Key Points
ADAMTS13 dissolves t-PA–resistant cerebral occlusions in a mouse model of stroke.
The thrombolytic activity of ADAMTS13 could become useful for more efficient and safer thrombolytic treatment of acute ischemic stroke.
Abstract
Rapid vascular recanalization forms the basis for successful treatment of cerebral ischemia. Currently, tissue plasminogen activator (t-PA) is the only approved thrombolytic drug for ischemic stroke. However, t-PA does not always result in efficient thrombus dissolution and subsequent blood vessel recanalization. To better understand thrombus composition, we analyzed thrombi retrieved from ischemic stroke patients and found a distinct presence of von Willebrand factor (VWF) in various samples. Thrombi contained on average 20.3% ± 10.1% VWF, and this was inversely correlated with thrombus red blood cell content. We hypothesized that ADAMTS13 can exert a thrombolytic effect in VWF-containing thrombi in the setting of stroke. To test this, we generated occlusive VWF-rich thrombi in the middle cerebral artery (MCA) of mice. Infusion of t-PA did not dissolve these MCA occlusions. Interestingly, administration of ADAMTS13 5 minutes after occlusion dose-dependently dissolved these t-PA–resistant thrombi resulting in fast restoration of MCA patency and consequently reduced cerebral infarct sizes (P < .005). Delayed ADAMTS13 administration 60 minutes after occlusion was still effective but to a lesser extent (P < .05). These data show for the first time a potent thrombolytic activity of ADAMTS13 in the setting of stroke, which might become useful in treatment of acute ischemic stroke.
Introduction
Ischemic stroke is one of the leading causes of death and permanent physical impairment worldwide. Early vessel recanalization is of crucial importance to improve final clinical outcome in acute ischemic stroke.1 Hence, the primary goal in stroke treatment is reopening of occluded cerebral blood vessels to establish reperfusion and salvation of threatened tissue. Notwithstanding the huge clinical relevance of ischemic stroke, treatment options are limited. The only 2 US Food and Drug Administration--approved therapies include pharmacological thrombolysis using tissue plasminogen activator (t-PA) and mechanical removal of the occluding thrombus via endovascular intervention. However, endovascular treatment is not always available or possible, and t-PA needs to be delivered IV within 3 hours of stroke onset. Despite the clinical benefits of early t-PA administration, t-PA treatment suffers from serious limitations.2 Use of t-PA has been associated with neurotoxic activity and damage of the blood-brain barrier, increasing the risk of hemorrhages and worsening stroke outcome.3 Most remarkably, t-PA results in recanalization only in less than half of the patients that receive it.1 The exact nature of this so-called t-PA resistance is not yet fully understood, but location, size, and composition of the occluding thrombus have been reported to be important factors influencing t-PA outcome.4-8 Interestingly, various studies have shown that in particular arterial platelet-rich clots are more resistant to thrombolysis with t-PA.7,9-11 Surprisingly little is known about the precise composition of human stroke clots. Nevertheless, such information is crucial for the design of novel thrombolytic strategies.
A crucial player in arterial thrombus formation is von Willebrand factor (VWF), a large multimeric plasma glycoprotein. VWF recruits platelets at sites of vascular injury by acting as a molecular bridge between circulating platelets and exposed components of the subendothelium. Together with fibrin(ogen), VWF links platelets together, further stabilizing the platelet plug.12,13 Interestingly, VWF can be cleaved by the metalloprotease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). As a result, ADAMTS13 digests large thrombogenic VWF molecules into smaller, less reactive multimers. Reduced or absent ADAMTS13 activity causes the microangiopathic disorder thrombotic thrombocytopenic purpura, characterized by VWF and platelet-rich microthrombi that cause multiple organ failure and even death when left untreated.14 Specific information on the presence of VWF in human stroke thrombi is currently lacking. However, the pathophysiological importance of the VWF/ADAMTS13 axis in ischemic stroke has become clear in the last few years.13 High VWF levels and low ADAMTS13 levels are important risk factors for ischemic stroke.15-17 In addition, various experimental animal models of cerebral and myocardial ischemia/reperfusion injury showed that absence of VWF is protective, whereas absence of ADAMTS13 exacerbates disease outcome.18-22
The therapeutic thrombolytic potential of targeting VWF by ADAMTS13 in the setting of acute stroke is currently not known. In this study, we therefore collected thrombi retrieved from stroke patients and subjected them to histological assessment with a specific focus on VWF. Furthermore, we investigated the thrombolytic activity of ADAMTS13 on VWF-rich thrombi in a mouse model of thrombotic stroke.
Materials and methods
Mice
All animal studies were performed in accordance with the local ethical law, the local ethical committees (KU Leuven, Leuven, Belgium; act no. 87–848), and guidelines for the care and use of laboratory animals. Experiments represented in Figure 4 were performed on 8- to 12-week-old male and female Adamts13−/− and their littermate Adamts13+/+ mice on the same mixed C57BL/6J, CASA/RK, and 129X1/SvJ background.23 The other experiments (represented in Figures 5 and 6) were performed on 8- to 12-week-old male and female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME).
Materials
t-PA (Actilyse) was purchased from Boehringer Ingelheim (Ingelheim am Rhein, Germany). Recombinant human ADAMTS13 (rhADAMTS13) was provided by Baxalta (Baxalta Innovations GmbH, Vienna, Austria). Vehicle was sodium chloride 0.9% (B. Braun Medical NV, Diegem, Belgium); this was also used to dilute both t-PA and rhADAMTS13.
Pharmacokinetics of rhADAMTS13
The pharmacokinetic and toxicological profile of rhADAMTS13 in mice was previously determined and described.20 Specifically, rhADAMTS13 has a terminal half-life of 24 hours in Adamts13−/− mice, and administration of rhADAMTS13 at doses almost 10 times higher than used in the current study did not change hematological parameters or induce pathological conditions.20
Thrombotic occlusion of the MCA
Acute ischemic stroke in mice was induced via the formation of an occlusive thrombus in the M3 segment of the right middle cerebral artery (MCA) as previously described with slight modifications.24 Briefly, via a skin incision between the right eye and ear, the temporal muscle was excised, and a small craniotomy was performed on the parietal bone to expose the MCA (see supplemental Figure 1, available on the Blood Web site). A small piece of Whatman filter paper (GE Healthcare, Buckinghamshire, UK) saturated with 20% ferric chloride (FeCl3; Sigma-Aldrich, St. Louis, MO) was placed for 4 minutes on top of the unharmed dura mater above the MCA. Distal MCA blood flow was determined by laser Doppler flow measurements (moorVMS-LDF1; Moor Instruments; Devon, UK) via a fiber-optic probe (diameter, 0.25 mm) placed directly above the MCA downstream of the thrombotic occlusion. Blood flow was continuously measured for 10 minutes before induction of MCA occlusion to set baseline flow (100%) and was monitored until a maximum of 2 hours after occlusion. Time to occlusion was defined as the time between FeCl3 application and a drop of blood flow below 25% of baseline value. Recanalization was defined as a return of averaged (over 60 seconds) blood flow above 25% of baseline value. A detailed description is provided in supplemental Methods.
Measurement of infarct volume
Cerebral infarct volumes were determined as described.25 Mice were sacrificed 24 hours after occlusion of the MCA. Brains were quickly removed and cut into 2-mm-thick coronal sections. Brain sections were stained with 2% 2,3,5-triphenyl-tetrazolium chloride (TTC; T8877; Sigma-Aldrich) in phosphate-buffered saline to visualize healthy tissue and unstained infarctions. Sections were photographed, and infarct areas (white) were analyzed via planimetry using ImageJ software (National Institutes of Health, Bethesda, MD; http://imagej.nih.gov/ij/) by an experimenter who was blinded to the treatment conditions.
Patient samples
Thrombectomy procedures were performed at the AZ Groeninge Hospital, Kortrijk, Belgium. Retrieved thrombi were immediately fixed overnight with 4% paraformaldehyde and embedded in paraffin. Approval was obtained from the medical ethics committee of the AZ Groeninge Hospital. All patients gave written informed consent. Collected clinical patient data included demographic features, cerebrovascular risk factors, National Institutes of Health Stroke Scores on admission and at discharge, use of IV thrombolysis, revascularization status, and likely stroke etiology. Stroke etiology was classified at discharge using TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.26 Patients classified as “other” included 2 patients with a patent foramen ovale and venous thrombotic complications; 1 patient classified in this group had a carotid artery dissection. For 6 patients, the likely stroke etiology could not be determined.
Histology procedures
Patient thrombi and mouse brain sections were stained with hematoxylin and eosin (H&E; HT110216; Sigma-Aldrich), Martius Scarlet Blue (MSB; fibrin and red blood cell staining), or anti-VWF antibodies (A0082; DAKO, Glostrup, Denmark). For the negative control, anti-VWF antibodies were omitted. Images were obtained using a light microscope (Primostar; Zeiss, Gottingen, Germany) and Zen 2012 (blue edition, version 1.1.2.0; Zeiss) acquisition software. Thrombus content was quantified using ImageJ software. A more detailed description is provided in supplemental Methods.
Statistical analysis
All data are presented as mean ± standard error of the mean. Statistical analysis was performed using GraphPad Prism (Version 6.0e). A D’Agostino-Pearson omnibus or Kolmogorov–Smirnov normality test was used to analyze normal distribution of the data sets. An unpaired Student t test was used to analyze time to first occlusion and the effect of thrombolysis on thrombus VWF and fibrin content. A Mann-Whitney U test was used to analyze the effect of thrombolysis on thrombus red blood cell content. To compare thrombus VWF content within stroke etiologies, a 1-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was used. An unpaired Student t test or 1-way ANOVA with Dunnett’s multiple comparison test was used for statistical comparison of infarct lesions when applicable. Changes in laser Doppler blood flow were analyzed and compared by repeated measures ANOVA.
Results
Presence of VWF in thrombi retrieved from stroke patients
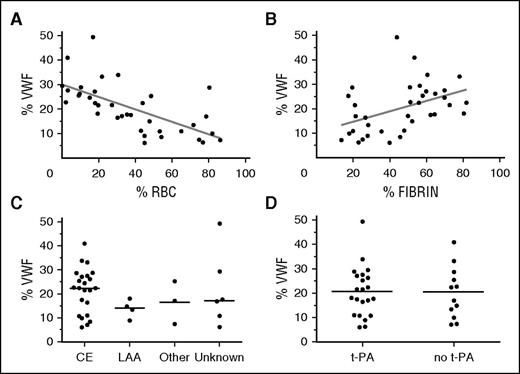
Because the occluding cerebral thrombus itself is the primary target of revascularization therapy, understanding its composition is essential for designing efficient thrombolytic therapies. Currently, little is known about the composition of occluding thrombi causing stroke, and no information is available on VWF content. We benefited from the unique opportunity of having access to thrombi retrieved via thrombectomy to directly investigate thrombus composition, with a specific focus on VWF content. Thrombi from 36 patients were collected and analyzed via H&E staining and MSB (fibrin/red blood cells) staining as well as via immunohistochemical staining for VWF. Quantification of various thrombus constituents revealed that the stroke thrombi contained on average 37.6% ± 26.1% red blood cells, 46.4% ± 20.5% fibrin, and 20.3% ± 10.1% VWF. Although VWF was present in all thrombi, substantial differences were observed within thrombi, with some thrombi containing little VWF (lowest value: 6.1%) and other thrombi being particularly rich in VWF (highest value: 49.3%) (Figures 1 and 2). Interestingly, an inverse linear correlation was observed between VWF and red blood cell content (R2 = 0.4248, P < .0001; Figure 2A), while a positive correlation was found between VWF and fibrin content; however, this correlation was less pronounced (R2 = 0.1867, P = .0085; Figure 2B). Another point of interest is a potential correlation between the amount of VWF in the thrombus and its origin. We did, however, not see a clear difference in VWF content between thrombi caused by large artery atherosclerosis and those derived from cardioembolisms. The small sample size of noncardioembolic thrombi however precludes good conclusions (Figure 2C). Thrombolytic treatment did not affect thrombus VWF content (Figure 2D). Furthermore, no correlation between thrombus VWF content and age, sex, target vessel, or stroke severity was observed (not shown).
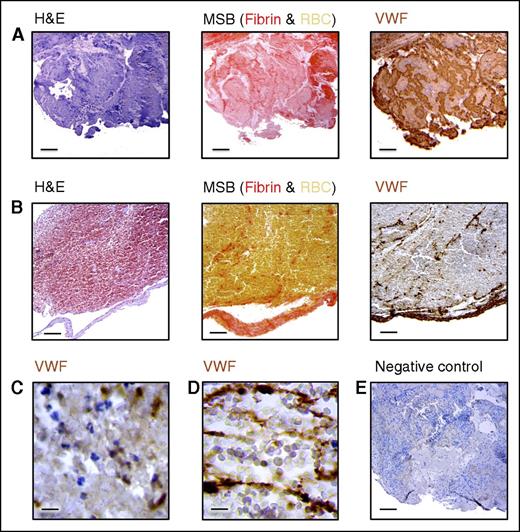
VWF staining on thrombi retrieved from stroke patients. Intracranial thrombi retrieved from stroke patients that underwent thrombectomy procedure were collected for histological analysis. Consecutive thrombi sections were stained with H&E, MSB, and anti-VWF antibodies. For the negative control, primary antibodies were omitted. Classical H&E staining was used to visualize overall thrombus composition and organization. On MSB staining, red areas show the presence of fibrin, whereas red blood cells (RBC) appear yellow. Interestingly, varying amounts of VWF (brown color) were found in all the thrombi analyzed. Two representative patient thrombi are shown illustrating a VWF-rich thrombus (A) and a red blood cell–rich, VWF-poor thrombus (B). Scale bar: 50 μm. (C) Higher magnification showing granular VWF staining. Scale bar: 5 μm. (D) A higher magnification of fibrillar/extracellular VWF. Scale bar: 5 μm. (E) Negative control for the anti-VWF staining. Scale bar: 50 μm.
VWF staining on thrombi retrieved from stroke patients. Intracranial thrombi retrieved from stroke patients that underwent thrombectomy procedure were collected for histological analysis. Consecutive thrombi sections were stained with H&E, MSB, and anti-VWF antibodies. For the negative control, primary antibodies were omitted. Classical H&E staining was used to visualize overall thrombus composition and organization. On MSB staining, red areas show the presence of fibrin, whereas red blood cells (RBC) appear yellow. Interestingly, varying amounts of VWF (brown color) were found in all the thrombi analyzed. Two representative patient thrombi are shown illustrating a VWF-rich thrombus (A) and a red blood cell–rich, VWF-poor thrombus (B). Scale bar: 50 μm. (C) Higher magnification showing granular VWF staining. Scale bar: 5 μm. (D) A higher magnification of fibrillar/extracellular VWF. Scale bar: 5 μm. (E) Negative control for the anti-VWF staining. Scale bar: 50 μm.
Correlation of VWF content with thrombus composition, stroke etiology, and treatment. VWF, red blood cell, and fibrin positive area were quantitatively analyzed for each of the thrombi. (A) A strong negative linear association was found between red blood cell content and VWF positive area. R2 = 0.4248; P < .0001. (B) A positive linear association was found between fibrin content and VWF positive area. R2 = 0.1867; P = .0085. (C) No significant difference in VWF staining was observed with different types of stroke etiology. (D) Thrombolysis did not affect thrombus VWF content (P = .9172). CE, cardioembolic; LAA, large artery atherosclerosis.
Correlation of VWF content with thrombus composition, stroke etiology, and treatment. VWF, red blood cell, and fibrin positive area were quantitatively analyzed for each of the thrombi. (A) A strong negative linear association was found between red blood cell content and VWF positive area. R2 = 0.4248; P < .0001. (B) A positive linear association was found between fibrin content and VWF positive area. R2 = 0.1867; P = .0085. (C) No significant difference in VWF staining was observed with different types of stroke etiology. (D) Thrombolysis did not affect thrombus VWF content (P = .9172). CE, cardioembolic; LAA, large artery atherosclerosis.
Mouse model of ischemic stroke with VWF-rich MCA occlusions
Based on our histological findings, we hypothesized that VWF-rich thrombi might be susceptible to thrombolysis by ADAMTS13. To test this, we used a mouse model that recapitulates acute ischemic stroke via thrombotic occlusion of the MCA after topical application of FeCl3 (supplemental Figure 1). Histological analysis of the occlusive thrombi formed in the MCA demonstrated that they are rich in VWF and poor in fibrin, as shown in Figure 3. In this study, we used 2 different sizes of thrombotic MCA occlusions (supplemental Figure 1). Using a small (0.5 × 0.5 mm) filter paper saturated with 20% FeCl3, relatively small occlusions were generated. When using a larger filter paper (0.5 × 1.5 mm) saturated with 20% FeCl3, larger MCA occlusions were formed. As described in the following results, the small occlusive thrombi are susceptible to spontaneous endogenous thrombolysis, whereas the larger occlusions are resistant to spontaneous thrombolysis.
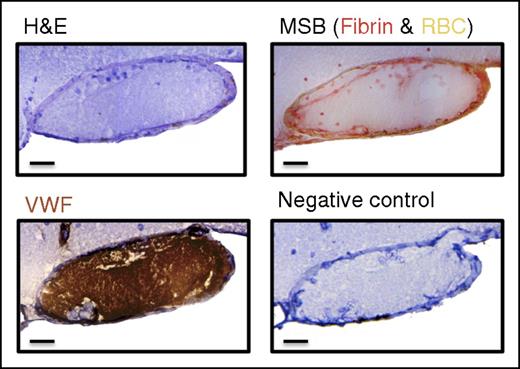
FeCl3-induced thrombotic occlusion of the right MCA is VWF-rich. Histological analysis of the induced MCA thrombus 5 minutes after occlusion via H&E, MSB, and VWF staining revealed a VWF-rich, fibrin-poor thrombus. Scale bar: 20 μm.
FeCl3-induced thrombotic occlusion of the right MCA is VWF-rich. Histological analysis of the induced MCA thrombus 5 minutes after occlusion via H&E, MSB, and VWF staining revealed a VWF-rich, fibrin-poor thrombus. Scale bar: 20 μm.
Absence of ADAMTS13 prevents spontaneous thrombus dissolution
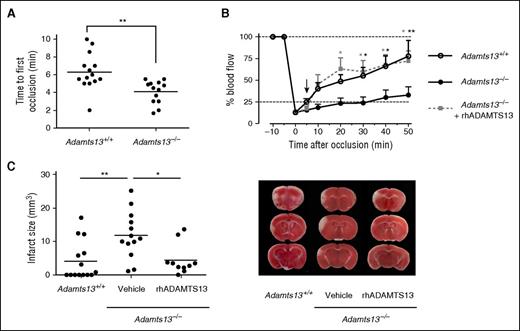
To assess the effect of ADAMTS13 on spontaneous thrombus dissolution, we generated a small occlusive thrombus (using a 0.5 × 0.5-mm filter paper) in both Adamts13+/+ and Adamts13−/− mice. Once formed, the occlusive thrombus reduced MCA blood flow to the same extent in Adamts13+/+ and Adamts13−/− mice (residual blood flow of 12.9% ± 1.9% vs 13.4% ± 1.4% of baseline, respectively, P = .86). Interestingly, most Adamts13+/+ mice showed fast spontaneous recanalization after initial occlusion as measured by laser Doppler flowmetry (Figure 4B). In contrast, however, spontaneous thrombus dissolution in Adamts13−/− mice occurred significantly later, or did not take place at all, within the experimental timeframe of 50 minutes. Indeed, whereas 85.7% of Adamts13+/+ mice (12 of 14) showed spontaneous recanalization within 2 minutes after occlusion, only 23.1% of Adamts13−/− mice showed a comparably fast recanalization (3 of 13 animals). At 50 minutes postocclusion, blood flow was restored to 77.6% ± 18.0% in Adamts13+/+ mice opposed to only 32.9% ± 9.6% in the Adamts13−/− mice (P < .01). Representative examples of individual blood flow profiles are shown in supplemental Figure 2A-D. These observations suggest that ADAMTS13 may promote thrombus dissolution and enhance recanalization of occluded blood vessels.
ADAMTS13 promotes MCA recanalization and reduces ischemic brain injury. A FeCl3-mediated injury was induced in the MCA of both Adamts13−/− and Adamts13+/+ animals to cause a small thrombotic occlusion of the MCA. (A) Absence of ADAMTS13 results in a faster occlusion of the MCA. Time to first occlusion was defined as the time after FeCl3 application until blood flow dropped below 25%. (B) Averaged postocclusion MCA blood flow profiles reveal that restoration of blood flow was significantly impaired in Adamts13−/− mice compared with Adamts13+/+ animals. Administration of rhADAMTS13 5 minutes after occlusion (arrow) restored blood flow. (C) Twenty-four hours after occlusion, cerebral infarctions were determined via TTC staining and planimetric analysis. Infarct sizes were significantly larger in Adamts13−/− mice than in Adamts13+/+ mice. Treatment of Adamts13−/− mice with rhADAMTS13 5 minutes after occlusion significantly reduced infarct sizes. Quantification of the infarct sizes is shown in the left panel, and representative brain sections are shown in the right panel. (n = 10-14 mice/group; *P < .05; **P < .01; compared with Adamts13−/− mice treated with vehicle.)
ADAMTS13 promotes MCA recanalization and reduces ischemic brain injury. A FeCl3-mediated injury was induced in the MCA of both Adamts13−/− and Adamts13+/+ animals to cause a small thrombotic occlusion of the MCA. (A) Absence of ADAMTS13 results in a faster occlusion of the MCA. Time to first occlusion was defined as the time after FeCl3 application until blood flow dropped below 25%. (B) Averaged postocclusion MCA blood flow profiles reveal that restoration of blood flow was significantly impaired in Adamts13−/− mice compared with Adamts13+/+ animals. Administration of rhADAMTS13 5 minutes after occlusion (arrow) restored blood flow. (C) Twenty-four hours after occlusion, cerebral infarctions were determined via TTC staining and planimetric analysis. Infarct sizes were significantly larger in Adamts13−/− mice than in Adamts13+/+ mice. Treatment of Adamts13−/− mice with rhADAMTS13 5 minutes after occlusion significantly reduced infarct sizes. Quantification of the infarct sizes is shown in the left panel, and representative brain sections are shown in the right panel. (n = 10-14 mice/group; *P < .05; **P < .01; compared with Adamts13−/− mice treated with vehicle.)
It is nonetheless important to point out that the use of an ADAMTS13-deficient background also has an impact on the process of thrombus formation itself due to the presence of thrombogenic ultralarge (UL)-VWF in the circulation of Adamts13−/− mice and the absence ADAMTS13-mediated destabilization of the growing thrombus.27 This prothrombotic phenotype is illustrated by the shorter time needed to reach full MCA occlusion in Adamts13−/− mice (4.2 ± 0.5 minutes) compared with Adamts13+/+ animals (6.4 ± 0.5 minutes, P < .01) (Figure 4A). However, administration of rhADAMTS13 (3500 U/kg) 5 minutes after a small occlusive MCA thrombus was formed in Adamts13−/− mice resulted in efficient restoration of MCA blood flow (72.1% ± 11.5% of baseline 50 minutes after occlusion) similar to Adamts13+/+ mice (Figure 4B and supplemental Figure 2E-F). Taken together, these data support the notion that ADAMTS13 is a determinant of arterial thrombus stability and that ADAMTS13 helps to safeguard vessel patency during an occlusive event.
Importantly, in these experiments fast recanalization of the MCA was associated with reduced cerebral stroke damage. Because the primary therapeutic goal of early vessel recanalization is to limit ischemic brain injury, we investigated whether the observed differences in blood flow restoration had a physiological effect on cerebral infarct size. Mouse brains were isolated 24 hours postocclusion and stained to visualize cerebral infarctions (Figure 4C). Brain infarctions observed in Adamts13+/+ animals were relatively small or even absent (4.1 ± 1.6 mm3). In line with the poor MCA recanalization observed in Adamts13−/− mice, cerebral infarctions in these animals were significantly larger (11.9 ± 1.9 mm3; P < .01). Notably, in Adamts13−/− mice that received rhADAMTS13 5 minutes after occlusion, infarct volumes were significantly reduced to values comparable to those of Adamts13+/+ animals (4.5 ± 1.4 mm3). Hence, ADAMTS13-mediated thrombus dissolution and restoration of MCA blood flow saves the brain from developing larger cerebral infarctions.
Thrombolytic effect of ADAMTS13 on large t-PA–resistant MCA occlusions
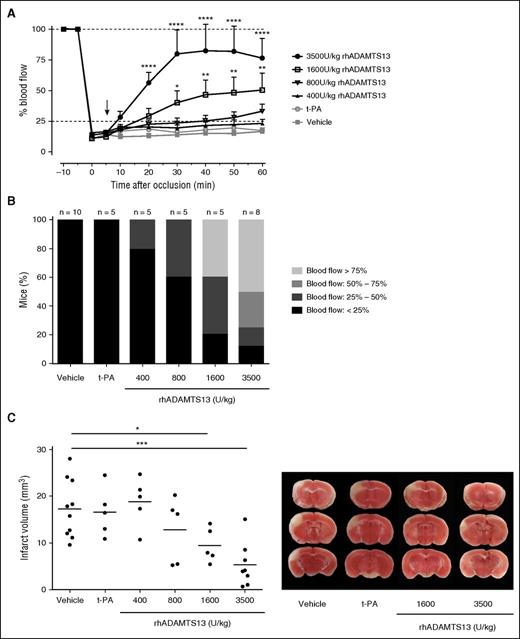
To better study the thrombolytic capacity of ADAMTS13 without potential strain-specific effects of Adamts13+/+ and especially Adamts13−/− mice, we next used wild-type C57BL/6J mice in our stroke model. In these mice, large VWF-rich thrombotic occlusions (0.5 × 1.5-mm filter paper saturated with 20% FeCl3) were generated. In contrast to the smaller occlusions, no spontaneous recanalization was observed after generation of large MCA thrombi in C57BL/6J mice for at least 2 hours after MCA occlusion (Figures 5 and 6). To test whether t-PA was able to lyse these large VWF-rich thrombi, we infused mice with 10 mg/kg t-PA (a bolus of 1 mg/kg 5 minutes after occlusion followed by an infusion of 9 mg/kg over 30 minutes). No changes in laser Doppler blood flow were observed, indicating that t-PA failed to induce recanalization (Figure 5A). These results illustrate the higher resistance of these larger occlusions to spontaneous or pharmacological t-PA-mediated thrombolysis. Using this t-PA–resistant model, we first tested whether rhADAMTS13 (3500 U/kg) could ameliorate MCA blood flow when administered 5 minutes after occlusion. Strikingly, after administration of rhADAMTS13, blood flow was gradually restored to more than 75% of baseline within 25 minutes after injection and remained stable afterward (76.6% ± 15.9% of baseline values 60 minutes after initial occlusion; Figure 5A). Although earlier pharmacological studies have shown that this relatively high dose of ADAMTS13 is safe and well tolerated,20 we next tested lower doses of rhADAMTS13 to determine the minimally effective dose in this model. As shown in Figure 5A, a dose-dependent effect was observed: a dose of 1600 U/kg still significantly improved blood flow when administered 5 minutes after occlusion (to 50.5% ± 13.6% of baseline values 60 minutes after occlusion), whereas a dose of 800 U/kg showed a limited improvement in blood flow (to 33.0% ± 6.0% of baseline values 60 minutes after occlusion). A dose of 400 U/kg rhADAMTS13 was ineffective, with blood flow remaining under 25% of baseline values (23.0% ± 3.6% 60 minutes postocclusion) in most mice, which was nevertheless still higher than in mice treated with vehicle (16.9% ± 2.3% 60 minutes postocclusion). For each dose of ADAMTS13, variation in the degree of recanalization was observed among individual animals, an effect that is masked by the above-mentioned average results. To better show the individual responses, we graded the degree of perfusion for each individual mouse at the end of blood flow monitoring (1 hour postocclusion), as shown in Figure 5B. The lower doses of rhADAMTS13 (400 U/kg and 800 U/kg) induced only partial reperfusion (between 25% and 50% in 1 of 5 mice and in 2 of 5 mice, respectively). The higher doses of rhADAMTS13 (1600 U/kg and 3500 U/kg) were able to recover MCA blood flow above 50% in 2 of 5 mice and 6 of 8 mice, respectively. These data show a clear dose-dependent thrombolytic activity of rhADAMTS13 in our model with large, t-PA–resistant MCA occlusions.
Dose-dependent thrombolytic activity of ADAMTS13 on large t-PA–resistant MCA occlusions. A large thrombus was generated in the right MCA of C57Bl/6J mice. This thrombus was resistant to spontaneous dissolution. Five minutes after occlusion, vehicle, t-PA, or different doses of rhADAMTS13 were IV administered (arrow), and MCA blood flow was monitored for 60 minutes. (A) Average blood flow profiles show that rhADAMTS13 restores MCA blood flow in a dose-dependent way, whereas t-PA was unable to restore blood vessel patency. (B) The proportion of animals in which blood flow is restored to more than 25%, 50%, or 75% increases with rhADAMTS13 dose. (C) When ischemic brain injury was assessed 24 hours after occlusion, a dose-dependent reduction of infarct size was observed with increasing amounts of rhADAMTS13. Quantification of the infarct sizes is shown in the left panel, and representative brain sections are shown in the right panel. (n = 10 and 8 mice for vehicle and 3500 U/kg rhADAMTS13, respectively, n = 5 for the lower doses of rhADAMTS13 and t-PA; *P < .05; **P < .01; ***P < .005; ****P < .001; compared with vehicle.)
Dose-dependent thrombolytic activity of ADAMTS13 on large t-PA–resistant MCA occlusions. A large thrombus was generated in the right MCA of C57Bl/6J mice. This thrombus was resistant to spontaneous dissolution. Five minutes after occlusion, vehicle, t-PA, or different doses of rhADAMTS13 were IV administered (arrow), and MCA blood flow was monitored for 60 minutes. (A) Average blood flow profiles show that rhADAMTS13 restores MCA blood flow in a dose-dependent way, whereas t-PA was unable to restore blood vessel patency. (B) The proportion of animals in which blood flow is restored to more than 25%, 50%, or 75% increases with rhADAMTS13 dose. (C) When ischemic brain injury was assessed 24 hours after occlusion, a dose-dependent reduction of infarct size was observed with increasing amounts of rhADAMTS13. Quantification of the infarct sizes is shown in the left panel, and representative brain sections are shown in the right panel. (n = 10 and 8 mice for vehicle and 3500 U/kg rhADAMTS13, respectively, n = 5 for the lower doses of rhADAMTS13 and t-PA; *P < .05; **P < .01; ***P < .005; ****P < .001; compared with vehicle.)
Delayed rhADAMTS13 administration 60 minutes after occlusion is able to restore MCA blood flow and reduce ischemic brain injury. A large thrombus was generated in the right MCA of C57Bl/6J mice. Sixty minutes after inducing a stable MCA occlusion, mice were treated with either rhADAMTS13 (3500 U/kg) or vehicle (arrow). MCA blood flow was monitored via laser Doppler flowmetry to assess recanalization of the MCA until 1 hour after injection. (A) In mice treated with rhADAMTS13, a significant increase in blood flow was observed. (B) Infarct sizes were significantly decreased in mice that received rhADAMTS13. Quantification of the infarct sizes is shown in the left panel, and representative brain sections are shown in the right panel. (n = 8 mice for each group; *P < .05; ****P < .001 compared with vehicle.)
Delayed rhADAMTS13 administration 60 minutes after occlusion is able to restore MCA blood flow and reduce ischemic brain injury. A large thrombus was generated in the right MCA of C57Bl/6J mice. Sixty minutes after inducing a stable MCA occlusion, mice were treated with either rhADAMTS13 (3500 U/kg) or vehicle (arrow). MCA blood flow was monitored via laser Doppler flowmetry to assess recanalization of the MCA until 1 hour after injection. (A) In mice treated with rhADAMTS13, a significant increase in blood flow was observed. (B) Infarct sizes were significantly decreased in mice that received rhADAMTS13. Quantification of the infarct sizes is shown in the left panel, and representative brain sections are shown in the right panel. (n = 8 mice for each group; *P < .05; ****P < .001 compared with vehicle.)
Importantly, we also assessed the effect of ADAMTS13-induced thrombolysis on cerebral infarct size. Twenty-four hours after initial occlusion, brains were collected, and infarct size was determined via TTC staining. In line with the dose-dependent restoration of MCA blood flow, we found a similar dose-dependent protective effect of ADAMTS13 on ischemic brain injury (Figure 5C). Administration of 400 U/kg rhADAMTS13 had no effect on infarct size compared with vehicle treatment (18.8 ± 2.3 mm3 vs 17.3 ± 2.2 mm3, respectively), but administration of higher doses reduced cerebral infarct volumes. This protective effect was statistically significant for the 2 highest doses of rhADAMTS13 (1600 U/kg and 3500 U/kg with infarct volumes of 9.4 ± 1.6 mm3 and 5.3 ± 1.7 mm3, respectively). In agreement with the absence of recanalization, administration of t-PA did not reduce ischemic brain injury (Figure 5C).
Successful thrombolysis and neuroprotection after delayed rhADAMTS13 administration
Immediate pharmacological thrombolysis after the occluding event is not always possible in the clinic. Moreover, aging of a thrombus is also known to affect its stability and susceptibility to thrombolysis.28,29 We therefore assessed the thrombolytic potential of ADAMTS13 in a more clinically realistic time window. One hour after inducing large t-PA–resistant MCA occlusions in C57BL/6J mice, rhADAMTS13 (3500 U/kg) was IV injected. Interestingly, even after this delayed administration, rhADAMTS13 was still able to lyse the thrombus, thereby partly restoring MCA patency (Figure 6A). Indeed, blood flow was restored to 43.9% ± 11.7% of baseline values 60 minutes after rhADAMTS13 injection (2 hours after occlusion). Again, MCA blood flow in the vehicle-treated group remained at 18.2% ± 1.7% 60 minutes after injection. Notably, this ADAMTS13-mediated partial restoration of blood flow was still sufficient to reduce brain injury by 40%. Infarct sizes of mice treated with rhADAMTS13 1 hour postocclusion were indeed significantly reduced when compared with mice that received vehicle (11.3 ± 1.6 mm3 vs 18.8 ± 2.9 mm3, respectively; Figure 6B). These results confirm a potent thrombolytic and protective activity of rhADAMTS13, even after delayed administration.
rhADAMTS13 dissolves VWF-platelet agglutinates
Data from the above-mentioned stroke models suggest that ADAMTS13 cleaves VWF inside the thrombus, thereby helping the dissolution of the thrombus and restoration of blood vessel patency. As a proof of concept, we performed in vitro ristocetin-induced platelet agglutinations (supplemental Figure 3). In this assay, platelets bind to VWF to form VWF-platelet agglutinations. After maximal agglutination was achieved, either vehicle or rhADAMTS13 was added, and breakdown of the complexes was monitored. The degree of agglutination reversed rapidly from its maximal value to 24.6% ± 3.7% 20 minutes after administration of rhADAMTS13, whereas vehicle had no effect (91.2% ± 3.6%). These data show that by cleaving VWF, ADAMTS13 can reverse the crosslinking of platelets.
Absence of bleeding
It is interesting to note that no bleeding complications were observed throughout our study. Upon isolation of mouse brains, each brain was carefully examined macroscopically to detect any potential hemorrhages. Also, when preparing the 2-mm-thick slices for TTC staining, each section was carefully screened for bleeding events. Bleeding was not observed in any of the animals, not even with the highest dose of rhADAMTS13. However, bleeding was also absent in all mice that received t-PA, so conclusions should be made with caution.
Discussion
As the main finding of this study, we first identified VWF-rich thrombi on histological analysis of stroke patient thrombi retrieved via thrombectomy. Second, using a mouse model of ischemic stroke, we showed that ADAMTS13 is able to dissolve an occlusive VWF-rich thrombus, thereby facilitating efficient thrombolysis and subsequent vessel recanalization.
ADAMTS13 was identified not so long ago as the protease that cleaves VWF, regulating VWF activity.30,31 Indeed, by cleaving the Y1605-M1606 bond in the VWF A2 domain, ADAMTS13 digests large VWF multimers into smaller, less reactive molecules. As a result, ADAMTS13 has been shown to negatively regulate thrombus formation, presumably by cleaving VWF bound to platelets in the growing thrombus.27,32,33 Our observation that occlusive MCA thrombus formation occurs faster in Adamts13−/− mice further supports this concept; however, the presence of unprocessed hyperactive UL-VWF in the circulation of these mice might also contribute to accelerated thrombosis.
Although improved guidelines, new techniques, and faster therapeutic interventions have improved overall patient outcome in stroke management, there is still an urgent need for new and improved thrombolytic agents. Interestingly, Crescente et al showed that ADAMTS13 was able to dissolve occlusive thrombi in the venous microcirculation in a dorsal skin fold chamber thrombosis model.34 However, the direct thrombolytic capacity of ADAMTS13 in a thrombotic stroke model was still largely unknown. Our observations that spontaneous recanalization occurs in Adamts13+/+ mice but not in Adamts13−/− animals after formation of a relatively small but initially occlusive thrombus in the MCA suggest that ADAMTS13 contributes to the overall endogenous thrombolytic capacity. Despite the presence of UL-VWF that could lead to more stable thrombi in Adamts13−/− mice, exogenous rhADAMTS13 was able to efficiently recanalize the MCA in these mice, indicating a thrombolytic potency of ADAMTS13. To fully assess the thrombolytic potential of rhADAMTS13, we further tested and confirmed efficient dissolution of large t-PA–resistant occlusions in C57BL/6J mice, even when ADAMTS13 was administered 1 hour after occlusion. Most probably, ADAMTS13 can promote thrombus dissolution by cleaving VWF that is linking platelets together in the thrombus. We indeed observed that ADAMTS13 is able to reverse crosslinking of platelets in vitro after ristocetin-induced platelet agglutinations. This concept is further supported by an elegant study of Shim et al, showing that platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress.35 Furthermore, Shida and coworkers visualized that ADAMTS13 specifically cleaves VWF on the surface of platelet thrombi in a shear force--dependent manner.36 Hence, by cleaving VWF in the thrombus, ADAMTS13 could exert a prothrombolytic activity. Recently, ADAMTS13 activity was shown to predict response to thrombolysis in patients with acute stroke,37 which strongly corroborates our experimental results on a prothrombolytic ADAMTS13 activity in the setting of acute stroke.
Of note, our results are also in agreement with 2 studies using similar thrombotic stroke models showing that blockade of the VWF-platelet glycoprotein Ib interaction induces thrombus dissolution after occlusive thrombus formation in the MCA.38,39 Thus, targeting VWF either via direct cleavage by ADAMTS13 or by inhibition of VWF-platelet binding might become an interesting alternative strategy in thrombolytic therapy. A combination of both strategies could even further improve thrombolytic success.38 A promising aspect of this approach, at least in experimental models, is the absence of bleeding, in contrast to t-PA.34,38,39 We did not observe any bleeding complications when treating mice with rhADAMTS13, which is in line with previous studies on the safe use of ADAMTS13 in stroke experiments.21 ADAMTS13 even reduced bleeding complications associated with t-PA in another murine stroke model.40
The current study focused on the prothrombolytic role of ADAMTS13 in the acute setting of stroke in which an occlusive thrombus is obstructing MCA blood flow. Previous studies in which a silicon-coated monofilament was used to block MCA blood flow already showed the beneficial effect of ADAMTS13 (and inhibitors of VWF–glycoprotein Ib) in cerebral ischemia/reperfusion injury by dampening both thrombosis and inflammation in the reperfused tissue.13,19,21,41 Hence, ADAMTS13 as a therapeutic agent would not only promote efficient thrombus dissolution as shown in the present study but also directly attenuate subsequent ischemia/reperfusion injury, thereby further improving overall stroke outcome.
In recent years, both clinical and experimental studies have undeniably shown the crucial importance of the VWF/ADAMTS13 axis in stroke: high VWF levels and low ADAMTS13 levels are indeed associated with an increased risk of stroke and even worse stroke outcome.13,18-22,25 Logically, thrombolytic activity of ADAMTS13 will be most effective in patients presenting with intracranial thrombi that are predominantly stabilized by VWF. Because little was known about the composition of human stroke thrombi, we assessed for the first time the presence of VWF in thrombi retrieved from stroke patients. Based on our histological analysis, VWF content in thrombi seems to vary, with some samples being particularly rich in VWF. Although VWF is also implicated in venous thrombus formation,42,43 it seems plausible that thrombi rich in VWF would especially be formed in arterial high-shear conditions, such as thrombi formed on atherosclerotic lesions or in stenosed arteries. Because of our limited number of noncardioembolic thrombi samples, we were not able to draw strong conclusions concerning VWF content and thrombus origin. Future studies using larger cohorts will have to elucidate whether VWF content or organization in stroke thrombi is associated with stroke etiology. Nevertheless, the inverse correlation of VWF presence with red blood cell content in the thrombus is particularly interesting because radiological imaging on admission was shown to predict thrombus red cell content.6,44 Determining thrombus composition from radiological signs may help identify patients who could benefit from specific thrombolytic approaches such as the use of ADAMTS13.
Combination therapy of ADAMTS13 together with t-PA could have a synergistic thrombolytic effect, allowing the use of lower doses of fibrinolytics to reduce the associated risk of bleeding in the acute setting of ischemic stroke. Because the thrombi in our model were completely t-PA–resistant, we were not able to test this in this study. Another limitation of our study was the discrepancy between the embolic nature of acute ischemic stroke in patients and the in situ FeCl3-induced thrombus in our stroke model. Although this model specifically generates VWF-rich thrombi, mimicking some patient thrombi we examined, this model does not recapitulate the variability of stroke thrombi formed in patients.
In conclusion, we here propose that ADAMTS13 has thrombolytic potency, which could become a promising addition to current pharmacological thrombolytic therapy, in particular, in those cases in which t-PA is ineffective.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the FWO (Fonds voor Wetenschappelijk Onderzoek Vlaanderen G.0A86.13 and 1509216N) (S.F.D.M), by an Onderzoekstoelage grant from KU Leuven (OT/14/099) (S.F.D.M.) and by the Deutsche Forschungsgemeinschaft, sonderforschungsbereiche 688 (Teilprojekt A13) (C.K.).
Authorship
Contribution: F.D. acquired, analyzed, and interpreted the data and wrote the manuscript. S.F.D.M. conceived and designed the study, analyzed and interpreted the data, and wrote the manuscript. L.D. and A.V. performed experiments. T.A. and O.F. performed thrombectomy procedures, collected clinical data from the patients, and reviewed the manuscript. H.R., B.P., and F.S. provided essential reagents and reviewed the manuscript. F.L. and C.K. helped setting up the stroke model and reviewed the manuscript. H.D. and K.V. reviewed the manuscript.
Conflict-of-interest disclosure: H.R., B.P., and F.S. are employees of Baxalta Innovations GmbH, Vienna, Austria. The remaining authors declare no competing financial interests.
Correspondence: Simon De Meyer, Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, E. Sabbelaan 53, 8500 Kortrijk, Belgium; e-mail: simon.demeyer@kuleuven-kulak.be.