic impact of MRD in T-ALL.
Outcomes of Allogeneic Stem Cell Transplantation According to T-ALL Subtype
| Subtype . | n (%) . | OS . | HR . | p-value . | PFS . | HR . | Progression . | p-value . |
|---|---|---|---|---|---|---|---|---|
| Early | 30 (33) | 41% | Ref | 41% | Ref | Ref | ||
| Cortical | 28 (31) | 26% | 1.3 | 0.4 | 24% | 0.8 | 1.8 | 0.1 |
| Mature | 17 (19) | 31% | 1.1 | 0.8 | 23% | NE | 2.4 | 0.04 |
| ETP | 16 (17) | 29% | 0.9 | 0.8 | 22% | 0.9 | 1.5 | 0.4 |
| Subtype . | n (%) . | OS . | HR . | p-value . | PFS . | HR . | Progression . | p-value . |
|---|---|---|---|---|---|---|---|---|
| Early | 30 (33) | 41% | Ref | 41% | Ref | Ref | ||
| Cortical | 28 (31) | 26% | 1.3 | 0.4 | 24% | 0.8 | 1.8 | 0.1 |
| Mature | 17 (19) | 31% | 1.1 | 0.8 | 23% | NE | 2.4 | 0.04 |
| ETP | 16 (17) | 29% | 0.9 | 0.8 | 22% | 0.9 | 1.5 | 0.4 |
Brammer:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal