Key Points
Cells expressing JAK2 E846D or R1063H exhibit pathologic STAT5 activation in the specific context of EPOR.
Cooperation of germ line JAK2 mutations E846D and R1063H defines a JAK2-signaling threshold for induction of erythrocytosis.
Abstract
The role of somatic JAK2 mutations in clonal myeloproliferative neoplasms (MPNs) is well established. Recently, germ line JAK2 mutations were associated with polyclonal hereditary thrombocytosis and triple-negative MPNs. We studied a patient who inherited 2 heterozygous JAK2 mutations, E846D from the mother and R1063H from the father, and exhibited erythrocytosis and megakaryocytic atypia but normal platelet number. Culture of erythroid progenitors from the patient and his parents revealed hypersensitivity to erythropoietin (EPO). Using cellular models, we show that both E846D and R1063H variants lead to constitutive signaling (albeit much weaker than JAK2 V617F), and both weakly hyperactivate JAK2/STAT5 signaling only in the specific context of the EPO receptor (EPOR). JAK2 E846D exhibited slightly stronger effects than JAK2 R1063H and caused prolonged EPO-induced phosphorylation of JAK2/STAT5 via EPOR. We propose that JAK2 E846D predominantly contributes to erythrocytosis, but is not sufficient for the full pathological phenotype to develop. JAK2 R1063H, with very weak effect on JAK2/STAT5 signaling, is necessary to augment JAK2 activity caused by E846D above a threshold level leading to erythrocytosis with megakaryocyte abnormalities. Both mutations were detected in the germ line of rare polycythemia vera, as well as certain leukemia patients, suggesting that they might predispose to hematological malignancy.
Introduction
Somatic JAK2 mutations are the most common disease-causing event in patients with myeloproliferative neoplasms (MPNs). In the majority of MPN patients, an acquired gain-of-function V617F JAK2 mutation leads to constitutive activation of the JAK2 kinase and subsequently to excessive activation of JAK/STAT signaling.1-4 Recently, germ line JAK2 mutations (different from V617F substitution) have been described in cases with familial MPNs exhibiting hereditary polyclonal thrombocytosis5-7 and triple-negative MPNs.8 All of these inherited JAK2 mutations, localized in the kinase and pseudokinase domain of JAK2, signal through the thrombopoietin receptor (MPL) rather than the erythropoietin (EPO) receptor (EPOR), explaining the phenotype of the polyclonal disease. It is proposed that differential signaling of STATs leads to different clinical phenotypes associated with different activating JAK2 mutations: essential thrombocythemia and hereditary thrombocytosis being promoted by MPL/STAT1 signaling; STAT5 activation resulting in excessive erythroid proliferation and polycythemia vera (PV).6,9
Here, we studied a patient who inherited 2 JAK2 mutations and presented with erythrocytosis and abnormal megakaryopoiesis in the bone marrow (BM), partially resembling PV cases with JAK2 exon 12 mutations.10 Thus, in contrast to previously studied germ line JAK2 mutations, which were all heterozygous, here mutations on both JAK2 alleles are present. We show that both of these mutations are activating in the context of EPOR, but with different characteristics. Functional analyses of the 2 germ line JAK2 mutations suggested the threshold of JAK2 activity necessary for induction of erythrocytosis.
Study design
Descriptions of assays and tests performed on patient’s samples can be found in supplemental Materials and methods (available on the Blood Web site).
Ba/F3-EPOR11 JAK2 stable transfectants or γ-2A cells were used in viability and proliferation assays, analyses of JAK2 signal transduction, and dual-luciferase assays for STAT transcriptional activities. Retrovirally transduced mouse BM cells were used for in vitro colony assay. Analysis of x-ray crystallography data was performed using PyMOL software. For detailed descriptions of individual procedures, see supplemental Materials and methods.
Results and discussion
Case presentation, EPO hypersensitivity of erythroid progenitors, and mutational analyses
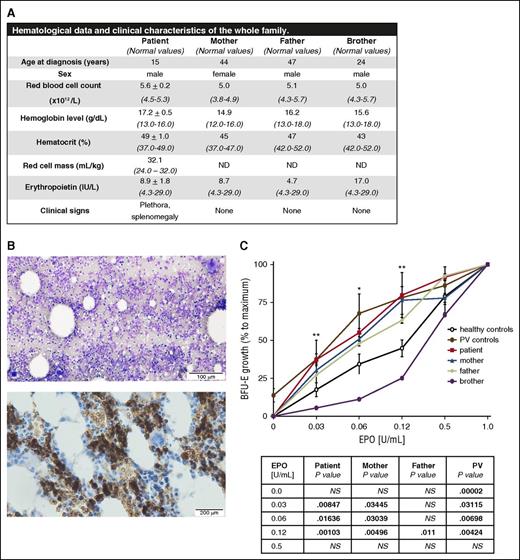
The diagnosis of polycythemia in a 15-year-old boy was based on the hematological and clinical data summarized in Figure 1A. The patient had increased hemoglobin/hematocrit level, higher red blood cell count, plethora, palpable spleen, and normal EPO level. Although the white blood cell differential and platelet count were normal (supplemental Table 1), the BM aspirate and BM biopsy showed hypercellularity, erythroid hyperplasia, and atypical, enlarged, and immature megakaryocytes, likely resulting from the stimulation of the megakaryocytic lineage (Figure 1B; supplemental Figure 1). The iron status parameters were normal (supplemental Table 2). The in vitro colony assay revealed hypersensitivity of the patient’s burst-forming unit-erythroid (BFU-E) progenitors to EPO (Figure 1C), a feature characteristic for primary polycythemia states.12 Both parents and the patient’s brother were clinically normal, with normal EPO levels and hematological (Figure 1A) and iron status parameters, with the exception of elevated ferritin in the father and brother likely due to infection (supplemental Table 2). Nevertheless, the sensitivity of BFU-E progenitors to EPO was significantly increased in the mother and slightly also in the father compared with healthy controls and the patient’s brother (Figure 1C). In addition, the colony assay revealed significantly increased numbers of circulating BFU-E progenitors in the patient and his parents compared with healthy controls (supplemental Figure 2A). These results suggested the hereditary nature of the propositus’s erythrocytosis. No somatic mutations or chromosomal aberrations were uncovered by either whole-exome sequencing or microarray analysis (supplemental Table 3i-ii), suggesting that the hematopoiesis was likely polyclonal. Targeted mutational screening (see supplemental Materials and methods) and search for germ line variants using whole-exome sequencing revealed a E846D substitution in JAK2 in a heterozygous state in the propositus and his mother and JAK2 R1063H in the propositus and his father (supplemental Results; supplemental Figure 2B); both variants were reported as polymorphisms with very low frequencies (supplemental Table 4). Subsequent screening of a cohort of 99 subjects (including healthy controls, MPN patients, and pediatric patients with essential thrombocythemia or hereditary erythrocytosis) detected JAK2 E846D substitution in 1 CALR+/V617F+ MPN patient and JAK2 R1063H in 1 V617F+ PV patient (supplemental Table 5) and in 1 triple-negative MPN patient (J.D.M.F. and R.K., unpublished data, August 29, 2015). The E846D substitution was previously reported in normal-karyotype acute myeloid leukemia13 and the JEG-3 cancer cell line14 ; R1063H was reported in 3 JAK2 V617F+ PV patients,2 as well as in acute myeloid leukemia13 and B-cell acute lymphoblastic leukemia cases.15 Altogether, these data suggest a much higher frequency of these 2 variants in MPN patients than in normal individuals.
Hematological parameters, clinical data, BM evaluation, and in vitro sensitivity assay of erythroid progenitors to EPO. (A) Hematological and clinical analysis of the propositus and his family members revealed erythrocytosis, plethora, and palpable spleen (splenic length of 12 cm based on ultrasound measurement) in the patient. ND, not done. (B) Patient’s BM aspirate (May-Grünwald-Giemsa staining, top) and BM biopsy (glycophorin C staining specific for erythroid lineage, brown color, bottom) showed hypercellularity, erythroid hyperplasia, normal granulopoiesis, and abnormal megakaryopoiesis (for details, see supplemental Figure 1). The images were visualized with an Olympus BX41 light microscope (Hamburg, Germany) and acquired with an Olympus DP73 camera driven by CellSens Entry software. Images were labeled using Adobe Photoshop software (Adobe Systems, San Jose, CA). Top: magnification, ×100; scale bar, 100 µm. Bottom: magnification, ×200; scale bar, 200 µm. (C) The EPO dose-response curves derived from the patient, patient’s brother, patient’s parents, normal controls, and PV patients. The growth of BFU-E colonies at the indicated concentrations of EPO was expressed as a percentage of maximal EPO stimulation (represented by EPO concentration of 1 U/mL). Erythroid progenitors from the patient (red curve) were hypersensitive to EPO; there was a relatively higher number of BFU-E colonies in comparison with healthy controls (black curve) in low EPO concentrations. The in vitro growth of the erythroid progenitors of both of the patient’s parents (blue and green curve, respectively) also showed slightly increased sensitivity to EPO when compared with normal controls (black curve). The progenitors of the patient’s brother (purple curve) showed normal growth. Two PV patients, positive for the V617F mutation (brown curve), were used as positive controls for hypersensitivity and formation of EPO-independent colonies (EECs). The table below the graph shows statistical evaluation of the BFU-E colony number at individual concentrations with respect to normal controls. P values were calculated using Origin 6.1 software (OriginLab Corporation, Norhampton, MA, USA). *P < .05; **P < .01. NS, not significant.
Hematological parameters, clinical data, BM evaluation, and in vitro sensitivity assay of erythroid progenitors to EPO. (A) Hematological and clinical analysis of the propositus and his family members revealed erythrocytosis, plethora, and palpable spleen (splenic length of 12 cm based on ultrasound measurement) in the patient. ND, not done. (B) Patient’s BM aspirate (May-Grünwald-Giemsa staining, top) and BM biopsy (glycophorin C staining specific for erythroid lineage, brown color, bottom) showed hypercellularity, erythroid hyperplasia, normal granulopoiesis, and abnormal megakaryopoiesis (for details, see supplemental Figure 1). The images were visualized with an Olympus BX41 light microscope (Hamburg, Germany) and acquired with an Olympus DP73 camera driven by CellSens Entry software. Images were labeled using Adobe Photoshop software (Adobe Systems, San Jose, CA). Top: magnification, ×100; scale bar, 100 µm. Bottom: magnification, ×200; scale bar, 200 µm. (C) The EPO dose-response curves derived from the patient, patient’s brother, patient’s parents, normal controls, and PV patients. The growth of BFU-E colonies at the indicated concentrations of EPO was expressed as a percentage of maximal EPO stimulation (represented by EPO concentration of 1 U/mL). Erythroid progenitors from the patient (red curve) were hypersensitive to EPO; there was a relatively higher number of BFU-E colonies in comparison with healthy controls (black curve) in low EPO concentrations. The in vitro growth of the erythroid progenitors of both of the patient’s parents (blue and green curve, respectively) also showed slightly increased sensitivity to EPO when compared with normal controls (black curve). The progenitors of the patient’s brother (purple curve) showed normal growth. Two PV patients, positive for the V617F mutation (brown curve), were used as positive controls for hypersensitivity and formation of EPO-independent colonies (EECs). The table below the graph shows statistical evaluation of the BFU-E colony number at individual concentrations with respect to normal controls. P values were calculated using Origin 6.1 software (OriginLab Corporation, Norhampton, MA, USA). *P < .05; **P < .01. NS, not significant.
JAK2 E846D and R1063H promote survival at low EPO concentrations by increasing EPO-induced phosphorylation of STAT5
Because the germ line JAK2 mutations emerged as the main disease-causing event in our patient, we created a cellular model: Ba/F3-EPOR cells expressing JAK2_E846D and JAK2_R1063H. Ba/F3-EPOR cells transfected with the wild-type human JAK2 (JAK2_wt) and V617F JAK2 were used as a negative and positive control, respectively.16 As expected, the JAK2_V617F transfectants exhibited survival advantage and supported constitutive proliferation in EPO-free or EPO-limiting conditions1,4,17 (supplemental Figure 3A; Figure 2A). The viability of JAK2_E846D and JAK2_R1063H transfectants in EPO-free medium was comparable to the JAK2_wt transfectants (supplemental Figure 3A); neither these transfectants expressing individual JAK2 genetic variants nor double transfectants with both E846D and R1063H (not shown) became growth factor independent. Nevertheless, JAK2_E846D–expressing cells showed significantly increased proliferation in EPO-limiting conditions compared with JAK2_wt (Figure 2A). Improved proliferation for JAK2_R1063H transfectants was observed only in 0.1 U/mL EPO concentration (Figure 2A). These results suggested that E846D and R1063H substitutions have a weaker effect on the survival and proliferation capacity of cells than the oncogenic V617F mutation, which is consistent with their germ line transmission and with nonclonal hematopoiesis observed in the JAK2 E846D+ mother (described in supplemental Materials and methods). This also showed a stronger effect of E846D substitution on improved proliferation in EPO-limiting conditions compared with R1063H.
Proliferation assay in cytokine-limiting conditions, immunoblot analysis of JAK2 signal transduction, luciferase assay, and colony assay of retrovirally transduced BM cells. (A) MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) test in Ba/F3-EPOR-IL-3–dependent cells stably transfected with different JAK2 vectors. The percentage of proliferating cells was calculated as the percentage of the maximal cell growth observed at EPO concentration of 1.0 U/mL. The transfectants were starved for 12 hours in IL-3–free media and then incubated for 48 hours in the media with different EPO concentration. Results are shown as the mean ± standard deviation (SD) (n = at least 4 tests performed in triplicates). The table below the graph shows statistical evaluation of the growth of individual Ba/F3 transfectants at tested EPO concentrations compared with cell-expressing JAK2 wt. P values were calculated using Origin 6.1 software (OriginLab Corporation). (B) JAK2 downstream signaling in stable Ba/F3-EPOR-IL-3–dependent transfectants. The cells were starved in IL-3–free media for 12 hours and then stimulated with indicated concentrations of EPO for 15 minutes. JAK2_V617F–expressing cells served as a positive control and showed constitutive activation of STAT5 (i-ii). Immunoblot and subsequent densitometry analysis revealed that JAK2_E846D transfectants and also R1063H-expressing cells showed increase in STAT5 activation (i-ii). Each bar represents the ratio of the density of phosphorylated STAT5 (p-STAT5) to the density of normalized total JAK2 and is presented as fold change against the ratio calculated for 1.0 U/mL EPO. For detailed information on JAK2 normalization, see supplemental Materials and methods. Activation of JAK2 and its targets was determined by antibodies recognizing specific phosphorylation sites (as detailed in supplemental Materials and methods); tubulin antibody was used as a loading control. (C) STAT5 transcriptional activity in JAK2-deficient γ-2A cells transfected with various JAK2 complementary DNAs (cDNAs) in the presence of EPOR, MPL, and G-CSF receptor (G-CSFR). The heterozygous configuration was mimicked by cotransfection of JAK2 wt cDNA with JAK2 V617F, JAK2 E846D, or JAK2 R1063H. Four hours after transfection, the cells were stimulated with different concentrations of EPO (i) or stimulated with 10 ng/mL thrombopoietin (ii) or 10 ng/mL G-CSF (iii) and luminescence was detected in cell lysates 24 hours (ii-iii) or 48 hours (i) after transfection using a PerkinElmer Victor X Light analyzer. (i) STAT5 transcriptional activity downstream of JAK2 E846D and R1063H was significantly increased in both EPO-free and EPO-limiting condition in comparison with JAK2 wt in the presence of EPOR; the double mutants expressing both JAK2 E846D and R1063H mutants showed increased STAT5 transcriptional activity over the single mutants in low EPO concentration. (ii-iii) Only JAK2 V617F significantly increased STAT5 transcriptional activity in the presence of MPL and G-CSFR. STAT5 transcriptional activity of JAK2 E846D and JAK2 R1063H cells was comparable to wt cells. The panels show the average of 3 independent experiment ± standard error of the mean (SEM). *P < .05, **P < .01, ***P < .001 using the 2-tailed Student t test. rlu, relative light unit. (D) In vitro colony growth of transduced murine BM. Murine BM cells were infected with retroviruses coding for murine JAK2 wt, V617F, E846D, or R1063H for single mutant configuration and with E846D and R1063H retroviral particles for double mutant configuration. Infected murine BM cells were then plated on methylcellulose media in the absence of EPO (i) or in the presence of 0.1 U/mL EPO (ii). After 2 and 7 days, the plates were evaluated for the formation of CFU-E and BFU-E, respectively. The BM cells infected with the virus containing the empty pMEGIX vector were used as a control. JAK2_E846D and JAK2_E846D/R1063H BM cells have significantly higher numbers of CFU-E and BFU-E colonies in both EPO-free and low EPO conditions when compared with JAK2_wt cells. Cells with JAK2_R1063H mutation had significantly increased CFU-E, but not BFU-E colony formation. The highest number of erythroid colonies was detected in the sample derived from JAK2_V617F positive control. Congruent results were obtained by determination of the replating capacity of primary human cells naturally harboring the JAK2 E846D and R1063H mutations (supplemental Figure 6). The panels show the average of 3 independent experiments. The BFU-E and CFU-E colony numbers are expressed as the number of colonies per transduced cells and are normalized to wt values ± SD. P values were calculated using Origin 6.1 software. *P < .05, **P < .01, ***P < .001. (E) Immunoblot analysis of prolonged activity of E846D-mutant JAK2 kinase. The JAK2 transfectants were after IL-3 starvation stimulated with 20 U/mL EPO for 15 minutes and then incubated in base Iscove modified Dulbecco medium (IMDM) with no additives for indicated periods of time. JAK2_E846D–expressing cells showed prolonged activation of STAT5 and EKR1/2 similarly to JAK2_V617F transfectants as predicted by in silico modeling (supplemental Figure 7).
Proliferation assay in cytokine-limiting conditions, immunoblot analysis of JAK2 signal transduction, luciferase assay, and colony assay of retrovirally transduced BM cells. (A) MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) test in Ba/F3-EPOR-IL-3–dependent cells stably transfected with different JAK2 vectors. The percentage of proliferating cells was calculated as the percentage of the maximal cell growth observed at EPO concentration of 1.0 U/mL. The transfectants were starved for 12 hours in IL-3–free media and then incubated for 48 hours in the media with different EPO concentration. Results are shown as the mean ± standard deviation (SD) (n = at least 4 tests performed in triplicates). The table below the graph shows statistical evaluation of the growth of individual Ba/F3 transfectants at tested EPO concentrations compared with cell-expressing JAK2 wt. P values were calculated using Origin 6.1 software (OriginLab Corporation). (B) JAK2 downstream signaling in stable Ba/F3-EPOR-IL-3–dependent transfectants. The cells were starved in IL-3–free media for 12 hours and then stimulated with indicated concentrations of EPO for 15 minutes. JAK2_V617F–expressing cells served as a positive control and showed constitutive activation of STAT5 (i-ii). Immunoblot and subsequent densitometry analysis revealed that JAK2_E846D transfectants and also R1063H-expressing cells showed increase in STAT5 activation (i-ii). Each bar represents the ratio of the density of phosphorylated STAT5 (p-STAT5) to the density of normalized total JAK2 and is presented as fold change against the ratio calculated for 1.0 U/mL EPO. For detailed information on JAK2 normalization, see supplemental Materials and methods. Activation of JAK2 and its targets was determined by antibodies recognizing specific phosphorylation sites (as detailed in supplemental Materials and methods); tubulin antibody was used as a loading control. (C) STAT5 transcriptional activity in JAK2-deficient γ-2A cells transfected with various JAK2 complementary DNAs (cDNAs) in the presence of EPOR, MPL, and G-CSF receptor (G-CSFR). The heterozygous configuration was mimicked by cotransfection of JAK2 wt cDNA with JAK2 V617F, JAK2 E846D, or JAK2 R1063H. Four hours after transfection, the cells were stimulated with different concentrations of EPO (i) or stimulated with 10 ng/mL thrombopoietin (ii) or 10 ng/mL G-CSF (iii) and luminescence was detected in cell lysates 24 hours (ii-iii) or 48 hours (i) after transfection using a PerkinElmer Victor X Light analyzer. (i) STAT5 transcriptional activity downstream of JAK2 E846D and R1063H was significantly increased in both EPO-free and EPO-limiting condition in comparison with JAK2 wt in the presence of EPOR; the double mutants expressing both JAK2 E846D and R1063H mutants showed increased STAT5 transcriptional activity over the single mutants in low EPO concentration. (ii-iii) Only JAK2 V617F significantly increased STAT5 transcriptional activity in the presence of MPL and G-CSFR. STAT5 transcriptional activity of JAK2 E846D and JAK2 R1063H cells was comparable to wt cells. The panels show the average of 3 independent experiment ± standard error of the mean (SEM). *P < .05, **P < .01, ***P < .001 using the 2-tailed Student t test. rlu, relative light unit. (D) In vitro colony growth of transduced murine BM. Murine BM cells were infected with retroviruses coding for murine JAK2 wt, V617F, E846D, or R1063H for single mutant configuration and with E846D and R1063H retroviral particles for double mutant configuration. Infected murine BM cells were then plated on methylcellulose media in the absence of EPO (i) or in the presence of 0.1 U/mL EPO (ii). After 2 and 7 days, the plates were evaluated for the formation of CFU-E and BFU-E, respectively. The BM cells infected with the virus containing the empty pMEGIX vector were used as a control. JAK2_E846D and JAK2_E846D/R1063H BM cells have significantly higher numbers of CFU-E and BFU-E colonies in both EPO-free and low EPO conditions when compared with JAK2_wt cells. Cells with JAK2_R1063H mutation had significantly increased CFU-E, but not BFU-E colony formation. The highest number of erythroid colonies was detected in the sample derived from JAK2_V617F positive control. Congruent results were obtained by determination of the replating capacity of primary human cells naturally harboring the JAK2 E846D and R1063H mutations (supplemental Figure 6). The panels show the average of 3 independent experiments. The BFU-E and CFU-E colony numbers are expressed as the number of colonies per transduced cells and are normalized to wt values ± SD. P values were calculated using Origin 6.1 software. *P < .05, **P < .01, ***P < .001. (E) Immunoblot analysis of prolonged activity of E846D-mutant JAK2 kinase. The JAK2 transfectants were after IL-3 starvation stimulated with 20 U/mL EPO for 15 minutes and then incubated in base Iscove modified Dulbecco medium (IMDM) with no additives for indicated periods of time. JAK2_E846D–expressing cells showed prolonged activation of STAT5 and EKR1/2 similarly to JAK2_V617F transfectants as predicted by in silico modeling (supplemental Figure 7).
We then focused on JAK2 downstream signal transduction pathways. Upon interleukin-3 (IL-3) starvation and short incubation in IL-3–free EPO-containing medium, JAK2_E846D– and JAK2_R1063H–expressing cells showed increased STAT5 phosphorylation (Figure 2B). Weak activation of STAT1 was also observed for both mutants (supplemental Figure 3B).
JAK2 E846D and R1063H activate STAT5 transcriptional activity specifically through EPOR
Next, we tested the effect of our JAK2 mutants on STAT transcriptional activity by dual-reporter luciferase assay in JAK2-deficient γ-2A cells.18 Figure 2Ci shows that JAK2 E846D and R1063H significantly increased STAT5 transcriptional activity in EPO-free and low EPO conditions in the presence of EPOR when compared with cells expressing JAK2 wt. In EPO-limiting conditions, the coexpression of these 2 JAK2 mutants further improved STAT5 transcriptional activity compared with cells expressing a single JAK2 mutant (E846D or R1063H). No effect of JAK2 E846D and R1063H was observed on basal STAT5 activation via MPL or the granulocyte colony-stimulating factor (G-CSF) receptor (Figure 2Cii-iii) or on the activation of other STAT members (supplemental Figure 4). This was in agreement with increased STAT5 phosphorylation in the patient’s BM detected by immunohistochemical analysis of STAT5 (supplemental Figure 5) and is consistent with the erythroid phenotype in our patient.
JAK2 E846D–transduced murine BM cells show increased capacity for CFU-E and BFU-E formation whereas JAK2 R1063H increases only CFU-E formation
To assess the effect of JAK2 mutants on primary cells, BM cells from C57BL/6 mice were retrovirally transduced with murine JAK2 expression vectors and used for in vitro colony assay. The cells expressing the E846D mutant formed significantly increased numbers of colony forming unit-erythroid (CFU-E) and BFU-E, when compared with cells with JAK2 wt in EPO-free or EPO-limiting conditions (Figure 2D). The observation that the R1063H mutant induced CFU-E rather than BFU-E colony growth (see also primary father’s progenitors in supplemental Figure 6) is reminiscent of the weak activation of EPOR by the anemic strain of the Friend virus complex gp55 envelope protein (gp55-A), which also specifically supported CFU-E formation.19,20 For the EPO-free condition, the co-occurrence of the 2 mutations was slightly more effective in colony growth than the single mutants alone (Figure 2D). This assay indicated that JAK2 E846D and R1063H mutations facilitate hypersensitive response of erythroid progenitors from the patient or patient’s parents to EPO.
In silico analysis predicted increased activity of JAK2 R1063H–mutant kinase and prolonged activation of JAK2 E846D–mutant kinase after cytokine stimulation
In silico modeling using the x-ray structures of the inactive and active JH1 domain of JAK221,22 suggested that the R1063H mutation would be presumed to facilitate the active conformation of JH1, whereas the JAK2 E846D mutant would show slower return to the ground state after cytokine stimulation (supplemental Figure 7). The effect of E846D substitution was confirmed by immunoblots analysis in which IL-3–starved JAK2_E846D transfectants showed prolonged activation of STAT5 and extracellular signal-regulated kinase 1/2 (ERK1/2) compared with JAK2_wt after high-dose EPO stimulation and subsequent EPO withdrawal (Figure 2E) resembling signaling downstream EPOR gain-of-function mutants.23 The prolonged STAT5 and ERK1/2 activation downstream JAK2_E846D can be effectively reduced by JAK2 inhibitors, ruxolitinib or AZ-960, but not by wortmannin, a phosphoinositide-3-kinase inhibitor (supplemental Figure 8).
In summary, we establish that E846D and R1063H are weakly activating mutations and conclude that the cooperation of these activating JAK2 mutations causes a polyclonal hereditary erythrocytosis with megakaryocytic atypia, clinically resembling JAK2 exon 12–positive PV.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michael Schuster and Donat Alpar (the Biomedical Sequencing Facility at CeMM) as well as Martin Tichy and Anna Lapcikova (University Hospital and Faculty of Medicine and Dentistry in Olomouc) for their technical assistance, and Srdan Verstovsek (MD Anderson, Houston, TX) for critical reading of the paper and discussions.
This work was supported by Czech Science Foundation Project P301/12/1503 (V.D.). J.F.K. was supported by CZ.1.07/2.3.00/30.0041. Support to S.N.C. was from the Fonds de la Recherche Scientifique–Fonds National de la Recherche Scientifique, Belgium, the Salus Sanguinis Foundation, the Action de Recherche Concertée projects MEXP31C1 and ARC10/15-027 of the University catholique de Louvain, Brussels, the Fondation contre le Cancer, Brussels, the Pôle d'Attraction Interuniversitaire Programs BCHM61B5, and Belgian Medical Genetics Initiative (BeMG). E.L. was supported by a Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture PhD fellowship. The Sonderforschungsbereich grant from the Austrian Science Fund F4702-B20 is acknowledged for its generous support to R.K. J.D.M.F. acknowledges support from L’ORÉAL Österreich, For Women in Science Fellowship.
Authorship
Contribution: K.K. and M.H. designed and performed research, analyzed data, and wrote the paper; C.P. designed and performed research, analyzed data, and reviewed the paper; J.F.K. performed research, analyzed data, and reviewed the paper; D.P. recruited the patient and contributed to the editing of the paper; E.L. performed research and contributed to the writing of the paper; B.K. performed research and analyzed data; J.D.M.F. designed and performed research and analyzed data; F.S. analyzed data; R.K. contributed to study design, analyzed data, reviewed the paper, and provided financial support; S.N.C. contributed to study design, analyzed data, wrote the paper, and provided financial support; and V.D. designed the study, analyzed data, wrote the paper, and provided financial support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vladimir Divoky, Department of Biology, Faculty of Medicine and Dentistry, Palacký University, Hnevotinska 3, Olomouc, 775 15, Czech Republic; e-mail: vladimir.divoky@upol.cz; or Stefan N. Constantinescu, Signal Transduction and Molecular Hematology Unit, Ludwig Institute for Cancer Research and de Duve Institute, Université catholique de Louvain, Avenue Hippocrate 74, UCL 75-4, Brussels B-1200, Belgium; e-mail: stefan.constantinescu@bru.licr.org.
References
Author notes
K.K., M.H., and C.P. contributed equally to this work.

![Figure 2. Proliferation assay in cytokine-limiting conditions, immunoblot analysis of JAK2 signal transduction, luciferase assay, and colony assay of retrovirally transduced BM cells. (A) MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) test in Ba/F3-EPOR-IL-3–dependent cells stably transfected with different JAK2 vectors. The percentage of proliferating cells was calculated as the percentage of the maximal cell growth observed at EPO concentration of 1.0 U/mL. The transfectants were starved for 12 hours in IL-3–free media and then incubated for 48 hours in the media with different EPO concentration. Results are shown as the mean ± standard deviation (SD) (n = at least 4 tests performed in triplicates). The table below the graph shows statistical evaluation of the growth of individual Ba/F3 transfectants at tested EPO concentrations compared with cell-expressing JAK2 wt. P values were calculated using Origin 6.1 software (OriginLab Corporation). (B) JAK2 downstream signaling in stable Ba/F3-EPOR-IL-3–dependent transfectants. The cells were starved in IL-3–free media for 12 hours and then stimulated with indicated concentrations of EPO for 15 minutes. JAK2_V617F–expressing cells served as a positive control and showed constitutive activation of STAT5 (i-ii). Immunoblot and subsequent densitometry analysis revealed that JAK2_E846D transfectants and also R1063H-expressing cells showed