Key Points
Staphylococcal enterotoxins activate oncogenic pathways in CTCL.
This discovery implies a novel role of microbes as drivers of disease progression.
Abstract
Cutaneous T-cell lymphoma (CTCL) is characterized by proliferation of malignant T cells in a chronic inflammatory environment. With disease progression, bacteria colonize the compromised skin barrier and half of CTCL patients die of infection rather than from direct organ involvement by the malignancy. Clinical data indicate that bacteria play a direct role in disease progression, but little is known about the mechanisms involved. Here, we demonstrate that bacterial isolates containing staphylococcal enterotoxin A (SEA) from the affected skin of CTCL patients, as well as recombinant SEA, stimulate activation of signal transducer and activator of transcription 3 (STAT3) and upregulation of interleukin (IL)-17 in immortalized and primary patient–derived malignant and nonmalignant T cells. Importantly, SEA induces STAT3 activation and IL-17 expression in malignant T cells when cocultured with nonmalignant T cells, indicating an indirect mode of action. In accordance, malignant T cells expressing an SEA-nonresponsive T-cell receptor variable region β chain are nonresponsive to SEA in monoculture but display strong STAT3 activation and IL-17 expression in cocultures with SEA-responsive nonmalignant T cells. The response is induced via IL-2 receptor common γ chain cytokines and a Janus kinase 3 (JAK3)-dependent pathway in malignant T cells, and blocked by tofacitinib, a clinical-grade JAK3 inhibitor. In conclusion, we demonstrate that SEA induces cell cross talk–dependent activation of STAT3 and expression of IL-17 in malignant T cells, suggesting a mechanism whereby SEA-producing bacteria promote activation of an established oncogenic pathway previously implicated in carcinogenesis.
Introduction
Cutaneous T-cell lymphoma (CTCL) comprises a group of heterogeneous lymphoproliferative disorders defined by the expansion of malignant skin-homing T cells in a chronic inflammatory environment. Mycosis fungoides and Sézary syndrome represent the most prevalent forms of CTCL.1,2 Despite intensive research, the CTCL etiology remains elusive and the pathogenesis is far from understood. Chromosomal instability, abnormal gene expression, gene duplication, and epigenetic deregulation have been implicated in CTCL, but no single underlying genetic or epigenetic event has yet been identified as a likely cause of the disease.3-9 Persistent activation of signal transducer and activator of transcription 3 (STAT3)10 has repeatedly been implicated in CTCL pathogenesis as a potent driver of survival and proliferation of malignant T cells.11-17 Importantly, STAT3 promotes malignant expression of the proinflammatory cytokine interleukin (IL)-17, including a range of cytokines associated with skin inflammation, immune deregulation, and disease progression.18-23
It is well established that STAT3 is tyrosine phosphorylated in vivo in CTCL skin lesions and in peripheral blood Sézary cells. The level of tyrosine phosphorylation in STAT3 increases in advanced disease.13,24 Activating mutations are sufficient to turn STAT3 into a full oncogene in experimental animals,10 and activating mutations in Janus kinases (JAKs) have been described in other hematologic malignancies.25-27 Recently, activating mutations have also been described in a subset (12.5%) of CTCL patients,28,29 but it remains unknown what drives aberrant activation of JAK/STAT signaling in the majority of patients. STAT3 activation may become further increased after loss of regulatory control by suppressor of cytokines signaling 3, by protein inhibitor of activated STAT3, and by other tyrosine protein phosphatases.19,30 However, presently, it remains unclear what drives the dramatic increase and chronic activation of STAT3 in advanced CTCL.
Although the etiology of this malignancy remains unclear, recent studies report on a significant geographical and occupational clustering of patient cohorts.31-36 Thus, cross-analysis of cancer databases in Texas identified several geographic clusters with a fivefold to 20-fold increased CTCL incidence.37 A potential etiologic agent is unknown, but the environmental factors appear to play an essential role in CTCL pathogenesis.36,37 For decades, microbes have been suspected to play a key role in CTCL, both as etiologic agents and as drivers of life-threatening complications.22,38-42 So far, firm evidence for a microbial etiology in CTCL is lacking,43,44 but clinical data indicate that bacteria may play an important role in progression and mortality in advanced disease.39,40,45 Whereas Staphylococcus aureus is a common commensal organism in healthy individuals, it is a major source of morbidity in CTCL because it causes persistent skin and life-threatening systemic infections39,42,46,47 seen in 44% to 76% of patients with advanced CTCL.40,45,48
Staphylococcal enterotoxins (SEs), including the A type (SEA), are bacterial superantigens that circumvent normal antigen processing and recognition. SEs bind directly to major histocompatibility complex class II molecules and cross-link T-cell receptors (TCRs) by binding to their TCR variable region β chains (TCR-Vbs) with very high affinity, which results in broad T-cell hyperactivation. Because SEs are restricted only by the TCR-Vbs of the TCR complex, they can activate up to 20% of all naïve T cells.49 The importance of SEs is emphasized by reports indicating that antibiotic therapy of staphylococcal infections in CTCL is associated with clinical improvement and, in some cases, remission of the lymphoma.40,45,50 However, the mechanisms involved in disease aggravation and progression mediated by S aureus and SEs are poorly understood.
In this study, we report that SEA induces STAT3 activation and IL-17 expression in malignant T cells via engagement of nonmalignant CD4 T cells. Our findings suggest that bacterial toxins play a central role in the activation of a key oncogenic pathway in CTCL.
Materials and methods
Antibodies and reagents
Enzyme-linked immunosorbent assay (ELISA) kits and IL-2-, IL-7-, and IL-15-blocking antibodies were purchased from R&D Systems (Minneapolis, MN). JAK3 and Erk1/2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), whereas STAT3 antibody was purchased from Cell Signaling Technology (Beverly, MA). Fluorochrome-conjugated CD3, CD4, CD25, CD26; major histocompatibility complex class II; and pY(705)-STAT3 and the respective fluorochrome-conjugated isotype control antibodies used for fluorescence-activated cell sorting (FACS) were provided by R&D Systems, BioLegend (San Diego, CA), BD Biosciences (San Jose, CA), and Leinco Technologies (St Louis, MO), respectively. Other reagents were obtained as follows: TCR-Vb kit from Ramcon (Bregnerød, Denmark), JAK3 inhibitor tofacitinib (CP-690550) from Selleck Chemicals (Houston, TX), small interfering RNA (siRNA) against JAK3 and STAT3 from ThermoFisher Scientific (Waltham, MA), and SEs from Toxin Technology (Sarasota, FL). SEA mutants were generously provided by Active Biotech (Lund, Sweden).
Patients and isolation of S aureus bacteria
Malignant and nonmalignant T cells were isolated from the blood of patients diagnosed with Sézary syndrome in accordance with the World Health Organization/European Organization for Research and Treatment of Cancer classification.1 Malignant Sézary syndrome T cells typically lack the expression of cell surface markers CD26 and/or CD7 and often display reduced expression of CD4 when compared with nonmalignant T cells.1,51,52 Accordingly, T cells were identified as malignant (CD4low/+/CD7−, CD4low/+/CD26−) and nonmalignant (CD4+/CD7+, CD4+/CD26+). Bacterial isolates were collected from CTCL patients using swabs wetted with 0.1% Triton X-100 in 0.075 M phosphate buffer, transferred to Stuart transport medium, and cultivated on blood agar overnight at 37°C at 5% carbon dioxide. In accordance with the Declaration of Helsinki, the samples were obtained with informed consent after approval by the Committee on Health Research Ethics.
Cell lines
The malignant T-cell line, SeAx, and the nonmalignant T-cell line, MF1850, were established from patients diagnosed with CTCL53 and cultured in media supplemented with 10% human serum (HS medium) and IL-2 as described elsewhere.54 Prior to experimental setup, the CTCL cell lines were starved overnight in HS medium without IL-2.
ELISA
The concentrations of IL-17A in cell culture supernatants were measured using the Human IL-17A DuoSet ELISA Development Kit from R&D Systems in accordance with the manufacturer’s instructions.
Detection of SEs in bacterial isolate supernatants
The presence of SEs in bacterial cultures was examined using the RIDASCREEN SET A, B, C, D, E kit (R-Biopharm, Darmstadt, Germany), with a toxin detection limit of 0.25 ng/mL and in accordance with the manufacturer’s instructions.
RNA purification, complementary DNA synthesis, and qPCR
Total cellular RNA was purified and reverse transcribed into complementary DNA as previously described.55 Quantitative polymerase chain reaction (qPCR) was performed using the TaqMan assay from ThermoFisher Scientific in accordance with the manufacturer’s instructions, and the samples were analyzed on an Mx3005P instrument (Stratagene).
Cell isolation, flow cytometry, and cell sorting
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of Sézary syndrome patients by Lymphoprep density-gradient centrifugation (Axis-Shield, Oslo, Norway) and (1) used directly for flow cytometric analysis,56 (2) cultured in HS medium with phosphate-buffered saline (PBS) or SEA, or (3) sorted by FACS using FACSAria (BD Biosciences) into populations of malignant and nonmalignant T cells based on CD4 and CD26 surface expression and then mono- or cocultured in HS medium with PBS or SEA. Purity of the sorted malignant and nonmalignant T cells was >99% and >95%, respectively. In experiments in which cocultured SeAx and MF1850 cells were sorted, the SeAx cells were stained prior culture with 1 μM carboxyfluorescein succinimidyl ester (CFSE) as previously described.24 The CFSE-positive (SeAx) and CFSE-negative (MF1850) cells were sorted by FACSAria, resulting in a purity >98%. Data acquisition and flow cytometric analysis were done on Fortessa flow cytometers (BD Biosciences) using FlowJo software (Tree Star, Ashland, OR).
Transient transfections
A total of 2 × 106 cells per sample were transfected with siRNA against JAK3, STAT3, or nontargeting control (ON-TARGETplus SMARTpool; Thermo Scientific, Lafayette, CO). Pellets were resuspended in 100 μL of transfection solution (Ingenio Electroporation Solution; Mirus Bio, Madison, WI) in the presence of 0.25 μM of the respective siRNAs and transfected with an Amaxa Nucleofector (Lonza, Cologne, Germany).
Statistics
For statistical analysis, a 2-tailed Student t test with a significance level of P = .05 was used, with P < .05 indicating a significant difference between a sample and control.
Results
SE-containing bacterial isolates from CTCL skin trigger expression of IL-17 by malignant cells
It has been a matter of controversy whether malignant T cells express IL-17 in CTCL. Thus, some studies have reported on IL-17A and/or IL-17F expression by malignant T cells in lesional skin or blood,21,55,57-59 whereas others did not find IL-17 family cytokines despite the presence of IL-22-producing T helper (Th)17 cells.60 Because IL-17 is typically produced by CD4 T cells in response to bacteria such as S aureus (reviewed in Lee et al61 ) and because SE-producing S aureus often colonizes lesional skin, we hypothesized that SE can trigger IL-17 expression in CTCL. Accordingly, we tested whether bacterial isolates from lesional skin induced IL-17 production in cocultures of malignant and nonmalignant T cells. We analyzed for the presence of common enterotoxins in 46 bacterial isolates from CTCL skin (N = 6) and found that SEA was present in 21 out of 46 isolates, whereas SEB, SEC2, SED, and TSST-1 were not detected, therefore, confirming previous findings by others that lesional skin is often colonized by SEA-producing staphylococci.40
Next, we performed cocultures of malignant and nonmalignant T-cell lines stimulated with SEA-positive and -negative bacterial isolates from CTCL skin. As shown in Figure 1A, SEA-containing isolates stimulated vigorous production of IL-17A protein (average, 1515 pg/mL; range, 485-3865 pg/mL; Figure 1A), whereas SEA-negative bacterial isolates did not (average, 195 pg/mL; range, 100-250 pg/mL; Figure 1A). To address whether malignant and/or nonmalignant T cells produced IL-17, we stimulated cocultures and separate cultures of malignant and nonmalignant T cells in the presence or absence of SEA-containing isolates prior to analysis of IL-17 protein in culture supernatants. As shown in Figure 1B, SEA-positive isolates induced a strong IL-17 response in cocultures of malignant and nonmalignant T-cell lines (Figure 1B), whereas IL-17 production was not observed in separate cultures of malignant and nonmalignant T cells (Figure 1B). The SEA-negative isolates induced only weak IL-17 response. Considering that SEA was by far the most prevalent SE in bacterial isolates from our patients, we tested whether recombinant SEA can also induce IL-17 production in cocultures of malignant and nonmalignant T-cells. Indeed, as shown in Figure 1C, recombinant SEA produced almost identical results as presented in Figure 1B. Notably, 2 nonstimulatory SEA-mutants (SEAD227A and SEAF47A/D227A)62 and SEB, SEC, SED, and TSST-1 did not elicit significant IL-17 production (Figure 1D), indicating that the IL-17 response was highly specific for intact SEA. The JAK3/STAT3 pathway drives IL-17 expression in malignant T cells,21 and as shown in Figure 1E, the clinical-grade JAK3 inhibitor tofacitinib profoundly (>70%) inhibited SEA-induced IL-17 production in cocultures of malignant and nonmalignant T cells.
Bacterial isolates from CTCL patients contain SEs. (A) Mixed bacterial isolates from patients were tested for SE expression (SEA, SEB, SEC, SED, and TSST-1) and categorized accordingly as either positive or negative. Cocultures with malignant T cells (SeAx) and nonmalignant T cells (MF1850) were then stimulated with SE-positive or SE-negative isolates and incubated for 24 hours. IL-17 concentration in the supernatants was determined by ELISA. (B) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured in the absence (Media) or presence (Isolate) of a mixed bacterial isolate from a CTCL patient. IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (C-D) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or recombinant SEA (50 ng/mL) (C), or with PBS, SEAwt, or the toxins SEAD227A, SEAF47A/D227A, SEB, SEC2, SED, SEE, SEI, or TSST-1 (50 ng/mL) (D). IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (E) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with SEA (50 ng/mL) and tofacitinib (0.3 μM) or vehicle (dimethylsulfoxide [DMSO]) for 24 hours. After incubation, IL-17A protein concentration was determined by ELISA. Error bars represent standard error of the mean of 3 independent experiments. *P < .05. Malign., malignant; Neg, negative; Non-malign., nonmalignant; Pos, positive.
Bacterial isolates from CTCL patients contain SEs. (A) Mixed bacterial isolates from patients were tested for SE expression (SEA, SEB, SEC, SED, and TSST-1) and categorized accordingly as either positive or negative. Cocultures with malignant T cells (SeAx) and nonmalignant T cells (MF1850) were then stimulated with SE-positive or SE-negative isolates and incubated for 24 hours. IL-17 concentration in the supernatants was determined by ELISA. (B) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured in the absence (Media) or presence (Isolate) of a mixed bacterial isolate from a CTCL patient. IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (C-D) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or recombinant SEA (50 ng/mL) (C), or with PBS, SEAwt, or the toxins SEAD227A, SEAF47A/D227A, SEB, SEC2, SED, SEE, SEI, or TSST-1 (50 ng/mL) (D). IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (E) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with SEA (50 ng/mL) and tofacitinib (0.3 μM) or vehicle (dimethylsulfoxide [DMSO]) for 24 hours. After incubation, IL-17A protein concentration was determined by ELISA. Error bars represent standard error of the mean of 3 independent experiments. *P < .05. Malign., malignant; Neg, negative; Non-malign., nonmalignant; Pos, positive.
SEA induces STAT3 activation in cocultures
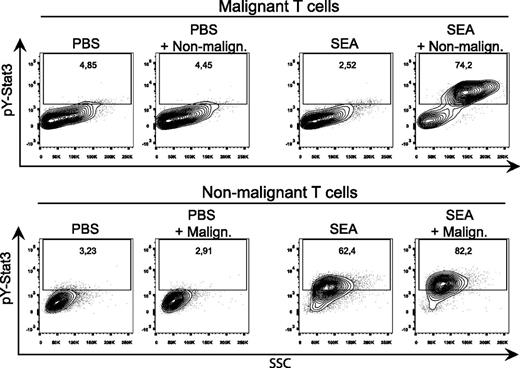
As shown in Figure 2, SEA induced a strong upregulation and phosphorylation (pY705) of STAT3 in both malignant and nonmalignant T cells after coculture when compared with cocultures stimulated with a vehicle control (PBS). STAT3 phosphorylation was also increased in nonmalignant T cells, but not in malignant T cells, after monoculture with SEA when compared with vehicle control.
SEs activate and phosphorylate STAT3 in both malignant and nonmalignant T cells. (A) Representative flow cytometric analysis of CFSE-stained malignant (SeAx) and nonmalignant (MF1850) T-cell lines mono- and cocultured with either vehicle (PBS) or recombinant SEA (50 ng/mL) for 24 hours. All samples were stained for pY(705)-STAT3. “PBS + Malign.” signifies gated nonmalignant T cells cocultured with malignant T cells, and vice versa for “SEA + Non-malign.”
SEs activate and phosphorylate STAT3 in both malignant and nonmalignant T cells. (A) Representative flow cytometric analysis of CFSE-stained malignant (SeAx) and nonmalignant (MF1850) T-cell lines mono- and cocultured with either vehicle (PBS) or recombinant SEA (50 ng/mL) for 24 hours. All samples were stained for pY(705)-STAT3. “PBS + Malign.” signifies gated nonmalignant T cells cocultured with malignant T cells, and vice versa for “SEA + Non-malign.”
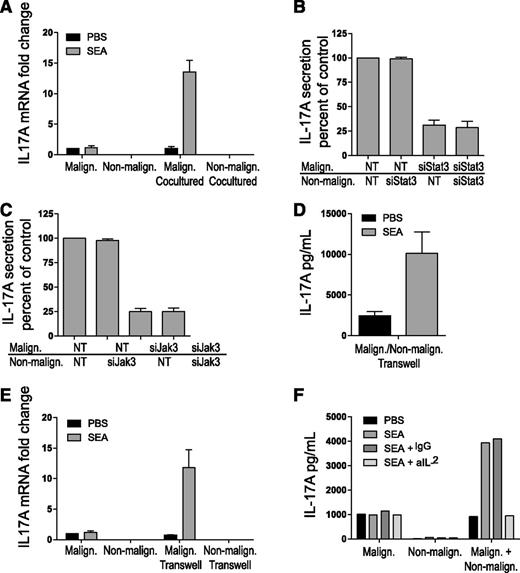
To address whether IL-17 in cocultures originated from malignant cells, nonmalignant cells, or both cell types, we separated the malignant and nonmalignant T cells by FACS after coculture in the presence or absence of SEA as above and measured IL-17. As shown in Figure 3A, SEA induced high expression of IL-17 mRNA in malignant T cells after coculture with nonmalignant T cells when compared with vehicle control. In contrast, SEA did not induce significant IL-17 mRNA expression in nonmalignant T cells after coculture with malignant T cells. Likewise, SEA did not induce IL-17 mRNA expression in monocultures of malignant and nonmalignant T cells. As shown in Figure 3B, siRNA-mediated depletion of STAT3 in malignant T cells profoundly inhibited IL-17 production in cocultures of malignant and nonmalignant T cells (Figure 3B, third column) when compared with the effect of nontargeting siRNA controls (Figure 3B, first column). In contrast, STAT3 knockdown in nonmalignant T cells had no effect on IL-17 production (Figure 3B, second column), and STAT3 depletion in both malignant and nonmalignant T cells had no additional effect when compared with siRNA-mediated depletion of STAT3 in malignant T cells alone (Figure 3B, third vs fourth column). In parallel, malignant and nonmalignant T cells were treated with JAK3 siRNA or a nontargeting control prior to coculture in the presence or absence of SEA as above. JAK3 depletion in malignant T cells strongly inhibited IL-17 production in cocultures (Figure 3C), whereas JAK3 depletion in nonmalignant T cells had no effect, indicating that SEA drives IL-17 expression through a JAK3/STAT3-dependent pathway in malignant T cells cocultured with nonmalignant T cells.
Enterotoxin induces IL-17 production in cocultured malignant T cells. (A) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were either mono- or cocultured with vehicle (PBS) or SEA (50 ng/mL) for 16 hours. The cocultured malignant and nonmalignant T cells were sorted by FACS, and the relative levels of IL-17A and GAPDH mRNA were determined in all samples by qPCR. In each sample, the level of IL-17A mRNA was normalized to that of GAPDH mRNA and depicted as fold change compared with monocultured malignant T cells with PBS. “Malign. Cocultured” signifies IL-17A expression in malignant T cells cocultured with nonmalignant T cells, and vice versa for “Non-malign. Cocultured.” (B-C) Malignant (SeAx) and nonmalignant (MF1850) T cells were transiently transfected with nontargeting (NT) or STAT3 specific siRNA (B) or JAK3-specific siRNA (C) and monocultured for 24 hours. Then, the transfected cells were washed and cocultured in the presence of SEA (50 ng/mL) for another 24 hours before the concentrations of IL-17A in cell culture supernatants were determined by ELISA. Data are presented as percentage of IL-17A secretion relative to cocultures of malignant and nonmalignant T cells transfected with NT siRNA. (D) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were cocultured separated by Transwells with vehicle (PBS) or SEA (50 ng/mL) for 24 hours. IL-17 concentrations in the supernatants were determined by ELISA. (E) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were either monocultured with Transwells or cocultured separated by Transwells for 24 hours. The relative levels of IL-17A and GAPDH mRNA were determined in all samples by qPCR. In each sample, the level of IL-17A mRNA was normalized to that of GAPDH mRNA and depicted as fold change compared with monocultured malignant T cells with PBS. “Malign. Transwell” signifies IL-17A expression in malignant T cells cocultured with nonmalignant T cells separated by a Transwell, and vice versa for “Non-malign. Transwell.” (F) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS), SEA, SEA plus immunoglobulin G (IgG) isotype control, or SEA plus neutralizing IL-2 antibody (aIL-2). IL-17 concentrations in the supernatants were determined by ELISA. Error bars represent standard error of the mean of 3 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA.
Enterotoxin induces IL-17 production in cocultured malignant T cells. (A) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were either mono- or cocultured with vehicle (PBS) or SEA (50 ng/mL) for 16 hours. The cocultured malignant and nonmalignant T cells were sorted by FACS, and the relative levels of IL-17A and GAPDH mRNA were determined in all samples by qPCR. In each sample, the level of IL-17A mRNA was normalized to that of GAPDH mRNA and depicted as fold change compared with monocultured malignant T cells with PBS. “Malign. Cocultured” signifies IL-17A expression in malignant T cells cocultured with nonmalignant T cells, and vice versa for “Non-malign. Cocultured.” (B-C) Malignant (SeAx) and nonmalignant (MF1850) T cells were transiently transfected with nontargeting (NT) or STAT3 specific siRNA (B) or JAK3-specific siRNA (C) and monocultured for 24 hours. Then, the transfected cells were washed and cocultured in the presence of SEA (50 ng/mL) for another 24 hours before the concentrations of IL-17A in cell culture supernatants were determined by ELISA. Data are presented as percentage of IL-17A secretion relative to cocultures of malignant and nonmalignant T cells transfected with NT siRNA. (D) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were cocultured separated by Transwells with vehicle (PBS) or SEA (50 ng/mL) for 24 hours. IL-17 concentrations in the supernatants were determined by ELISA. (E) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were either monocultured with Transwells or cocultured separated by Transwells for 24 hours. The relative levels of IL-17A and GAPDH mRNA were determined in all samples by qPCR. In each sample, the level of IL-17A mRNA was normalized to that of GAPDH mRNA and depicted as fold change compared with monocultured malignant T cells with PBS. “Malign. Transwell” signifies IL-17A expression in malignant T cells cocultured with nonmalignant T cells separated by a Transwell, and vice versa for “Non-malign. Transwell.” (F) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS), SEA, SEA plus immunoglobulin G (IgG) isotype control, or SEA plus neutralizing IL-2 antibody (aIL-2). IL-17 concentrations in the supernatants were determined by ELISA. Error bars represent standard error of the mean of 3 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA.
To address whether the cell cross talk–dependent induction of IL-17 requires cell-to-cell contact or was mediated through soluble factors, malignant and nonmalignant T cells were cocultured as above but separated by a cytokine-permeable membrane in Transwell plates. SEA induced high levels of IL-17 protein in supernatants isolated from malignant and nonmalignant T cells cocultured in Transwell plates (Figure 3D). Likewise, SEA induced a significant increase in IL-17 mRNA expression in malignant T cells, but not in nonmalignant T cells, after coculture in Transwell plates (Figure 3E). Because IL-2 induces IL-17 expression in malignant T cells21 and because SEA induces IL-2 expression in nonmalignant T cells,22 cocultures were performed with and without SEA and IL-2-blocking and control antibodies. As shown in Figure 3F, inhibition of IL-2 almost completely blocked IL-17 production in cocultures, indicating the key role of IL-2 in SEA-mediated cross talk between malignant and nonmalignant T-cell lines.
STAT3 activation and IL-17 expression in primary T cells from CTCL patients
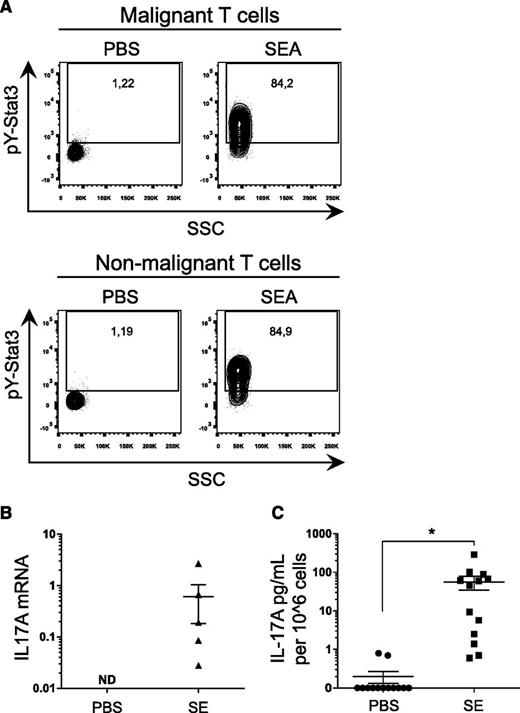
To address whether SEA also triggered STAT3 activation and IL-17 expression in primary T cells derived from CTCL patients, PBMCs were cultured in the presence or absence of SEA prior to FACS analysis of STAT3 activation in malignant (CD4+/CD26−) and nonmalignant (CD4+/CD26+) T-cell populations. As observed from pY(705)-STAT3 staining, SEA induced a profound activation of STAT3 in both malignant (CD4+/CD26−) and nonmalignant (CD4+/CD26+) T cells (Figure 4A). Analysis of IL-17 expression showed induction of both mRNA (Figure 4B) and protein (Figure 4C), demonstrating significant IL-17A upregulation by SE in 5 of 6 patients tested.
SE treatment leads to STAT3 phosphorylation and subsequent IL-17 secretion in primary T cells from CTCL patients. (A) Representative flow cytometric analysis of PBMCs freshly purified from a CTCL patient and cultured for 24 hours with SEA (200 ng/mL) or vehicle (PBS). After incubation, cells were stained for pY-STAT3 and CD3, CD4, and CD26. Nonmalignant T cells stain CD3+, CD4+, and CD26+, whereas malignant T cells stain CD3+, CD4+, and CD26−. (B) PBMCs from CTCL patients were stimulated with an SE cocktail of SEA, SEB, SEC2, SEE, SEI, and TSST-1 (200 ng/mL) or vehicle (PBS) for 24 hours. After incubation, IL17A expression and GAPDH expression were determined by qPCR. In each sample, IL17A expression is normalized to GAPDH. (C) Pooled data of PBMCs from CTCL patients stimulated for 24 hours with an SE cocktail of SEA, SEB, SEC2, SEE, SEI, and TSST-1 (200 ng/mL) or vehicle (PBS). IL-17A concentrations were determined by ELISA and normalized to 106 cells. *P < .05. ND, not detected.
SE treatment leads to STAT3 phosphorylation and subsequent IL-17 secretion in primary T cells from CTCL patients. (A) Representative flow cytometric analysis of PBMCs freshly purified from a CTCL patient and cultured for 24 hours with SEA (200 ng/mL) or vehicle (PBS). After incubation, cells were stained for pY-STAT3 and CD3, CD4, and CD26. Nonmalignant T cells stain CD3+, CD4+, and CD26+, whereas malignant T cells stain CD3+, CD4+, and CD26−. (B) PBMCs from CTCL patients were stimulated with an SE cocktail of SEA, SEB, SEC2, SEE, SEI, and TSST-1 (200 ng/mL) or vehicle (PBS) for 24 hours. After incubation, IL17A expression and GAPDH expression were determined by qPCR. In each sample, IL17A expression is normalized to GAPDH. (C) Pooled data of PBMCs from CTCL patients stimulated for 24 hours with an SE cocktail of SEA, SEB, SEC2, SEE, SEI, and TSST-1 (200 ng/mL) or vehicle (PBS). IL-17A concentrations were determined by ELISA and normalized to 106 cells. *P < .05. ND, not detected.
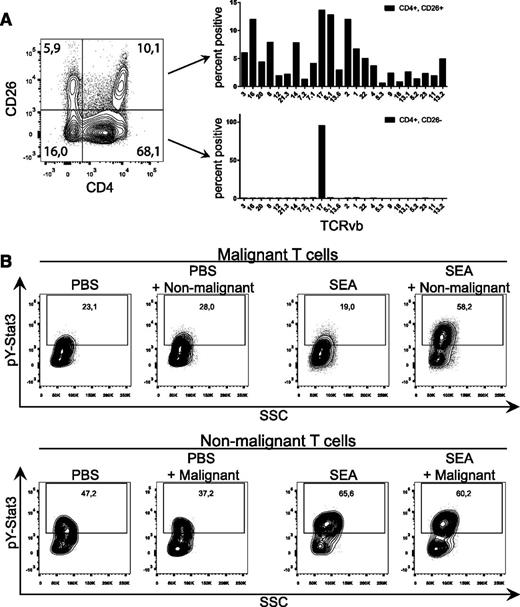
To further investigate SEA-mediated activation of primary malignant T cells, we performed TCR-Vb staining of malignant (CD4+/CD26−) and nonmalignant (CD4+/CD26+) T-cell compartments. As shown in a representative image in Figure 5A, CD4+/CD26− T cells expressed only the TCR-Vb17, whereas CD4+/CD26+ T cells displayed a typical gaussian distribution of TCR-Vbs, indicating that the CD4+/CD26− compartment consisted of only 1 malignant T-cell clone, whereas the CD4+/CD26+ compartment contained a nonmalignant T-cell population with a normal TCR-Vb distribution.
SEs induce STAT3 phosphorylation in primary malignant T cells cultured with nonmalignant T cells. (A) Representative flow cytometric analysis of freshly purified PBMCs from a CTCL patient stained with CD3, CD4, CD26, and a TCR-Vb panel. Bar plots demonstrate TCR-Vb repertoire of the malignant (CD3+, CD4+, CD26−) T-cell compartment and the nonmalignant (CD3+, CD4+, CD26+) compartment. (B) CD4+/CD26− (malignant T cells) and CD4+/CD26+ (normal T cells) were separated by FACS from freshly purified PBMCs from a CTCL patient. CD4+/CD26− and CD4+/CD26+ T cells were mono- and cocultured with either vehicle (PBS) or SEA (200 ng/mL) for 24 hours. After incubation, cells were stained for pY-STAT3. Intensity of pY-STAT3 staining is shown in the contour plot. “PBS + Non-malignant” signifies gated malignant T cells cocultured with nonmalignant T cells and stimulated with vehicle, and vice versa for “SEA + Malignant.”
SEs induce STAT3 phosphorylation in primary malignant T cells cultured with nonmalignant T cells. (A) Representative flow cytometric analysis of freshly purified PBMCs from a CTCL patient stained with CD3, CD4, CD26, and a TCR-Vb panel. Bar plots demonstrate TCR-Vb repertoire of the malignant (CD3+, CD4+, CD26−) T-cell compartment and the nonmalignant (CD3+, CD4+, CD26+) compartment. (B) CD4+/CD26− (malignant T cells) and CD4+/CD26+ (normal T cells) were separated by FACS from freshly purified PBMCs from a CTCL patient. CD4+/CD26− and CD4+/CD26+ T cells were mono- and cocultured with either vehicle (PBS) or SEA (200 ng/mL) for 24 hours. After incubation, cells were stained for pY-STAT3. Intensity of pY-STAT3 staining is shown in the contour plot. “PBS + Non-malignant” signifies gated malignant T cells cocultured with nonmalignant T cells and stimulated with vehicle, and vice versa for “SEA + Malignant.”
Using FACS, we separated CD4+/CD26− and CD4+/CD26+ T cells and performed mono- and cocultures with or without SEA prior to analysis of STAT3 phosphorylation. As shown in Figure 5B, both malignant and nonmalignant T cells displayed a considerable baseline STAT3 phosphorylation in primary malignant and nonmalignant T cells, which is in agreement with our previous findings.13 Notably, SEA triggered a profound upregulation of STAT3 phosphorylation in malignant T cells after coculture with nonmalignant T cells and in the presence of SEA, whereas SEA had little effect on STAT3 phosphorylation in monoculture of malignant T cells. In contrast, SEA induced a strong upregulation of STAT3 phosphorylation in nonmalignant T cells, and this phosphorylation level was not further affected by addition of malignant T cells (Figure 5B).
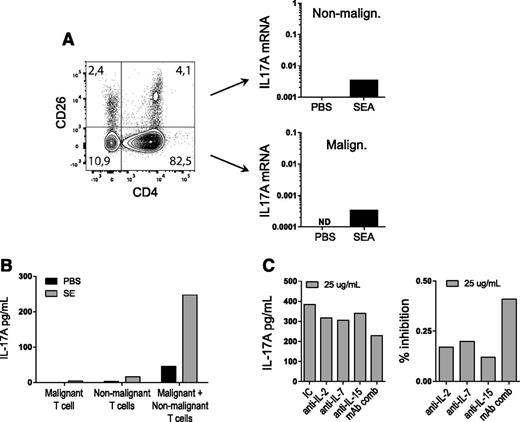
To address whether SEA triggered IL-17 expression in primary malignant T cells, PBMCs were cultured with and without SEA prior to qPCR analysis of IL-17A expression in CD4+/CD26− malignant T cells (Figure 6A, lower right) and CD4+/CD26+ nonmalignant T cells (Figure 6A, upper right). Notably, SEA induced IL-17A expression in both the large fraction (86%) of malignant T cells and the small fraction (5%) of nonmalignant T cells (Figure 6A). Next, malignant T cells (CD4+/CD26−) were cultured in the presence and absence of SEA in monoculture and coculture with nonmalignant CD4 T cells. As shown in Figure 6B, SEA induced IL-17 production in primary malignant T cells when cocultured with nonmalignant T cells (but not in monocultures of malignant T cells), showing that IL-17A expression in primary malignant T cells depended on SEA-driven cross talk between malignant and nonmalignant T cells. Next, cocultures were treated with neutralizing antibodies against IL-2, IL-7, and IL-15, as well as against a combination of the 3 antibodies, prior to stimulation with SEA. As shown in Figure 6C, each individual antibody inhibited the IL-17A response by 15% to 20%, whereas the combination of antibodies inhibited the response by >40%, indicating that the IL-17A response was at least partly driven by IL-2 receptor common γ gamma chain (IL-2Rg) cytokines.
SEs induce IL-17 production from cocultures of primary malignant T cells and nonmalignant CD4 T cells. (A) PBMCs from a CTCL patient were stimulated with either vehicle (PBS) or SEA (200 ng/mL) for 24 hours and then sorted by CD4 and CD26. IL17A gene expression from malignant and nonmalignant cells was determined by qPCR and normalized to GAPDH expression. (B) Primary malignant T cells from a CTCL patient and nonmalignant CD4 T cells were mono- and cocultured with either vehicle (PBS) or SEA (200 ng/mL). IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (C) Primary malignant T cells from a CTCL patient and nonmalignant CD4 T cells were cocultured with SEA and blocking antibodies against IL-2, IL-7, or IL-15 or against a combination of IL-2, IL-7, and IL-15 (mAb comb) for 24 hours. IL-17A concentrations were determined by ELISA and normalized to 106 cells and are shown in absolute concentrations and in percent inhibition of IC control.
SEs induce IL-17 production from cocultures of primary malignant T cells and nonmalignant CD4 T cells. (A) PBMCs from a CTCL patient were stimulated with either vehicle (PBS) or SEA (200 ng/mL) for 24 hours and then sorted by CD4 and CD26. IL17A gene expression from malignant and nonmalignant cells was determined by qPCR and normalized to GAPDH expression. (B) Primary malignant T cells from a CTCL patient and nonmalignant CD4 T cells were mono- and cocultured with either vehicle (PBS) or SEA (200 ng/mL). IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (C) Primary malignant T cells from a CTCL patient and nonmalignant CD4 T cells were cocultured with SEA and blocking antibodies against IL-2, IL-7, or IL-15 or against a combination of IL-2, IL-7, and IL-15 (mAb comb) for 24 hours. IL-17A concentrations were determined by ELISA and normalized to 106 cells and are shown in absolute concentrations and in percent inhibition of IC control.
Discussion
In this study, we demonstrate for the first time that SEA induces STAT3 activation and IL-17 expression in immortalized and primary malignant T cells derived from CTCL patients. SEA-containing isolates of bacteria from CTCL skin, as well as recombinant SEA, triggered STAT3 activation and robust IL-17 production in malignant T cells when cocultured with nonmalignant T cells, but not with SEA alone. Activated STAT3 is oncogenic in animal models10 and believed to foster CTCL.12-17 STAT3 provides survival signals through upregulation of proto-oncogenes such as Bcl-2 and survivin,11,15 IL-2 receptor,63 and pro-oncogenic microRNAs,64,65 and through downregulation of tumor-suppressive microRNAs such as miR-22.66 In addition, STAT3 drives expression of suppressor of cytokines signaling,19 cytokines of the Th2 (IL-5 and IL-13),67 Th17 (IL-17, IL-22),21 regulatory T-cell (IL-10) phenotype,22 and other factors.
Our finding that SEA induced strong STAT3 activation in primary malignant T cells provides direct evidence linking bacterial toxins with activation of an oncogene in CTCL. Moreover, it suggests a mechanism whereby toxin-producing bacteria (via the activation of STAT3) can augment an array of pathological processes in the lymphomagenesis. This finding is important because, for decades, SEs have been suspected to play a tumor-promoting role in CTCL.39,40,45,50,68-70 We now propose that SEA-mediated cross talk between malignant and nonmalignant T cells triggers oncogenic STAT3 activation in vivo. Our findings provide a plausible explanation for clinical observations indicating that SE-producing staphylococci promote tumor growth and aggravate the disease and, conversely, that antibiotic therapy may halt disease progression and even induce tumor regression in some CTCL patients.40,45,50
Despite the well-established role of STAT3 in CTCL pathogenesis, it has not been clear what drives malignant STAT3 activation in vivo. Recently, activating mutations have been described in a subset (12.5%) of CTCL patients,28,29 but it remains unknown what drives aberrant STAT3 activation in the majority of patients. Early on, it was discovered that malignant T cells under ex vivo conditions rapidly lost expression of activated STAT3, indicating that in vivo signals and factors (such as IL-2Rg cytokines) present in the local environment play a key role in malignant STAT3 activation in CTCL patients.14 In support, IL-2 and other IL-2Rg-binding cytokines such as IL-7, IL-15, and IL-21 induce STAT3 activation in primary malignant T cells and immortalized T-cell lines,71-73 suggesting that these cytokines may also drive STAT3 activation in vivo. Although malignant and nonmalignant T cells, as well as stromal cells and keratinocytes, may produce IL-2Rg-binding cytokines in vivo, the actual cells producing these factors and relative contribution by different sources remain unknown.
The present findings showing that SEA triggers STAT3 activation and IL-17 expression via an indirect mechanism involving nonmalignant (ie, infiltrating) T cells and soluble factors such as IL-2 and other IL-2Rg cytokines suggest that enterotoxins may also trigger IL-2Rg-mediated STAT3 activation in vivo. SE-producing S aureus skin infection is more common in advanced disease compared with less-advanced CTCL. In fact, S aureus was isolated from skin, blood, and other foci from the majority of CTCL patients with advanced disease and, in half of these patients, the bacteria produced SEA, SEB, and/or TSST-1.40 If the proposed mechanism is at play in these patients, then higher loads of SE-producing bacteria in skin and blood in advanced disease would be predicted to translate into higher levels of activated STAT3 and may partially explain why malignant STAT3 activation is increased in advanced disease.13
As mentioned earlier, SEs have long been suspected to drive chronic activation of malignant T cells.40,50,68-70,74 Originally, it was thought that toxins triggered proliferation and expansion of malignant T cells through direct binding and activation of malignant T-cell clones expressing the appropriate TCR-Vb, even though little data were available to support this hypothesis; others, however, contradicted this view (reviewed in Willerslev-Olsen et al38 ). Our findings presented in this study have significant implications for the understanding of the interplay between bacterial toxins and malignant T cells. An indirect mode of action implies that toxin-mediated activation of malignant T cells does not rely on the expression of a single, toxin-specific TCR-Vb by these malignant T cells but on expression of multiple toxin-binding TCR-Vbs expressed by nonmalignant infiltrating T cells. Consistent with this hypothesis, we observed that SEA induced STAT3 activation in a primary malignant T-cell clone expressing an SEA-nonresponsive TCR-Vb (TCR-Vb17) only when cocultured with nonmalignant T cells expressing a full TCR-Vb repertoire including several SEA-binding TCR-Vbs (such as TCR-Vb5).
In principle, this observation implies that it is not only the few patients who harbor a single malignant T-cell clone expressing an SEA-responsive TCR-Vb but all patients who carry nonmalignant T cells with SEA-responsive TCR-Vbs who are susceptible to SEA-mediated STAT3 activation in malignant T cells. Thus, bacterial toxins might have a dramatic impact on malignant T-cell activation in a much broader range of patients than previously thought. Moreover, our findings show that malignant T cells engage in a complex and delicate cross talk with nonmalignant T cells that dramatically changes their response to signals and factors in the microenvironment. By inference, our data therefore indicate that conventional in vitro models using monocultures of purified malignant T cells have fundamental limitations when it comes to mimicking the pathogenesis in vivo. Furthermore, it is likely that cytokines and factors other than IL-2Rg cytokines also influence toxin-mediated cross talk between malignant and nonmalignant T cells. Indeed, SEA triggers IL-10 expression in cocultures of malignant and nonmalignant T cells;75 IL-13 inhibits IL-17, but not IL-22 and IL-26, expression by Th17 cells;76 and prostaglandins (eg, prostaglandin E2) produced by malignant T cells are known to modulate differentiation and cytokine production by nonmalignant T cells.75 Accordingly, our data suggest that an inclusion of nonmalignant T cells (and possibly stromal cells and keratinocytes) into cultures of malignant T cells would critically improve future in vitro models of CTCL to better mimic the dynamic interactions seen in CTCL patients.
It has been a matter of controversy whether IL-17 is expressed in CTCL. Some studies have reported IL-17 mRNA and/or protein expression in situ and ex vivo, whereas others reported its absence, despite the presence of IL-22-producing Th17-like cells.21,57-60 The present findings offer a possible explanation for these opposing results; specifically, that the differences in frequency and severity of skin colonization and infection by SE-producing bacteria between different cohorts of patients and even within a single cohort may explain why IL-17 expression differed between these studies and between patients within a single cohort.21,57,60 The finding that SEA induces IL-17 expression in nonmalignant primary T cells was not unexpected, given that SEA mediates STAT3 activation in these cells,77 but it was important because it suggests that both malignant and nonmalignant T cells may contribute to IL-17 expression in vivo. Because psoriasis is also associated with IL-17, deregulated STAT3 signaling, and skin colonization by superantigen-producing bacteria like S aureus, it is tempting to speculate whether similar pathological mechanisms are involved in psoriasis and CTCL disorders, which have many histologic and clinical features in common. Yet, it is an open question whether IL-17 is involved in the antimicrobial defense and/or lymphomagenesis in CTCL patients displaying skin colonization by enterotoxin-producing S aureus.
In conclusion, we show that SEA induces a cross talk–dependent activation of STAT3 and expression of IL-17 in malignant T cells, suggesting a mechanism whereby SEA-producing bacteria promote activation of an established oncogenic pathway (STAT3) previously implicated in the pathogenesis of CTCL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Keld Kaltoft for the cell lines and Thomas Leanderson, Gunnar Hedlund, and Karin Leanderson for providing essential materials.
This study was supported by the Danish Cancer Society (Kræftens Bekæmpelse), the Fight Cancer program (Knæk Cancer), the Lundbeck Foundation, the Danish Council for Independent Research (Det Frie Forskningsråd/Sundhed og Sygdom), the Novo Nordisk Research Foundation, LINAK A/S, Kræftfonden, and the Dansk Kræftforsknings Fond.
Authorship
Contribution: A.W.-O. performed the experiments; A.W.-O. and N.O. analyzed and made the figures; L.M.L., R.G., L.I., and M.K. provided the essential materials and patient samples; and A.W.-O., T.K., I.V.L., S.F., D.L.P., C.N., N.P.M., D.S., M.A.W., C.M.B., C.G., A.W., S.K., and N.O. designed the research and wrote the paper. All authors read, commented on, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niels Odum, Department of Immunology and Microbiology, University of Copenhagen, Blegdamsvej 3c, DK-2200 Copenhagen N, Denmark; e-mail: ndum@sund.ku.dk.

![Figure 1. Bacterial isolates from CTCL patients contain SEs. (A) Mixed bacterial isolates from patients were tested for SE expression (SEA, SEB, SEC, SED, and TSST-1) and categorized accordingly as either positive or negative. Cocultures with malignant T cells (SeAx) and nonmalignant T cells (MF1850) were then stimulated with SE-positive or SE-negative isolates and incubated for 24 hours. IL-17 concentration in the supernatants was determined by ELISA. (B) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured in the absence (Media) or presence (Isolate) of a mixed bacterial isolate from a CTCL patient. IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (C-D) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with vehicle (PBS) or recombinant SEA (50 ng/mL) (C), or with PBS, SEAwt, or the toxins SEAD227A, SEAF47A/D227A, SEB, SEC2, SED, SEE, SEI, or TSST-1 (50 ng/mL) (D). IL-17A protein was measured in the supernatant after 24 hours of incubation with ELISA. (E) Malignant (SeAx) and nonmalignant (MF1850) T-cell lines were mono- and cocultured with SEA (50 ng/mL) and tofacitinib (0.3 μM) or vehicle (dimethylsulfoxide [DMSO]) for 24 hours. After incubation, IL-17A protein concentration was determined by ELISA. Error bars represent standard error of the mean of 3 independent experiments. *P < .05. Malign., malignant; Neg, negative; Non-malign., nonmalignant; Pos, positive.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/10/10.1182_blood-2015-08-662353/5/m_1287f1.jpeg?Expires=1763524747&Signature=q5hzztAGWuSfeOT1xIXFcuXWIsGZ3KiFgtx32Dy4s7hlHNidf-6Vv0XEqO3C6goKetwbCiYIU8Hy2LUfBl5wjY-Hwclw-re0WmKMwD4IANcQlRu4M6U7rwWRvXsMgohsVwx0Be~NOCjX4CYrhy5CqVZP9Tgz63AGjWwyh2VML7IJ5LtDnMtmTjCeJaUK7Xo8tHq~MOPlAEzmFsx1HKKCIQPkEG8wiFB4n4yGydUfdTc~Ono4WRaP1mS6JZR20uqpd9mbt6wGP0wC3IBcKJD2PimBW3OdMnDy49pVsTWHq4~FSz0scEeaMhSIDkZMb9bjYdXtUwr5C4OYXSFEBtHBtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)