Abstract
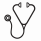
Background: Serious morbidity and mortality in sickle cell anemia (SCA) begins early in life during the physiologic decline of fetal hemoglobin (HbF) and its replacement with sickle hemoglobin (HbSS). In older children (>5 years), hydroxyurea (HU) is safe and provides similar laboratory benefits as in adults when escalated to a mean (standard deviation, SD) maximal tolerated dose (MTD) of 25.6 ± 6.2 mg/kg/day (Kinney, Blood 1999). In infants, a fixed-dose of 20 mg/kg/day is safe, improves hematologic parameters and provides substantial clinical benefits (Wang, Lancet 2011). However, the hematologic benefits of intensifying HU to MTD in young children have not been evaluated. We aimed to describe the initial and long-term hematologic changes provided by initiation of HU and escalation to MTD in young children (<5 years of age) who have not yet completed their physiologic decline of HbF.
Methods: The Hydroxyurea Study of Long-Term Effects (HUSTLE, NCT00305175) is a prospective observational evaluation of HU in children with SCA. After enrollment, all children were escalated to MTD, defined by an absolute neutrophil count goal of 2,000-4,000x106/L or other hematologic toxicity. Medication possession ratios (MPR) were calculated as [(days medication in family's possession ÷ days for which medication was prescribed) x 100] and MPR values >80% were used as a surrogate for good adherence (Estepp, Pediatr Blood Cancer 2014). Children who initiated HU prior to 5 years of age (younger group) were compared to those who initiated at ≥5 years of age (older group) at three time points: prior to initiation of HU (baseline), when MTD was established, and 5 years after MTD was reached. Laboratory parameters for the two groups (means and SD) were compared using the two sample t-test or Wilcoxon Rank Sum test based on the normality of the data. Correlation between changes in HbF and age at baseline was tested using Spearman correlation test. P-values <0.05 were considered statistically significant, and all statistical tests and tables were performed with SAS version 9.3 (Cary, N.C.).
Results: In total, 151 children provided nearly 700 patient-years of follow up with a mean MPR of 106% (0.9) (median [IQR]; 100% [84.8-107.1%]). The young group (n=49) initiated HU at a mean (SD) of 2.6 (1.1) years old, and the old group (n=102) at 11.1 (3.7) years (p<0.001). The young group was escalated over a longer time period (13.6 versus 10.6 months; p=0.04) and achieved a higher average MTD compared to the older group (28.8 versus 24.7 mg/kg/day; p<0.001). At baseline, the young group had a higher mean HbF level (14.9 versus 7.5%; p<0.001), absolute reticulocyte count (ARC) (300 versus 270 x103, p=0.02)and white blood cell count (WBC) (15.6 versus 12.6 x103/μL, p<0.001), and similar relationships were observed at MTD (Table 1). No differences between the two age groups were observed in the direction or magnitude of changes after escalation of HU between groups (Table 1). For example, the young group had an average increase of 13.3% in HbF compared to 13.4% in the older group (p=0.87) (Table 1; Figure 1). Following 5 years of therapy at MTD, the mean (SD) age of the young group was 9.1 (1.0) years and 15.7 (3.7) in the older group. At this time, only hemoglobin levels differed between groups (slightly higher in the old group), but HbF levels were similar between groups as HbF declined with age (Table 1).
Figure 1. Increase in fetal hemoglobin (HbF) at maximal tolerated dose (MTD) from baseline versus age (years) at the time hydroxyurea was initiated. The solid green line stands for linear regression line; the solid and dash read lines stand for nonparametric regression and its smoothed conditional spread lines, respectively.
Conclusions: Intensifying HU to MTD in young children with SCA is safe and efficacious, providing stable long-term improvement in numerous hematologic parameters. At young ages, there appears to be a short-term additive effect of HU to the elevated baseline HbF, but this is not sustained. Thus, initiating HU at an early age does not prevent the normal physiological decline of HbF. The clinical impact of intensifying HU to MTD in very young children warrants further exploration and is to be addressed by the recently NHLBI-funded pilot HUGKISS trial (hydroxyurea management in kids: intensive versus stable dosage strategies trial).
Hankins:Novartis: Research Funding. Estepp:Pfizer: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; National Institute of Health: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal