Key Points
Mpl agonist, but not granulocyte colony-stimulating factor, induces self-renewing HSC divisions and expansions.
Abstract
In steady-state adult hematopoiesis, most hematopoietic stem cells (HSCs) are in the resting phase of the cell cycle. Upon enhanced hematopoietic demand, HSCs can be induced to divide and self-renew or differentiate. However, the cell-extrinsic signals inducing HSC cycling remain to be elucidated. Using in vivo high-resolution single HSC divisional tracking, we directly demonstrate that clinically applied thrombopoietin receptor but not granulocyte colony-stimulating factor (G-CSF) receptor agonists drive HSCs into self-renewing divisions leading to quantitative expansion of functional HSC as defined by their in vivo serial multilineage and long-term repopulating potential. These results suggest that thrombopoietin mimetics might be applicable to expand HSCs in vivo and to sensitize thrombopoietin receptor–expressing HSCs to cell cycle–dependent cytotoxic agents.
Introduction
During steady-state hematopoiesis, 80% to 90% of hematopoietic stem cells (HSCs) are quiescent (ie, are in the resting [G0] phase of the cell cycle).1-4 Thus, at any given time, steady-state hematopoiesis is sustained by a small fraction of actively cycling HSCs and downstream hematopoietic progenitor cells (HPCs).5,6 However, upon demand (eg, massive blood loss, severe infection), blood production can be enhanced several-fold.1-4,7-10 Although this is in part accomplished by HPCs, quiescent HSCs are also temporarily recruited into the cell cycle.3-7 Identification of factors that trigger self-renewing HSC divisions and do not cause HSC loss might inform on new therapeutic interventions. However, revealing HSC self-renewal factors that are altered upon hematopoietic demand remains technically challenging because of the difficulty in dissecting HSC divisional behavior and function in vivo. We recently established an in vivo assay allowing high-resolution single HSC divisional tracking and subsequent assessment of HSC biology by serial transplantation.4 Here, we have applied this assay in order to address the long-standing question of how the clinically used agonists to granulocyte colony-stimulating factor (G-CSF) receptor (CSF3R; Csf3r [Filgrastim]), FMS-like tyrosine kinase 3 (also fetal liver tyrosine kinase 3 [FLT3; Flt3]), and thrombopoietin (TPO; Tpo) receptor (MPL; Mpl [ (Romiplostim]) as well as an antagonist to the chemokine receptor CXCR4 (Cxcr4 [Plerixafor]), affect in vivo maintenance and proliferation of bona fide HSCs.
Study design
Mice
C57BL/6J (CD45.2+) and B6.SJL (CD45.1+) were obtained from The Jackson Laboratories, and both were intercrossed to generate CD45.1/2+ mice.
CFSE chasing, drug challenge, analysis, and serial transplantation
Lin−c-Kit+Sca-1+ cells (LKS) were fluorescence-activated cell sorted (FACS), labeled with 5- and 6- carboxyfluorescein diacetate succinimidyl ester (CFSE), and IV transplanted as described previously.4 One week after transplantation, mice were injected intraperitoneally daily with either phosphate-buffered saline (PBS) or with human to mouse cross-reactive human G-CSF (Filgrastim; 7.5 μg/mouse), human MPL agonist (Romiplostim; 2.5 μg/mouse), human FLT3-ligand (FLT3L-hFc; 30 μg/mouse), or subcutaneously with a human CXCR4 antagonist (Plerixafor; 125 μg/mouse) as specified in the supplemental Methods, available on the Blood Web site. Donor cells were sorted based on CFSE dilution-defined divisional history 1 week after final injections (ie, 3 weeks after initial transfer) and transplanted into lethally irradiated secondary recipients. Four months later, whole bone marrow (BM) cells were serially transplanted into lethally irradiated tertiary recipient mice.
Results and discussion
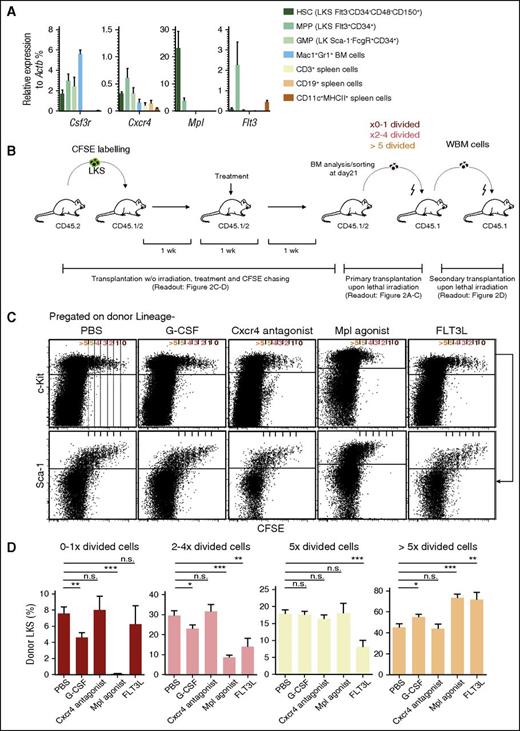
Direct response to a given agonist or antagonist requires respective receptor expression. We compared the relative messenger RNA (mRNA) expression of Csf3r, Cxcr4, Mpl, and Flt3 on phenotypically defined HSCs, multipotent progenitors (MPPs), and granulocyte-macrophage progenitors to mature BM and spleen cells (Figure 1A). Our findings are in line and extend prior reports on respective receptor expression.10-12 They suggest, but do not definitively prove, that HSCs can respond directly to Mpl and to some extent to Csf3r agonists and Cxcr4 antagonist, but not to Flt3-ligand.
Receptor expression and proliferation of LKS upon extrinsic stimulation. (A) Quantitative polymerase chain reaction analysis on mRNA expression of the indicated genes in FACS-sorted BM HSCs (LKS Flt3−CD34−CD48−CD150+), MPPs (LKS Flt3+CD34+), GMPs (LK Sca1−FcgR+CD34+), and granulocytes (Mac1+Gr1+), as well as spleen T (CD3+), B (CD19+), and dendritic cells (CD11c+MHCII+). Relative expression of the indicated genes to the expression of Actb is shown in the respective cell populations (mean ± standard error of the mean), data obtained from 3 independent experiments, n = 4. (B) Experimental scheme: steady-state, nonirradiated mice were transplanted with 0.8 to 1.1 × 105 CFSE-labeled LKS cells; 1 week posttransplantation, they were injected over 1 week as indicated in “Study design.” One week after the final injection and 3 weeks after transplantation of CFSE-labeled LKS, mice were analyzed and cells were subsequently sorted for transplantation as indicated. For secondary transplantation, whole BM was used as indicated. (C) Representative dot plot analysis of lineage-depleted BM cells pregated on donor Lin− (upper) or donor Lin−c-Kit+ cells (lower). (D) Percentage of donor LKS in subsequent divisional groups. Graphs show mean ± standard error of the mean (n = 6-24 mice from 3 to 14 independent experiments). Data analyzed with paired, 2-tailed Student t test. ***P < .001, **P < .01, *P < .05. GMPs, granulocyte-macrophage progenitors; ns, not significant; w/o, without.
Receptor expression and proliferation of LKS upon extrinsic stimulation. (A) Quantitative polymerase chain reaction analysis on mRNA expression of the indicated genes in FACS-sorted BM HSCs (LKS Flt3−CD34−CD48−CD150+), MPPs (LKS Flt3+CD34+), GMPs (LK Sca1−FcgR+CD34+), and granulocytes (Mac1+Gr1+), as well as spleen T (CD3+), B (CD19+), and dendritic cells (CD11c+MHCII+). Relative expression of the indicated genes to the expression of Actb is shown in the respective cell populations (mean ± standard error of the mean), data obtained from 3 independent experiments, n = 4. (B) Experimental scheme: steady-state, nonirradiated mice were transplanted with 0.8 to 1.1 × 105 CFSE-labeled LKS cells; 1 week posttransplantation, they were injected over 1 week as indicated in “Study design.” One week after the final injection and 3 weeks after transplantation of CFSE-labeled LKS, mice were analyzed and cells were subsequently sorted for transplantation as indicated. For secondary transplantation, whole BM was used as indicated. (C) Representative dot plot analysis of lineage-depleted BM cells pregated on donor Lin− (upper) or donor Lin−c-Kit+ cells (lower). (D) Percentage of donor LKS in subsequent divisional groups. Graphs show mean ± standard error of the mean (n = 6-24 mice from 3 to 14 independent experiments). Data analyzed with paired, 2-tailed Student t test. ***P < .001, **P < .01, *P < .05. GMPs, granulocyte-macrophage progenitors; ns, not significant; w/o, without.
To determine the effects of these receptor agonists/antagonists on HSC proliferation and function, we used an in vivo CFSE dilution assay previously demonstrated to faithfully evaluate HSC divisional behavior.4 One week after transplantation of CFSE-labeled HSC-containing LKS into nonconditioned recipient mice, mice were injected repetitively with either PBS, G-CSF, a Cxcr4 antagonist, a Mpl agonist, or FLT3-ligand, followed by BM analysis after 1 week of subsequent rest (ie, 3 weeks after initial LKS cell transfer; experimental scheme depicted in Figure 1B). In PBS-injected mice, few LKS cells remained in the CFSEhi 0×- to 1×-divided fraction, but increasingly more appeared in the subsequent CFSElow/− 2×- to 4×-, 5×-, and >5×-divided fractions, accompanied by a gradual loss of Sca-1 and c-Kit expression4 (Figure 1C-D; supplemental Figure 1). A similar pattern as in PBS-injected mice was observed in Cxcr4 antagonist-receiving mice, whereas G-CSF injections resulted in relatively less 0×- to 1×-, 2×- to 4×-, and equal or increased 5×- or >5×-divided fractions (Figure 1C-D; supplemental Figure 1). In strong contrast, Mpl agonist-injected mice showed very few, if any, 0×- to 1×-divided cells, significantly less or unchanged relative numbers in the 2×- to 4×- and 5×-, and increased numbers in the >5×-divided cellular fraction with higher maintenance of LKS Flt3−Mpl+CD48−CD150+ phenotypes as compared with G-CSF injection (supplemental Figure 2). FLT3 ligand–injected mice showed unchanged 0×- to 1×-, decreased 2×- to 5×-, and increased >5×-divided cells.
Because Mpl agonist–induced in vivo divisional changes of LKS cells were the most striking, we evaluated this in more detail. Mpl-mediated LKS cell proliferation was dose-dependent and led to an increase of divided cells with HSC and MPP immunophenotypes (supplemental Figure 3). Similarly, Mpl stimulation expanded phenotypic HSC-containing fractions in mice without prior transfer of LKS cells (supplemental Figure 4A-B), whereas G-CSF did so to a lesser extent but efficiently mobilized HSCs/HPCs to the spleen (supplemental Figure 4A-D). To test if Mpl agonist–induced HSC/HPC proliferation leads to increased susceptibility to DNA synthesis-targeting drugs,13 we cotreated mice with a sublethal dose of 5-fluorouracil. A significant fraction of Mpl agonist-treated but not PBS- or G-CSF–treated mice died, most likely from hematopoietic failure13 (supplemental Figure 5).
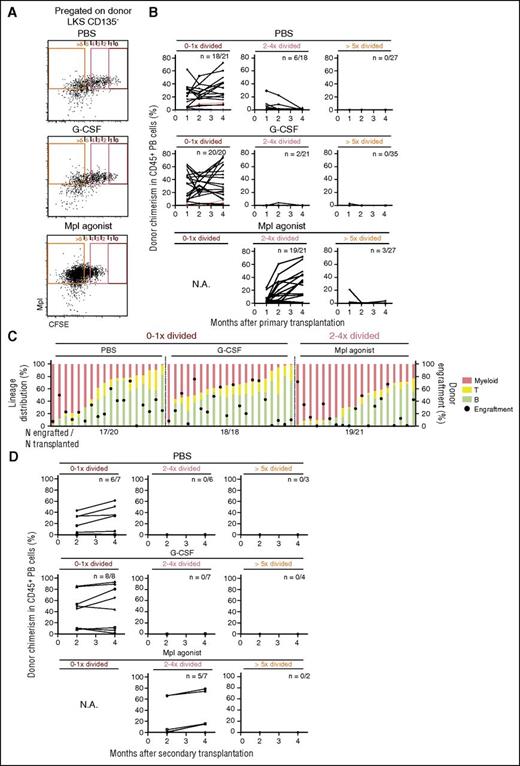
To definitively test whether Mpl stimulation leads to division and expansion of bona fide, functionally defined HSCs, we sorted 7 to 22 LKS Flt3−Mpl+ cells from each of the 0×- to 1×-, 2×- to 4×-, and >5×-divided fractions from PBS-, G-CSF–, or Mpl agonist–treated animals from the experiments in Figure 1B-D and transplanted them into lethally irradiated recipient mice (primary transplantation upon lethal irradiation, Figure 1B and Figure 2A). Subsequent monthly donor chimerism analysis revealed long-term multilineage repopulating HSCs in 0×- to 1×-divided (1 in 11.5 cells), much less in 2×- to 4×-divided (1 in 53.3 cells), and none in >5×-divided cells in PBS-treated mice, confirming prior data on steady-state HSC dormancy (Figure 2B-C; supplemental Figure 6; supplemental Table 1).4 Similar results as in PBS-treated mice were found in G-CSF–treated animals. Again, in strong contrast, Mpl agonist–treated animals showed high repopulating activity in 2×- to 4×-divided (1 in 9.4 cells) and low activity in >5×-divided cells (1 in 178.8 cells) (Figure 2B-C; supplemental Figure 6; supplemental Table 1). Although HSC-defining serial transplantation activity (ie, transplantation activity in secondary recipients upon lethal irradiation as depicted in Figure 1B) was maintained only in the 0×- to 1×- but not 2×- to 4×-divided fractions from PBS- and G-CSF–treated donors, Mpl agonist treatment led to a transition of serially transplantable HSCs to the 2×- to 4×-divided fractions (Figure 2D). Although the estimated HSC frequency in the 0×- to 1×- and 2×- to 4×-divided populations of PBS- and Mpl agonist–treated animals, respectively, was almost the same, the total number of functional HSCs was estimated to be 3.8-fold higher in Mpl agonist–treated animals (Figure 2C; supplemental Figure 1; supplemental Table 1). Thus, Mpl agonist treatment induced division and numeric expansion of serially transplantable HSCs.
Mpl agonist treatment induces HSC division and self-renewal. (A) Representative BM FACS plots and sorting gates (pregated on donor LKS Flt3−) from animals transplanted 3 weeks prior with CFSE-labeled LKS cells and subsequently treated with PBS, G-CSF, or Mpl agonist as indicated in “Study design” and Figure 1B. (B) Donor chimerism in peripheral blood (PB) observed over 4 months of recipients of cells sorted from 0×- to 1×-, 2×- to 4×-, and >5×-divided cells from PBS, G-CSF, and Mpl agonist-treated animals as indicated. Graphs show individual donor chimerism from animals transplanted with 7 cells (blue line, n = 1), 12 cells (red lines, n = 2), 17 cells (green line, n = 1), and 20 to 22 cells (black lines). Number of animals engrafted (positive cutoff >0.4%)/number of animals transplanted is indicated on each graph. (C) Donor cell engraftment (cutoff >0.4%) and lineage distribution (myeloid lineage, T cells and B cells) in recipient mice PB at month 4. (D) Donor chimerism in PB over 4 months in recipients of whole BM from animals in panels B and C. Number of engrafted vs transplanted animals (positive cutoff >0.4%) is shown in each graph. N.A., not applicable.
Mpl agonist treatment induces HSC division and self-renewal. (A) Representative BM FACS plots and sorting gates (pregated on donor LKS Flt3−) from animals transplanted 3 weeks prior with CFSE-labeled LKS cells and subsequently treated with PBS, G-CSF, or Mpl agonist as indicated in “Study design” and Figure 1B. (B) Donor chimerism in peripheral blood (PB) observed over 4 months of recipients of cells sorted from 0×- to 1×-, 2×- to 4×-, and >5×-divided cells from PBS, G-CSF, and Mpl agonist-treated animals as indicated. Graphs show individual donor chimerism from animals transplanted with 7 cells (blue line, n = 1), 12 cells (red lines, n = 2), 17 cells (green line, n = 1), and 20 to 22 cells (black lines). Number of animals engrafted (positive cutoff >0.4%)/number of animals transplanted is indicated on each graph. (C) Donor cell engraftment (cutoff >0.4%) and lineage distribution (myeloid lineage, T cells and B cells) in recipient mice PB at month 4. (D) Donor chimerism in PB over 4 months in recipients of whole BM from animals in panels B and C. Number of engrafted vs transplanted animals (positive cutoff >0.4%) is shown in each graph. N.A., not applicable.
Our data and that of others demonstrate that HSCs do not express Flt3,10 explaining the lack of FLT3-ligand action on HSCs (Figure 1A). In contrast, it has been suggested that ∼80% of Rho123lowLKS (containing HSCs with a 25% purity)14 express Csf3r,15 and G-CSF induces divisions of immunophenotypically defined HSCs.3,16 Although we confirmed low Csfr3 mRNA expression in immunophenotypic-defined HSCs, we did not observe G-CSF–driven self-renewing divisions of functional HSCs (Figure 1A,C; Figure 2B). This is not a result of insufficient G-CSF dosing, because in fact G-CSF led to massive neutrophilia (data not shown) and hematopoietic stem and progenitor cell mobilization (supplemental Figure 4)3,16,17 ; this might be explained by limitations of previous labeling assays to test HSCs by functional readouts. The data presented here together with data on no/low CSF3R expression on human HSCs18 might explain why clinical coapplication of chemotherapy with G-CSF to “prime” acute myeloid leukemia cells toward chemotherapy did not result in the loss of healthy HSCs, permitting normal but not delayed hematopoietic recovery.19
In contrast to Flt3 and Csf3r, it has been clearly demonstrated that Cxcr4 and Mpl are expressed on HSCs11,12,20 (Figure 1A; Figure 2A), and that Cxcr4 signaling is required to maintain HSC quiescence in adult mice.11,20 Our data directly demonstrate that HSCs are not activated from dormancy by Cxcr4-antagonist application, even at doses that exceed doses used to mobilize HSCs in mice and in humans.21 In strong contrast, we directly demonstrate that supra-steady-state enhanced Mpl stimulation drives dormant HSCs into division and expansion, a situation that might occur, for example, in sepsis-mediated inflammation.22,23 Indeed, prior studies demonstrated that Tpo and Mpl signaling is essential for HSC homeostasis24,25 and their expansion after transplantation,25 whereas, seemingly contradictorily, other studies demonstrated that Tpo and Mpl signaling is critical for keeping HSCs in a quiescent state.12,24 We suggest that this discrepancy might result from Mpl signaling strength: although data on HSC quiescence stem from loss-of-function studies,12,24 we examined here the effect of supra-steady-state Mpl signaling, more comparable to studies on higher doses of thrombopoietin administration12 or on genetic deficiency of lymphocyte adaptor protein, a negative regulator of Tpo-induced signaling.26 However, because the CFSE-dilution assay informs on divisional history of cells, but not on cell-cycle status at the moment of analysis, our data do not exclude the possibility that, after several Mpl-induced cell divisions, HSCs return to G0 and become more quiescent than before.
Given the enhancement of multilineage regeneration, possibly via reexpansion of human HSCs in aplastic anemia,27 and our data on supra-steady-state Mpl-stimulation-mediated HSC sensitization to chemotherapy, we speculate that MPL agonist treatment might be tested in clinical settings as an add-on to conditioning chemotherapy before allogeneic HSC transplantation in order to reduce endogenous HSCs. Furthermore, we speculate that, if MPL is expressed on malignant counterparts of HSCs, these could also be targeted by MPL agonist chemosensitization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Steffen Boettcher for critical comments on the manuscript.
This work was supported in part by the Forschungskredit of the University of Zurich (L.V.K.), the Japanese Society for the Promotion of Science (KAKENHI; grant 15H01519) (H.T.), the Swiss National Science Foundation (grant 310030_146528), and the Promedica Foundation (Chur, Switzerland) (M.G.M.).
Authorship
Contribution: L.V.K. and H.T. designed research, performed experiments, analyzed results, and wrote the paper; L.V.K. made the figures; and M.G.M. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Hematology, University Hospital and University of Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland; e-mail: markus.manz@usz.ch; and Hitoshi Takizawa, International Research Center for Medical Sciences, Kumamoto University, Kumamoto 860-0811, Japan; e-mail: htakizawa@kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal