Abstract
The caudal-related homeobox gene CDX2 is ectopically expressed in 90% of human acute myeloid leukaemia (AML), but not normal haematopoietic stem and progenitor cells (HSPC). Retroviral expression of Cdx2 causes a highly penetrant, lethal AML in mouse models, while short hairpin RNA-mediated targeted knockdown of CDX2 in vitro impairs growth and clonogenic potential of AML cell lines (Scholl et al. 2007). These findings implicate Cdx2 overexpression as a clinically relevant model of myeloid leukaemogenesis, however existing studies have been limited by the requirement for ex vivo manipulation, retroviral overexpression and transplantation.
To understand the role of Cdx2 expression in de novo leukaemic transformation of HSC, we generated an inducible transgenic mouse model of Cdx2 expression linked to the mCherry fluorescent reporter. Cdx2 was specifically activated in adult HSC using the tamoxifen-inducible SclCreERT:Cdx2 model and compared with LysMCre:Cdx2 mice that selectively activated Cdx2 in the myeloid lineage, commencing at committed myeloid progenitors. SclCreERT:Cdx2 mice developed a lethal myelodysplastic phenotype with a long latency (median survival, 184 days) characterised by leukopenia, anaemia, thrombocytopenia and reduced marrow cellularity. Bone marrow histology revealed prominent megakaryocytic dysplasia. Competitive transplantation assays showed dramatic loss of long-term HSC self-renewal after tamoxifen induction of Cdx2 in the secondary recipients. Conversely, LysMCre:Cdx2 mice developed a myeloproliferative phenotype with leukocytosis, splenomegaly and extramedullary haematopoiesis. Interestingly, both cohorts exhibited marked neutrophil hypersegmentation compared to non-Cdx2 controls, a characteristic that is compatible with myelodysplasia, and suggests that Cdx2 not only controls HSC development and differentiation, but is required for granulocyte maturation. In contrast to retroviral models, AML was never observed with Cdx2 expression alone.
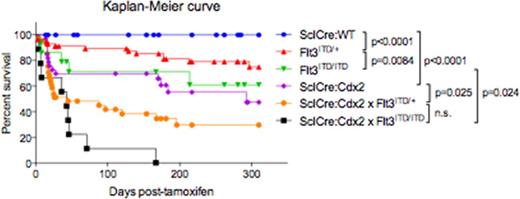
FLT3-internal tandem duplication (ITD) represents a tyrosine kinase mutation that is present in 35% of AML patients, making it one of the most common genetic alterations found in AML. Transgenic Flt3ITD mice develop myelodysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN) with long latency (approx. 10-15 months) but do not develop AML (Lee et al. 2007), suggesting that additional mutations are required for complete leukaemogenesis. Preliminary data has shown a correlation between CDX2 and FLT3 expression in AML. We therefore crossed SclCreERT:Cdx2 with Flt3ITD/+ and Flt3ITD/ITD mice. SclCreERT:Cdx2xFlt3ITD/+ mice developed a rapidly progressive lethal MDS with marked anaemia and thrombocytopenia, splenomegaly, morphological dysplasia and a shorter latency (median survival, 45 days) with a low incidence of transformation to AML. Strikingly, SclCreERT:Cdx2xFlt3ITD/ITD mice developed a fully penetrant AML characterised by marked leukocytosis, splenomegaly and hepatomegaly, and >20% circulating blast cells, demonstrating that Cdx2 cooperates with Flt3ITD to induce AML in a Flt3ITD gene dosage-dependent manner (Fig 1). Transplantation experiments of these samples into lethally irradiated, syngeneic wildtype (WT) recipients revealed that cells from SclCreERT:Cdx2xFlt3ITD/+ or SclCreERT:Cdx2xFlt3ITD/ITD but not WT, Flt3ITD/+ or Flt3ITD/ITD resulted in direct phenocopy of the primary disease with a short latency lethality, demonstrating the disease is intrinsic to transformed HSPC populations.
Mechanistically, SclCreERT:Cdx2xFlt3ITD/+ and SclCreERT:Cdx2xFlt3ITD/ITD samples showed marked depletion of long-term HSC, abnormal cell cycle regulation and widespread gene expression changes leading to the activation of a leukaemia stem cell (LSC) program, including the aberrant expression of several Hox genes.
Altogether, this work demonstrates that conditional Cdx2 expression has a critical role in the transformation of HSPC populations to AML LSC. This is a novel, inducible model of de novo leukaemic transformation and reflects common genetic aberrations seen in human AML. Thus, this model may help to identify tractable susceptibilities to target LSC populations and improve clinical outcomes.
Survival curves for SclCreERT:WT, Flt3ITD/+, Flt3ITD/ITD, SclCreERT:Cdx2, SclCreERT:Cdx2xFlt3ITD/+ and SclCreERT:Cdx2xFlt3ITD/ITD mice.
Survival curves for SclCreERT:WT, Flt3ITD/+, Flt3ITD/ITD, SclCreERT:Cdx2, SclCreERT:Cdx2xFlt3ITD/+ and SclCreERT:Cdx2xFlt3ITD/ITD mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal