Abstract
Background: Primary malignant disease relapse, graft-versus-host disease (GvHD) and infection are the most common causes of death following allogeneic hematopoietic cell transplantation (alloHCT). We investigated whether infection following initial alloHCT influenced subsequent risk for hematologic malignant disease relapse.
Methods: Consecutive adult patients (N=324) with acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML) or myelodysplasia (MDS) who received initial matched sibling (MSD) or unrelated donor (MUD) bone marrow (BM, n=33), peripheral blood stem cell (PBSC, n=253) or umbilical cord blood (UCB, n=38) grafts from January 2010 through December 2014 at The Ohio State University Comprehensive Cancer Center were included. Patient-, transplant-, and infection-related data were retrospectively obtained. Categories for microbiologically-documented infections included bacterial blood stream infection (BSI), viral reactivation (VRA), invasive fungal infection (IFI), and respiratory viral infection (RVI). Correlation between development of infection and relapse was assessed using multi-variable (MV) proportional sub-distribution hazards models, where infection was treated as a time‐dependent covariate and death from any cause as a competing risk. Landmark analyses (LMA) at D14, D28, D56 and D100, which included only those patients who survived without relapse at the indicated time and infection status as a covariate, were performed to predict future relapse.
Results: Most transplant recipients had first complete remission AML, received MUD PBSC grafts following myeloablative conditioning, and were given methotrexate and calcineurin-based GvHD prophylaxis. Following alloHCT, 107 (33%) patients experienced disease relapse. Median progression-free survival (PFS) for the entire patient cohort was nearly two years (703 days). Grades 2-4 acute GvHD developed in 164 (51%) patients, and 145 (45%) patients developed limited or extensive chronic GvHD. One- and three-year overall survival (OS) rates for the entire cohort were 63% and 45%, respectively.
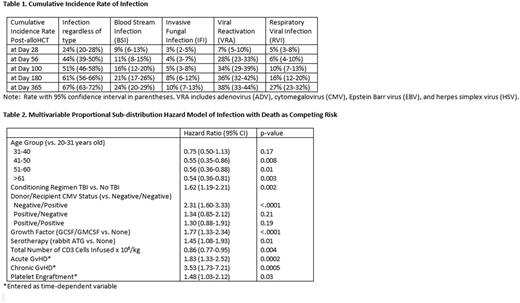
Cumulative incidence of infection, overall and by infection type, is depicted in Table 1. Patient- and transplant-related factors affecting development of infection are listed in Table 2. Patients who developed infection post-transplant had future relapse risk that was 46% (HR 0.54, 95% CI: 0.36-0.80, p=0.003) less than those patients who did not develop infection, regardless of infection type, in MV proportional sub-distribution hazards model analyses. LMA showed similar patterns in reduced risk for relapse following infection. Although not all reached statistical significance, LMA showed reduced relapse risk associated with early infection: D14 (HR 0.54, 95% CI: 0.27-1.05, p=0.07), D28 (HR 0.53, 95% CI: 0.30-0.93, p=0.03), D56 (HR 0.57, 95% CI: 0.37-0.90, p=0.01), and D100 (HR 0.63, 95% CI: 0.37-1.09, p=0.10).
Using infection as a time-dependent covariate, patients with infection had higher risk for infection-related mortality (IRM, HR 2.10, 95% CI: 1.45-3.05, p<0.0001) and non-relapse mortality (NRM, HR 6.42, 95% CI: 3.17-12.99, p<0.0001) versus patients not developing infection, but no difference in risk for relapse-related mortality (RRM, HR 0.97, 95% CI: 0.62-1.51, p=0.89). Interestingly, LMA at early post-HCT time points showed that infection may actually be protective for RRM: D14 (HR 0.69, 95% CI: 0.37-1.30, p=0.25), D28 (HR 0.59, 95% CI: 0.35-0.99, p=0.05), D56 (HR 0.57, 95% CI: 0.37-0.87, p=0.01), and D100 (HR 0.71, 95% CI 0.46-1.11, p=0.13).
Conclusion: Early infection post-alloHCT may confer protection against hematologic malignant disease relapse, but also increases NRM and IRM. Further investigation is required to define underlying immunologic mechanisms that may mediate such putative protection in order to promote presumed enhancement in graft-versus-leukemia effect.
Auletta:Shire Pharmaceuticals: Consultancy. Lozanski:Boehringer Ingelheim: Research Funding; Genentech: Research Funding; Beckman Coulter: Research Funding; Stemline Therapeutics Inc.: Research Funding. Hofmeister:Arno Therapeutics, Inc.: Research Funding; Janssen: Pharmaceutical Companies of Johnson & Johnson: Research Funding; Celgene: Research Funding; Incyte, Corp: Membership on an entity's Board of Directors or advisory committees; Signal Genetics, Inc.: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Research Funding; Takeda Pharmaceutical Company: Research Funding; Teva: Membership on an entity's Board of Directors or advisory committees. Andritsos:Hairy Cell Leukemia Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal