Abstract
The first edition of the European LeukemiaNet (ELN) recommendations for diagnosis and management of acute myeloid leukemia (AML) in adults, published in 2010, has found broad acceptance by physicians and investigators caring for patients with AML. Recent advances, for example, in the discovery of the genomic landscape of the disease, in the development of assays for genetic testing and for detecting minimal residual disease (MRD), as well as in the development of novel antileukemic agents, prompted an international panel to provide updated evidence- and expert opinion-based recommendations. The recommendations include a revised version of the ELN genetic categories, a proposal for a response category based on MRD status, and criteria for progressive disease.
Introduction
In 2010, an international expert panel, on behalf of the European LeukemiaNet (ELN), published recommendations for diagnosis and management of acute myeloid leukemia (AML).1 These recommendations have been widely adopted in general practice, within clinical trials, and by regulatory agencies. During recent years, considerable progress has been made in understanding disease pathogenesis, and in development of diagnostic assays and novel therapies.2 This article provides updated recommendations that parallel the current update to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia.3,4 For diagnosis and management of acute promyelocytic leukemia, readers are referred to the respective recommendations.5
Methods
The panel included 22 international members with recognized clinical and research expertise in AML. The panel met 3 times. Literature searches, categorization of evidence, and arrival at consensus were done as previously.1 Relevant abstracts presented at the 2013 to 2015 meetings of the American Society of Hematology, and the 2013 to 2016 meetings of the American Association for Cancer Research, the European Hematology Association, and the American Society of Clinical Oncology were reviewed.
WHO classification
The current update of the WHO classification provides few changes to the existing disease categories (Table 1). Most importantly, a new category “myeloid neoplasms with germ line predisposition” was added (Table 2).6
Myeloid neoplasms with germ line predisposition, AML and related precursor neoplasms, and acute leukemias of ambiguous lineage (WHO 2016)
| Myeloid neoplasms with germ line predisposition (see Table 2) . | |
|---|---|
| AML and related neoplasms | AML and related neoplasms (cont'd) |
| AML with recurrent genetic abnormalities | Acute myelomonocytic leukemia |
| AML with t(8;21)(q22;q22.1); RUNX1-RUNX1T1 | Acute monoblastic/monocytic leukemia |
| AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | Pure erythroid leukemia# |
| Acute promyelocytic leukemia with PML-RARA* | Acute megakaryoblastic leukemia |
| AML with t(9;11)(p21.3;q23.3); MLLT3-KMT2A† | Acute basophilic leukemia |
| AML with t(6;9)(p23;q34.1); DEK-NUP214 | Acute panmyelosis with myelofibrosis |
| AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1) | Myeloid sarcoma |
| AML (megakaryoblastic) with t(1;22)(p13.3;q13.3); RBM15-MKL1‡ | Myeloid proliferations related to Down syndrome |
| Provisional entity: AML with BCR-ABL1 | Transient abnormal myelopoiesis |
| AML with mutated NPM1§ | Myeloid leukemia associated with Down syndrome |
| AML with biallelic mutations of CEBPA§ | Blastic plasmacytoid dendritic cell neoplasm |
| Provisional entity: AML with mutated RUNX1 | Acute leukemias of ambiguous lineage |
| AML with myelodysplasia-related changes|| | Acute undifferentiated leukemia |
| Therapy-related myeloid neoplasms¶ | MPAL with t(9;22)(q34.1;q11.2); BCR-ABL1** |
| AML, NOS | MPAL with t(v;11q23.3); KMT2A rearranged |
| AML with minimal differentiation | MPAL, B/myeloid, NOS |
| AML without maturation | MPAL, T/myeloid, NOS |
| AML with maturation | |
| Myeloid neoplasms with germ line predisposition (see Table 2) . | |
|---|---|
| AML and related neoplasms | AML and related neoplasms (cont'd) |
| AML with recurrent genetic abnormalities | Acute myelomonocytic leukemia |
| AML with t(8;21)(q22;q22.1); RUNX1-RUNX1T1 | Acute monoblastic/monocytic leukemia |
| AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | Pure erythroid leukemia# |
| Acute promyelocytic leukemia with PML-RARA* | Acute megakaryoblastic leukemia |
| AML with t(9;11)(p21.3;q23.3); MLLT3-KMT2A† | Acute basophilic leukemia |
| AML with t(6;9)(p23;q34.1); DEK-NUP214 | Acute panmyelosis with myelofibrosis |
| AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1) | Myeloid sarcoma |
| AML (megakaryoblastic) with t(1;22)(p13.3;q13.3); RBM15-MKL1‡ | Myeloid proliferations related to Down syndrome |
| Provisional entity: AML with BCR-ABL1 | Transient abnormal myelopoiesis |
| AML with mutated NPM1§ | Myeloid leukemia associated with Down syndrome |
| AML with biallelic mutations of CEBPA§ | Blastic plasmacytoid dendritic cell neoplasm |
| Provisional entity: AML with mutated RUNX1 | Acute leukemias of ambiguous lineage |
| AML with myelodysplasia-related changes|| | Acute undifferentiated leukemia |
| Therapy-related myeloid neoplasms¶ | MPAL with t(9;22)(q34.1;q11.2); BCR-ABL1** |
| AML, NOS | MPAL with t(v;11q23.3); KMT2A rearranged |
| AML with minimal differentiation | MPAL, B/myeloid, NOS |
| AML without maturation | MPAL, T/myeloid, NOS |
| AML with maturation | |
For a diagnosis of AML, a marrow blast count of ≥20% is required, except for AML with the recurrent genetic abnormalities t(15;17), t(8;21), inv(16), or t(16;16). Adapted from Arber et al.3
MPAL, mixed phenotype acute leukemia; NK, natural killer.
Other recurring translocations involving RARA should be reported accordingly: for example, AML with t(11;17)(q23;q12); ZBTB16-RARA; AML with t(11;17)(q13;q12); NUMA1-RARA; AML with t(5;17)(q35;q12); NPM1-RARA; or AML with STAT5B-RARA (the latter having a normal chromosome 17 on conventional cytogenetic analysis).
Other translocations involving KMT2A (MLL) should be reported accordingly: for example, AML with t(6;11)(q27;q23.3); MLLT4-KMT2A; AML with t(11;19)(q23.3;p13.3); KMT2A-MLLT1; AML with t(11;19)(q23.3;p13.1); KMT2A-ELL; AML with t(10;11)(p12;q23.3); MLLT10-KMT2A.
Rare leukemia most commonly occurring in infants.
Diagnosis is made irrespective of the presence or absence of multilineage dysplasia.
At least 20% (≥20%) blood or marrow blasts AND any of the following: previous history of MDS or MDS/MPN; myelodysplasia-related cytogenetic abnormality (see list below); multilineage dysplasia; AND absence of both prior cytotoxic therapy for unrelated disease and aforementioned recurring genetic abnormalities. Cytogenetic abnormalities sufficient to diagnose AML with myelodysplasia-related changes are: Complex karyotype (defined as 3 or more chromosomal abnormalities in the absence of 1 of the WHO-designated recurring translocations or inversions, that is, t(8;21), inv(16) or t(16;16), t(9;11), t(v;11)(v;q23.3), t(6;9), inv(3) or t(3;3); AML with BCR-ABL1); Unbalanced abnormalities: −7 or del(7q); −5 or del(5q); i(17q) or t(17p); −13 or del(13q); del(11q); del(12p) or t(12p); idic(X)(q13); Balanced abnormalities: t(11;16)(q23.3;p13.3); t(3;21)(q26.2;q22.1); t(1;3)(p36.3;q21.2); t(2;11)(p21;q23.3); t(5;12)(q32;p13.2); t(5;7)(q32;q11.2); t(5;17)(q32;p13.2); t(5;10)(q32;q21.2); t(3;5)(q25.3;q35.1).
Cases should be classified with the related genetic abnormality given in the diagnosis.
The former subgroup of acute erythroid leukemia, erythroid/myeloid type (≥50% bone marrow erythroid precursors and ≥20% myeloblasts among nonerythroid cells) was removed; myeloblasts are now always counted as percentage of total marrow cells. The remaining subcategory AML, NOS, pure erythroid leukemia requires the presence of >80% immature erythroid precursors with ≥30% proerythroblasts.
BCR-ABL1+ leukemia may present as MPAL; treatment should include a tyrosine kinase inhibitor.
WHO classification of myeloid neoplasms with germ line predisposition and guide for molecular genetic diagnostics
| WHO classification . |
|---|
| Classification* |
| Myeloid neoplasms with germ line predisposition without a preexisting disorder or organ dysfunction |
| AML with germ line CEBPA mutation |
| Myeloid neoplasms with germ line DDX41 mutation† |
| Myeloid neoplasms with germ line predisposition and preexisting platelet disorders |
| Myeloid neoplasms with germ line RUNX1 mutation† |
| Myeloid neoplasms with germ line ANKRD26 mutation† |
| Myeloid neoplasms with germ line ETV6 mutation† |
| Myeloid neoplasms with germ line predisposition and other organ dysfunction |
| Myeloid neoplasms with germ line GATA2 mutation |
| Myeloid neoplasms associated with bone marrow failure syndromes |
| Juvenile myelomonocytic leukemia associated with neurofibromatosis, Noonan syndrome, or Noonan syndrome-like disorders |
| Myeloid neoplasms associated with Noonan syndrome |
| Myeloid neoplasms associated with Down syndrome† |
| Guide for molecular genetic diagnostics‡ |
| Myelodysplastic predisposition/acute leukemia predisposition syndromes |
| CEBPA, DDX41, RUNX1, ANKRD26, ETV6, GATA2, SRP72, 14q32.2 genomic duplication (ATG2B/GSKIP) |
| Cancer predisposition syndromes§ |
| Li Fraumeni syndrome (TP53) |
| Germ line BRCA1/BRCA2 mutations |
| Bone marrow failure syndromes |
| Dyskeratosis congenita (TERC, TERT) |
| Fanconi anemia |
| WHO classification . |
|---|
| Classification* |
| Myeloid neoplasms with germ line predisposition without a preexisting disorder or organ dysfunction |
| AML with germ line CEBPA mutation |
| Myeloid neoplasms with germ line DDX41 mutation† |
| Myeloid neoplasms with germ line predisposition and preexisting platelet disorders |
| Myeloid neoplasms with germ line RUNX1 mutation† |
| Myeloid neoplasms with germ line ANKRD26 mutation† |
| Myeloid neoplasms with germ line ETV6 mutation† |
| Myeloid neoplasms with germ line predisposition and other organ dysfunction |
| Myeloid neoplasms with germ line GATA2 mutation |
| Myeloid neoplasms associated with bone marrow failure syndromes |
| Juvenile myelomonocytic leukemia associated with neurofibromatosis, Noonan syndrome, or Noonan syndrome-like disorders |
| Myeloid neoplasms associated with Noonan syndrome |
| Myeloid neoplasms associated with Down syndrome† |
| Guide for molecular genetic diagnostics‡ |
| Myelodysplastic predisposition/acute leukemia predisposition syndromes |
| CEBPA, DDX41, RUNX1, ANKRD26, ETV6, GATA2, SRP72, 14q32.2 genomic duplication (ATG2B/GSKIP) |
| Cancer predisposition syndromes§ |
| Li Fraumeni syndrome (TP53) |
| Germ line BRCA1/BRCA2 mutations |
| Bone marrow failure syndromes |
| Dyskeratosis congenita (TERC, TERT) |
| Fanconi anemia |
Classification portion of table is adopted from Arber et al.3
Recognition of familial myeloid neoplasms requires that physicians take a thorough patient and family history to assess for typical signs and symptoms of known syndromes, including data on malignancies and previous bleeding episodes. See also Churpek and Godley27 for how to identify, test, and counsel individuals and families suspected of having an inherited myeloid malignancy syndrome.
Lymphoid neoplasms also reported.
Molecular genetic diagnostics are guided by a detailed patient and family history27 ; diagnostics should be performed in close collaboration with a genetic counselor; patients with a suspected heritable myeloid neoplasm, who test negative for known predisposition genes, should ideally be entered on a research study to facilitate new syndrome discovery.
Mutations in genes associated with cancer predisposition genes such as TP53 and BRCA1/2 appear to be frequent in therapy-related myeloid neoplasms.256
AML with recurrent genetic abnormalities
The molecular basis of AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2) was revisited showing that repositioning of a GATA2 enhancer element leads to overexpression of the MECOM (EVI1) gene and to haploinsufficiency of GATA2.7,8 A new provisional entity “AML with BCR-ABL1” was introduced to recognize that patients with this abnormality should receive therapy with a tyrosine kinase inhibitor. Distinction from blast phase of chronic myeloid leukemia may be difficult; preliminary data suggest that deletion of antigen receptor genes (immunoglobulin heavy chain and T-cell receptor), IKZF1, and/or CDKN2A may support a diagnosis of AML rather than chronic myeloid leukemia blast phase.9 AML with mutated NPM1 and AML with biallelic mutations of CEBPA have become full entities; the latter category was restricted to cases with biallelic mutations because recent studies have shown that only those cases define the entity and portend a favorable outcome.10-16 Both entities now subsume cases with multilineage dysplasia because presence of dysplasia lacks prognostic significance.17-19 Finally, a new provisional entity “AML with mutated RUNX1” (excluding cases with myelodysplasia-related changes) was added; it has been associated with distinct clinicopathologic features and inferior outcome.20-24
AML with myelodysplasia-related changes
Presence of multilineage dysplasia, preexisting myeloid disorder, and/or myelodysplasia-related cytogenetic changes remain diagnostic criteria for this disease category. Deletion 9q was removed from the list of myelodysplasia-related cytogenetic changes because, in addition to its association with t(8;21), it also frequently occurs in AML with NPM1 and biallelic CEBPA mutations.16,25
AML, not otherwise specified
The former subgroup acute erythroid leukemia, erythroid/myeloid type (≥50% bone marrow erythroid precursors and ≥20% myeloblasts among nonerythroid cells) was removed; myeloblasts are now always counted as percentage of total marrow cells. The remaining subcategory AML, not otherwise specified (NOS), pure erythroid leukemia requires >80% immature erythroid precursors with ≥30% proerythroblasts. French-American-British (FAB) subclassification does not seem to provide prognostic information for “AML, NOS” cases if data on NPM1 and CEBPA mutations are available.26
Myeloid neoplasms with germ line predisposition (synonyms: familial myeloid neoplasms; familial myelodysplastic syndromes/acute leukemias)
Inclusion of this new category reflects the increasing recognition that some cases of myeloid neoplasms, including myelodysplastic syndrome (MDS) and AML, arise in association with inherited or de novo germ line mutations (Table 2).6,27-30 Recognition of familial cases requires that physicians take a thorough patient and family history, including information on malignancies and previous bleeding episodes. Awareness of these cases is of clinical relevance because patients may need special clinical care.27 Affected patients, including their families, should be offered genetic counseling with a counselor familiar with these disorders.
Molecular landscape
The advent of high-throughput sequencing techniques has allowed new insights into the molecular basis of myeloid neoplasms.31-37 Similar to most sporadic human malignancies, AML is a complex, dynamic disease, characterized by multiple somatically acquired driver mutations, coexisting competing clones, and disease evolution over time.
The Cancer Genome Atlas AML substudy profiled 200 clinically annotated cases of de novo AML by whole-genome (n = 50) or whole-exome (n = 150) sequencing, along with RNA and microRNA sequencing and DNA-methylation analysis.31 Twenty-three genes were found to be commonly mutated, and another 237 were mutated in 2 or more cases, in nonrandom patterns of co-occurrence and mutual exclusivity. Mutated genes were classified into 1 of 9 functional categories: transcription factor fusions, the NPM1 gene, tumor suppressor genes, DNA methylation-related genes, signaling genes, chromatin-modifying genes, myeloid transcription factor genes, cohesin complex genes, and spliceosome complex genes.
The use of genetic data to inform disease classification and clinical practice is an active field of research. Recently, 1540 patients, intensively treated in prospective trials, were analyzed using targeted resequencing of 111 myeloid cancer genes, along with cytogenetic profiles.37 Patterns of comutations segregated AML cases into 11 nonoverlapping classes, each with a distinct clinical phenotype and outcome. Beyond known disease classes, 3 additional, heterogeneous classes emerged: AML with mutations in chromatin and RNA-splicing regulators; AML with TP53 mutations and/or chromosomal aneuploidies; and, provisionally, AML with IDH2R172 mutations.
Mutant allele fractions can be used to infer the phylogenetic tree leading to development of overt leukemia. Clonal evolution studies in patients and patient-derived xenograft models indicate that mutations in genes involved in regulation of DNA modification and of chromatin state, most commonly DNMT3A, TET2, and ASXL1, are often present in preleukemic stem or progenitor cells and occur early in leukemogenesis.38-41 Such mutations are present in ancestral cells capable of multilineage engraftment, may persist after therapy, lead to clonal expansion during remission, and cause recurrent disease.
Recent studies in large, population-based cohorts have identified recurrent mutations in epigenetic regulators (DNMT3A, ASXL1, TET2), and less frequently in splicing factor genes (SF3B1, SRSF2), to be associated with clonal hematopoietic expansion in elderly seemingly healthy subjects.42-46 The term “clonal hematopoiesis of indeterminate potential”47 has been proposed to describe this phenomenon which seems associated with increased risks of hematologic neoplasms. Preliminary data indicate that the rate of progression of clonal hematopoiesis of indeterminate potential to hematologic disease may be similar to the rate of progression of other premalignant states, such as monoclonal gammopathy of undetermined significance to multiple myeloma.
Diagnostic procedures
Morphology
At least 200 leukocytes on blood smears and 500 nucleated cells on spiculated marrow smears should be counted. A marrow or blood blast count of ≥20% is required, except for AML with t(15;17), t(8;21), inv(16), or t(16;16). Myeloblasts, monoblasts, and megakaryoblasts are included in the blast count. In AML with monocytic or myelomonocytic differentiation, monoblasts and promonocytes, but not abnormal monocytes, are counted as blast equivalents.
Immunophenotyping
Table 3 provides a list of markers helpful for establishing the diagnosis of AML,48 as well as specific lineage markers useful for defining mixed-phenotype acute leukemia.3,4
Expression of cell-surface and cytoplasmic markers for the diagnosis of AML and MPAL
| Expression of cell-surface and cytoplasmic markers . | |
|---|---|
| Diagnosis of AML* | |
| Precursors† | CD34, CD117, CD33, CD13, HLA-DR |
| Granulocytic markers‡ | CD65, cytoplasmic MPO |
| Monocytic markers§ | CD14, CD36, CD64 |
| Megakaryocytic markers|| | CD41 (glycoprotein IIb/IIIa), CD61 (glycoprotein IIIa) |
| Erythroid markers | CD235a (glycophorin A), CD36 |
| Diagnosis of MPAL¶ | |
| Myeloid lineage | MPO (flow cytometry, immunohistochemistry, or cytochemistry) or monocytic differentiation (at least 2 of the following: nonspecific esterase cytochemistry, CD11c, CD14, CD64, lysozyme) |
| T-lineage | Strong# cytoplasmic CD3 (with antibodies to CD3 ε chain) or surface CD3 |
| B-lineage** | Strong# CD19 with at least 1 of the following strongly expressed: cytoplasmic CD79a, cCD22, or CD10 or weak CD19 with at least 2 of the following strongly expressed: CD79a, cCD22, or CD10 |
| Expression of cell-surface and cytoplasmic markers . | |
|---|---|
| Diagnosis of AML* | |
| Precursors† | CD34, CD117, CD33, CD13, HLA-DR |
| Granulocytic markers‡ | CD65, cytoplasmic MPO |
| Monocytic markers§ | CD14, CD36, CD64 |
| Megakaryocytic markers|| | CD41 (glycoprotein IIb/IIIa), CD61 (glycoprotein IIIa) |
| Erythroid markers | CD235a (glycophorin A), CD36 |
| Diagnosis of MPAL¶ | |
| Myeloid lineage | MPO (flow cytometry, immunohistochemistry, or cytochemistry) or monocytic differentiation (at least 2 of the following: nonspecific esterase cytochemistry, CD11c, CD14, CD64, lysozyme) |
| T-lineage | Strong# cytoplasmic CD3 (with antibodies to CD3 ε chain) or surface CD3 |
| B-lineage** | Strong# CD19 with at least 1 of the following strongly expressed: cytoplasmic CD79a, cCD22, or CD10 or weak CD19 with at least 2 of the following strongly expressed: CD79a, cCD22, or CD10 |
MPO, myeloperoxidase. Other abbreviations are explained in Table 1.
The markers proposed in this table are according to European LeukemiaNet Work Package 10 recommendations.48
CD38 and other markers such as CD123 or CD133 can be added to identify leukemic stem cells, but do not contribute to diagnosis.
Of note, cells engaged in granulocytic maturation will retain the expression of CD13 and CD33 at various fluorescence levels. Seeking for the expression of CD15 and CD11b can provide further information. CD16 is only present on normal mature granulocytes. The absence of MPO together with myeloid markers defines AML with minimal differentiation which is different from acute undifferentiated leukemia.
Of note, cells engaged in monocytic differentiation will retain the expression of CD13 and CD33. Seeking the expression of CD64 and CD11b can provide additional information, notably for promonocytes.
CD42 (glycoprotein 1b) can also be used.
The category MPAL includes leukemias with expression of antigens of >1 lineage. They can either contain distinct blast populations of different lineages, or 1 blast population with expression of antigens of different lineages on the same cells, or a combination. The proposal in this table includes the modifications brought in the current update of the WHO classification of hematopoietic tumors.3,4
Strong defined as equal or brighter than the normal B or T cells in the sample.
Other markers can be used to confirm B-lineage involvement.
Cytogenetics and molecular cytogenetics
Conventional cytogenetic analysis remains mandatory in the evaluation of suspected AML. Eight balanced translocations and inversions, and their variants, are included in the WHO category “AML with recurrent genetic abnormalities”.3,4 Nine balanced rearrangements and multiple unbalanced abnormalities are sufficient to establish the WHO diagnosis of “AML with myelodysplasia-related changes” when ≥20% blood or marrow blasts are present (Table 1).