increase in STAT5 activation (i-ii). Each bar represents the ratio of the density of phosphorylated STAT5 (p-STAT5) to the density of normalized total JAK2 and is presented as fold change against the ratio calculated for 1.0 U/mL EPO. For detailed information on JAK2 normalization, see supplemental Materials and methods. Activation of JAK2 and its targets was determined by antibodies recognizing specific phosphorylation sites (as detailed in supplemental Materials and methods); tubulin antibody was used as a loading control. (C) STAT5 transcriptional activity in JAK2-deficient γ-2A cells transfected with various JAK2 complementary DNAs (cDNAs) in the presence of EPOR, MPL, and G-CSF receptor (G-CSFR). The heterozygous configuration was mimicked by cotransfection of JAK2 wt cDNA with JAK2 V617F, JAK2 E846D, or JAK2 R1063H. Four hours after transfection, the cells were stimulated with different concentrations of EPO (i) or stimulated with 10 ng/mL thrombopoietin (ii) or 10 ng/mL G-CSF (iii) and luminescence was detected in cell lysates 24 hours (ii-iii) or 48 hours (i) after transfection using a PerkinElmer Victor X Light analyzer. (i) STAT5 transcriptional activity downstream of JAK2 E846D and R1063H was significantly increased in both EPO-free and EPO-limiting condition in comparison with JAK2 wt in the presence of EPOR; the double mutants expressing both JAK2 E846D and R1063H mutants showed increased STAT5 transcriptional activity over the single mutants in low EPO concentration. (ii-iii) Only JAK2 V617F significantly increased STAT5 transcriptional activity in the presence of MPL and G-CSFR. STAT5 transcriptional activity of JAK2 E846D and JAK2 R1063H cells was comparable to wt cells. The panels show the average of 3 independent experiment ± standard error of the mean (SEM). *P < .05, **P < .01, ***P < .001 using the 2-tailed Student t test. rlu, relative light unit. (D) In vitro colony growth of transduced murine BM. Murine BM cells were infected with retroviruses coding for murine JAK2 wt, V617F, E846D, or R1063H for single mutant configuration and with E846D and R1063H retroviral particles for double mutant configuration. Infected murine BM cells were then plated on methylcellulose media in the absence of EPO (i) or in the presence of 0.1 U/mL EPO (ii). After 2 and 7 days, the plates were evaluated for the formation of CFU-E and BFU-E, respectively. The BM cells infected with the virus containing the empty pMEGIX vector were used as a control. JAK2_E846D and JAK2_E846D/R1063H BM cells have significantly higher numbers of CFU-E and BFU-E colonies in both EPO-free and low EPO conditions when compared with JAK2_wt cells. Cells with JAK2_R1063H mutation had significantly increased CFU-E, but not BFU-E colony formation. The highest number of erythroid colonies was detected in the sample derived from JAK2_V617F positive control. Congruent results were obtained by determination of the replating capacity of primary human cells naturally harboring the JAK2 E846D and R1063H mutations (supplemental Figure 6). The panels show the average of 3 independent experiments. The BFU-E and CFU-E colony numbers are expressed as the number of colonies per transduced cells and are normalized to wt values ± SD. P values were calculated using Origin 6.1 software. *P < .05, **P < .01, ***P < .001. (E) Immunoblot analysis of prolonged activity of E846D-mutant JAK2 kinase. The JAK2 transfectants were after IL-3 starvation stimulated with 20 U/mL EPO for 15 minutes and then incubated in base Iscove modified Dulbecco medium (IMDM) with no additives for indicated periods of time. JAK2_E846D–expressing cells showed prolonged activation of STAT5 and EKR1/2 similarly to JAK2_V617F transfectants as predicted by in silico modeling (supplemental Figure 7).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/10/10.1182_blood-2016-02-698951/4/m_1418f2.jpeg?Expires=1767925635&Signature=olY8GUFMGQu7qNScemwsK80LNUU268rCF5ywX-eTqY-bwACisWnyqOPxlzglYJU8f8TZAPBcNq8Z0RWjS46Vh9tM~Npkgw-T975AjsrcljYyaML-zYbvd6dWeyspO5JVZemkjAml0BGRZKzsJUHOfTg6pZumrbUT5MyTc2uTmhUwJzJgFdcSPy6MX~bGQzIy8NkE1HT-LIBsWMsvg8BRZBnqalZvTQrq54yJuhE58IZChCr5fi3526xFyRSpJHhCnKeMWp~ipoaABTST7M3f0s-HzQzNuymPoyWWLCiTDpuHU5XITf5uETniu1Z~dyaUkzuUGer2n1ic6YocZcRQ7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)