Other rare balanced rearrangements are recognized.49,50 Although considered disease-initiating events, they do not formally define disease categories. They involve genes, for example, encoding epigenetic regulators (eg, KMT2A [MLL], CREBBP, NSD1) or components of the nuclear pore complex (NUP98, NUP214) (Figure 1). Some rearrangements are cytogenetically cryptic, such as t(5;11)(q35.2;p15.4); NUP98-NSD1, which occurs in ∼1% of AML in younger adults and predicts a poor prognosis.51-53 Recent studies have highlighted the potential of novel sequencing technologies to discover additional AML-associated fusion genes.54-56
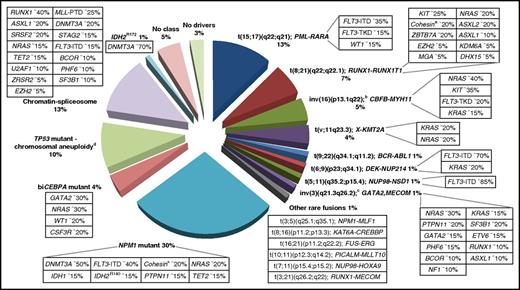
Molecular classes of AML and concurrent gene mutations in adult patients up to the age of ∼65 years. Class definition is based on the study by Papaemmanuil et al.37 For each AML class denoted in the pie chart, frequent co-occurring mutations are shown in the respective boxes. Data on the frequency of genetic lesions are compiled from the databases of the British Medical Research Council (MRC), the German-Austrian AML Study Group (AMLSG), and from selected studies.37,87,88,299 a indicates cohesin genes including RAD21 (∼10%), SMC1A (∼5%), and SMC3 (∼5%); b, inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11; c, inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1); and d, TP53 mutations are found in ∼45%, and complex karyotypes in ∼70% of this class. The structure of the pie chart is adapted from Grimwade et al,50 generated by Adam Ivey (King’s College London, London, United Kingdom).
Molecular classes of AML and concurrent gene mutations in adult patients up to the age of ∼65 years. Class definition is based on the study by Papaemmanuil et al.37 For each AML class denoted in the pie chart, frequent co-occurring mutations are shown in the respective boxes. Data on the frequency of genetic lesions are compiled from the databases of the British Medical Research Council (MRC), the German-Austrian AML Study Group (AMLSG), and from selected studies.37,87,88,299 a indicates cohesin genes including RAD21 (∼10%), SMC1A (∼5%), and SMC3 (∼5%); b, inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11; c, inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1); and d, TP53 mutations are found in ∼45%, and complex karyotypes in ∼70% of this class. The structure of the pie chart is adapted from Grimwade et al,50 generated by Adam Ivey (King’s College London, London, United Kingdom).
If cytogenetic analysis fails, fluorescence in situ hybridization is an option to detect gene rearrangements, such as RUNX1-RUNX1T1, CBFB-MYH11, KMT2A (MLL), and MECOM (EVI1) gene fusions, or loss of chromosome 5q, 7q, or 17p material.
Molecular genetic testing
Diagnostic workup should include screening for (a) mutations in NPM1, CEBPA, and RUNX1 genes because they define disease categories (provisionally for RUNX1); (b) mutations in FLT3 (both for internal tandem duplications [ITDs] together with data on the mutant–to–wild-type allelic ratio,57-60 and tyrosine kinase domain mutations at codons D835 and I836); activating mutations of FLT3 are not only prognostic, but may beneficially be affected by tyrosine kinase inhibition61 ; and (c) mutations in TP53 and ASXL1 because they consistently have been associated with poor prognosis (Table 4).62-70
Tests/procedures for a patient with AML
| For a patient with AML . | |
|---|---|
| Tests to establish the diagnosis | Additional tests/procedures at diagnosis (cont'd) |
| Complete blood count and differential count | Analysis of comorbidities |
| Bone marrow aspirate | Biochemistry, coagulation tests, urine analysis** |
| Bone marrow trephine biopsy* | Serum pregnancy test†† |
| Immunophenotyping | Information on oocyte and sperm cryopreservation‡‡ |
| Genetic analyses | Eligibility assessment for allogeneic HCT (including HLA typing)a |
| Cytogenetics† | Hepatitis A, B, C; HIV-1 testing |
| Screening for gene mutations including‡ | Chest radiograph, 12-lead electrocardiogram, and echocardiography or MUGA (on indication) |
| NPM1, CEBPA, RUNX1, FLT3, TP53, ASXL1 | Lumbar punctureb |
| Screening for gene rearrangements§ | Biobankingc |
| PML-RARA, CBFB-MYH11, RUNX1-RUNX1T1, BCR-ABL1, other fusion genes (if available) | Sensitive assessment of response by RT-qPCR or MFCd |
| Additional tests/procedures at diagnosis | RT-qPCRe,f for NPM1 mutation, CBFB-MYH11, RUNX1-RUNX1T1, BCR-ABL1, other fusion genes (if available)d |
| Demographics and medical history|| | MFCf,g |
| Detailed family history¶ | |
| Patient bleeding history# | |
| Performance status (ECOG/WHO score) |
| For a patient with AML . | |
|---|---|
| Tests to establish the diagnosis | Additional tests/procedures at diagnosis (cont'd) |
| Complete blood count and differential count | Analysis of comorbidities |
| Bone marrow aspirate | Biochemistry, coagulation tests, urine analysis** |
| Bone marrow trephine biopsy* | Serum pregnancy test†† |
| Immunophenotyping | Information on oocyte and sperm cryopreservation‡‡ |
| Genetic analyses | Eligibility assessment for allogeneic HCT (including HLA typing)a |
| Cytogenetics† | Hepatitis A, B, C; HIV-1 testing |
| Screening for gene mutations including‡ | Chest radiograph, 12-lead electrocardiogram, and echocardiography or MUGA (on indication) |
| NPM1, CEBPA, RUNX1, FLT3, TP53, ASXL1 | Lumbar punctureb |
| Screening for gene rearrangements§ | Biobankingc |
| PML-RARA, CBFB-MYH11, RUNX1-RUNX1T1, BCR-ABL1, other fusion genes (if available) | Sensitive assessment of response by RT-qPCR or MFCd |
| Additional tests/procedures at diagnosis | RT-qPCRe,f for NPM1 mutation, CBFB-MYH11, RUNX1-RUNX1T1, BCR-ABL1, other fusion genes (if available)d |
| Demographics and medical history|| | MFCf,g |
| Detailed family history¶ | |
| Patient bleeding history# | |
| Performance status (ECOG/WHO score) |
CMV, cytomegalovirus; ECOG, Eastern Cooperative Oncology Group; MUGA, multigated acquisition.
In patients with a dry tap (punctio sicca).
Results from cytogenetics should be obtained preferably within 5 to 7 days. At least 20 bone marrow metaphases are needed to define a normal karyotype, and recommended to describe an abnormal karyotype. Abnormal karyotypes may be diagnosed from blood specimens.
Results from NPM1 and FLT3 mutational screening should be available within 48 to 72 hours (at least in patients eligible for intensive chemotherapy), and results from additional molecular genetics within the first treatment cycle. Screening for gene mutations is an evolving field of research; screening for single genes may be replaced by gene panel diagnostics.
Screening for gene rearrangements should be performed if rapid information is needed for recommendation of suitable therapy, if chromosome morphology is of poor quality, or if there is typical morphology but the suspected cytogenetic abnormality is not present.
Including race or ethnicity, prior exposure to toxic agents, prior malignancy, therapy for prior malignancy, information on smoking.
Thorough family history needed to identify potential myeloid neoplasms with germ line predisposition.
History of bleeding episodes may inform cases of myeloid neoplasms with germ line predisposition and preexisting platelet disorders.
Biochemistry: glucose, sodium, potassium, calcium, creatinine, aspartate amino transferase, alanine amino transferase, alkaline phosphatase, lactate dehydrogenase, bilirubin, urea, total protein, uric acid, total cholesterol, total triglycerides, creatinine phosphokinase. Coagulation tests: prothrombin time, international normalized ratio where indicated, activated partial thromboplastin time. Urine analysis: pH, glucose, erythrocytes, leukocytes, protein, nitrite.
In women with childbearing potential.
Cryopreservation to be done in accordance with the wish of the patient.
HLA typing and CMV testing should be performed in those patients eligible for allogeneic HCT.
Required in patients with clinical symptoms suspicious of CNS involvement; patient should be evaluated by imaging study for intracranial bleeding, leptomeningeal disease, and mass lesion; lumbar puncture considered optional in other settings (eg, high white blood cell count).
Pretreatment leukemic bone marrow and blood sample; for further optional storing, see “Biobanking.”
Sensitive assessment of response can be performed at early time points, for example, following induction and consolidation courses to assess remission status and determine kinetics of disease response, and sequentially beyond consolidation to detect impending morphologic relapse. No generally applicable time points can be defined because kinetics of MRD response differs by treatment given, marker analyzed, and method used.
Monitoring of response by RT-qPCR recommended in clinical trials and clinical practice.
Sensitivity of response assessment varies by method used, and by marker tested; test used and sensitivity of the assay should always be reported; analyses should be done in experienced laboratories (centralized diagnostics).
Increasing evidence that response assessment by MFC qualitatively provides a better remission status than morphologic assessment and is of high prognostic impact.
Molecular testing by reverse transcriptase–polymerase chain reaction (RT-PCR) for recurring rearrangements can be useful (Table 4).
Although only a few of the recently identified molecular markers inform current clinical practice, the list (from the previous paragraph) will likely be expanded with testing for single genes replaced by gene panel diagnostics, or diagnostic platforms that simultaneously test for gene mutations and gene rearrangements.55,56
Biobanking
If possible, pretreatment leukemic marrow and blood should be stored within a biobank. Informed consent preferably should allow a broad array of correlative laboratory studies including analysis of germ line DNA. Pretreatment samples should include nucleic acid (DNA and RNA, stored at −80°C) and viable cells (stored at −196°C). Optimally, a plasma sample, a methanol/acetic acid-fixed cell pellet (from cytogenetic analysis), and frozen cell pellets from various time points during and after treatment (eg, at time of complete remission [CR], relapse, and for minimal residual disease [MRD] monitoring at defined time points during remission) should be obtained and stored under appropriate conditions.
Buccal swabs and sputum have been previously recommended for the analysis of germ line DNA; samples should preferably be obtained during remission to reduce the risk of contaminating DNA from leukemic cells. Skin fibroblasts may be the preferred tissue source. A skin biopsy can be performed using a punch biopsy or by taking a small biopsy at the site of skin incision during bone marrow aspiration or biopsy. When obtained at diagnosis, skin cells should be grown from the biopsy to avoid contamination of the specimen with leukemic cells; alternatively, the biopsy can be taken during remission without growing of fibroblasts. Other sources include finger nails and hair follicles, although the amount of DNA that can be extracted may be limited. Finally, bone marrow fibroblasts can be grown from viably frozen mononuclear cells.72
Other diagnostic tests
Tests and procedures for a patient with AML are described in Table 4.
Prognostic factors
Pretreatment factors
Recent studies have explored the relative contribution of genetic and clinical variables to prediction of event-free survival (EFS) and overall survival (OS).36,37,73,74 Genomic lesions account for about two-thirds of explained variation, with the other third contributed by demographic, clinical, and treatment variables. However, models incorporating all of these factors and aimed at predicting whether a patient with a given set of covariates will have a longer remission or life expectancy than another patient with a different set of covariates are correct in only 75% to 80% of cases. This emphasizes the need not only to identify other pretreatment prognostic factors but also to focus on posttreatment events, in particular the presence of MRD (see “Factors after diagnosis”).
Patient-related factors.
Increasing age is independently associated with poorer outcomes. Performance status, general health, and specific comorbidities modulate the effect of age on tolerance of chemotherapy (see also “Current therapy” and “Older patients not considered candidates for intensive chemotherapy”), whereas specific age-related AML-associated genetic abnormalities increase the likelihood of resistance, as do previous MDS, chronic myelomonocytic leukemia, myeloproliferative neoplasm (MPN), or prior exposure to cytotoxic therapy for other disorders. Hence, age should not be the sole determinant of treatment decisions.
AML-related genetic factors.
Genetic abnormalities are powerful prognostic factors.36,37,50,73,75,76 Results from conventional cytogenetics and from NPM1, FLT3, and CEBPA mutational screening are currently being used in routine practice following 2010 ELN recommendations.1
Recent data have led to several changes in these recommendations (see “2017 ELN genetic risk stratification” and Table 5). RUNX1 mutations although occurring with unfavorable features, such as older age, antecedent myeloid disorder, and concurrent gene mutations (eg, SRSF2, ASXL1), identify patients with poor prognosis.20-23,37,70,73 Likewise, ASXL1 mutations are more common in older patients and associated with inferior survival.36,37,62-65,69,70 TP53 mutations are associated with complex karyotype, monosomal karyotype, and specific chromosomal aneuploidies (eg, −5/5q−, −7/7q−), and predict for very poor outcome.37,66-70,73 TP53 mutation and complex karyotype provide independent prognostic information, with the combination of both having the worst outcome.37
2017 ELN risk stratification by genetics
| Risk category* . | Genetic abnormality . |
|---|---|
| Favorable | t(8;21)(q22;q22.1); RUNX1-RUNX1T1 |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | |
| Mutated NPM1 without FLT3-ITD or with FLT3-ITDlow† | |
| Biallelic mutated CEBPA | |
| Intermediate | Mutated NPM1 and FLT3-ITDhigh† |
| Wild-type NPM1 without FLT3-ITD or with FLT3-ITDlow† (without adverse-risk genetic lesions) | |
| t(9;11)(p21.3;q23.3); MLLT3-KMT2A‡ | |
| Cytogenetic abnormalities not classified as favorable or adverse | |
| Adverse | t(6;9)(p23;q34.1); DEK-NUP214 |
| t(v;11q23.3); KMT2A rearranged | |
| t(9;22)(q34.1;q11.2); BCR-ABL1 | |
| inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1) | |
| −5 or del(5q); −7; −17/abn(17p) | |
| Complex karyotype,§ monosomal karyotype|| | |
| Wild-type NPM1 and FLT3-ITDhigh† | |
| Mutated RUNX1¶ | |
| Mutated ASXL1¶ | |
| Mutated TP53# |
| Risk category* . | Genetic abnormality . |
|---|---|
| Favorable | t(8;21)(q22;q22.1); RUNX1-RUNX1T1 |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | |
| Mutated NPM1 without FLT3-ITD or with FLT3-ITDlow† | |
| Biallelic mutated CEBPA | |
| Intermediate | Mutated NPM1 and FLT3-ITDhigh† |
| Wild-type NPM1 without FLT3-ITD or with FLT3-ITDlow† (without adverse-risk genetic lesions) | |
| t(9;11)(p21.3;q23.3); MLLT3-KMT2A‡ | |
| Cytogenetic abnormalities not classified as favorable or adverse | |
| Adverse | t(6;9)(p23;q34.1); DEK-NUP214 |
| t(v;11q23.3); KMT2A rearranged | |
| t(9;22)(q34.1;q11.2); BCR-ABL1 | |
| inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1) | |
| −5 or del(5q); −7; −17/abn(17p) | |
| Complex karyotype,§ monosomal karyotype|| | |
| Wild-type NPM1 and FLT3-ITDhigh† | |
| Mutated RUNX1¶ | |
| Mutated ASXL1¶ | |
| Mutated TP53# |
Frequencies, response rates, and outcome measures should be reported by risk category, and, if sufficient numbers are available, by specific genetic lesions indicated.
Prognostic impact of a marker is treatment-dependent and may change with new therapies.
Low, low allelic ratio (<0.5); high, high allelic ratio (≥0.5); semiquantitative assessment of FLT3-ITD allelic ratio (using DNA fragment analysis) is determined as ratio of the area under the curve “FLT3-ITD” divided by area under the curve “FLT3-wild type”; recent studies indicate that AML with NPM1 mutation and FLT3-ITD low allelic ratio may also have a more favorable prognosis and patients should not routinely be assigned to allogeneic HCT.57-59,77
The presence of t(9;11)(p21.3;q23.3) takes precedence over rare, concurrent adverse-risk gene mutations.
Three or more unrelated chromosome abnormalities in the absence of 1 of the WHO-designated recurring translocations or inversions, that is, t(8;21), inv(16) or t(16;16), t(9;11), t(v;11)(v;q23.3), t(6;9), inv(3) or t(3;3); AML with BCR-ABL1.
Defined by the presence of 1 single monosomy (excluding loss of X or Y) in association with at least 1 additional monosomy or structural chromosome abnormality (excluding core-binding factor AML).116
These markers should not be used as an adverse prognostic marker if they co-occur with favorable-risk AML subtypes.
The prognostic impact of many markers is context-dependent with the effect of a given abnormality dependent on the presence/absence of another.37 Simple examples of such gene-gene interactions are that a NPM1 mutation conveys a “favorable” prognosis only in the absence of a FLT3-ITD (or FLT3-ITD with a low allelic ratio),57-59,77 whereas mutations in both ASXL1 and RUNX1 confer a particularly poor prognosis.37,65 Furthermore, tightly correlated clusters of mutated genes, that is, mutations in RNA splicing (SRSF2, SF3B1, U2AF1, ZRSR2), chromatin (ASXL1, STAG2, BCOR, KMT2APTD, EZH2), or transcription (RUNX1) regulators are found in high-risk MDS, high-risk MPN as well as secondary AML, indicating gene signatures identify high-risk myeloid disorders that cross-conventional diagnostic boundaries.37,78-82
In core-binding factor (CBF) AML, in particular in AML with t(8;21), the presence of KIT mutations, especially if higher mutant KIT levels are present, appear to be associated with poorer prognosis.83-87 Nevertheless, presence of a KIT mutation should not assign a patient to a different genetic risk category; rather, patients should be monitored for MRD, whose absence abrogates the effect of KIT.85 Although both types of CBF-AML are associated with mutations in signaling genes (NRAS, KIT, NF1, FLT3, KRAS), recent comprehensive mutation profiling studies have revealed a different spectrum of cooperating mutations (Figure 1).87,88 AML with RUNX1-RUNX1T1 is significantly enriched for mutations in chromatin-modifying genes (42%-44%), including ASXL2, and for mutations in cohesin complex genes (18%-20%), whereas they are nearly absent in AML with CBFB-MYH11.87-89
Although a genetic marker may currently not be prognostic, its presence may provide a target for new therapies as with IDH1, IDH2, and KMT2A (MLL).2 Likewise, a recent study in primary human samples identified co-occurrence of biallelic CEBPA mutations and mutations in the granulocyte colony-stimulating factor receptor gene CSF3R (signaling through the JAK-STAT pathway) as uniformly responsive to JAK inhibitors.90
Factors after diagnosis
Monitoring of MRD.
Two approaches can be used to detect MRD, that is, multiparameter flow cytometry (MFC) and molecular techniques, including real-time quantitative PCR (RT-qPCR), digital PCR, and next-generation sequencing–based technologies. Standardized RT-qPCR assays are now available to detect AML-associated genetic lesions (Table 4). Each methodology differs in the proportion of patients to whom it can be applied and in its sensitivity to detect MRD.91,92 It is expected that integrated evaluation of baseline factors and assessment of MRD will improve risk assessment and inform postremission therapy.91-93
MRD can be assessed (1) at early time points, for example, following induction and consolidation courses to assess remission status and determine kinetics of disease response, and (2) sequentially beyond consolidation to detect impending morphologic relapse. Remission status as assessed by MFC (which is informative in ∼90% of AML patients) provides a more reliable predictor of outcome than conventional morphology-based CR assessment.92-99 MFC can be used to assess “CR without MRD” (CRMRD−) (see “Response criteria and outcome measures” and Table 6). The depth of response assessed by MFC has been consistently shown to provide independent prognostic information and thus may inform risk stratification. Currently, analyses should be performed in experienced laboratories, until MFC techniques have been further standardized.
Response criteria in AML
| Category . | Definition . | Comment . |
|---|---|---|
| Response | ||
| CR without minimal residual disease (CRMRD−) | If studied pretreatment, CR with negativity for a genetic marker by RT-qPCR, or CR with negativity by MFC | Sensitivities vary by marker tested, and by method used; therefore, test used and sensitivity of the assay should be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
| Complete remission (CR) | Bone marrow blasts <5%; absence of circulating blasts and blasts with Auer rods; absence of extramedullary disease; ANC ≥1.0 × 109/L (1000/µL); platelet count ≥100 × 109/L (100 000/µL) | MRD+ or unknown |
| CR with incomplete hematologic recovery (CRi) | All CR criteria except for residual neutropenia (<1.0 × 109/L [1000/µL]) or thrombocytopenia (<100 × 109/L [100 000/µL]) | |
| Morphologic leukemia-free state (MLFS) | Bone marrow blasts <5%; absence of blasts with Auer rods; absence of extramedullary disease; no hematologic recovery required | Marrow should not merely be “aplastic”; at least 200 cells should be enumerated or cellularity should be at least 10% |
| Partial remission (PR) | All hematologic criteria of CR; decrease of bone marrow blast percentage to 5% to 25%; and decrease of pretreatment bone marrow blast percentage by at least 50% | Especially important in the context of phase 1-2 clinical trials |
| Treatment failure | ||
| Primary refractory disease | No CR or CRi after 2 courses of intensive induction treatment; excluding patients with death in aplasia or death due to indeterminate cause | Regimens containing higher doses of cytarabine (see Table 8) are generally considered as the best option for patients not responding to a first cycle of 7+3; the likelihood of responding to such regimens is lower after failure of a first |
| Death in aplasia | Deaths occurring ≥7 d following completion of initial treatment while cytopenic; with an aplastic or hypoplastic bone marrow obtained within 7 d of death, without evidence of persistent leukemia | |
| Death from indeterminate cause | Deaths occurring before completion of therapy, or <7 d following its completion; or deaths occurring ≥7 d following completion of initial therapy with no blasts in the blood, but no bone marrow examination available | |
| Response criteria for clinical trials only | ||
| Stable disease | Absence of CRMRD−, CR, CRi, PR, MLFS; and criteria for PD not met | Period of stable disease should last at least 3 mo |
| Progressive disease (PD)*,† | Evidence for an increase in bone marrow blast percentage and/or increase of absolute blast counts in the blood: | Category mainly applies for older patient given low-intensity or single-agent “targeted therapies” in clinical trials |
| • >50% increase in marrow blasts over baseline (a minimum 15% point increase is required in cases with <30% blasts at baseline; or persistent marrow blast percentage of >70% over at least 3 mo; without at least a 100% improvement in ANC to an absolute level (>0.5 × 109/L [500/µL], and/or platelet count to >50 × 109/L [50 000/µL] nontransfused); or | In general, at least 2 cycles of a novel agent should be administered | |
| • >50% increase in peripheral blasts (WBC × % blasts) to >25 × 109/L (>25 000/μL) (in the absence of differentiation syndrome)†; or | Some protocols may require blast increase in 2 consecutive marrow assessments at least 4 wk apart; the date of progression should then be defined as of the first observation date | |
| • New extramedullary disease | Some protocols may allow transient addition of hydroxyurea to lower blast counts | |
| “Progressive disease” is usually accompanied by a decline in ANC and platelets and increased transfusion requirement and decline in performance status or increase in symptoms | ||
| Relapse | ||
| Hematologic relapse (after CRMRD−, CR, CRi) | Bone marrow blasts ≥5%; or reappearance of blasts in the blood; or development of extramedullary disease | |
| Molecular relapse (after CRMRD−) | If studied pretreatment, reoccurrence of MRD as assessed by RT-qPCR or by MFC | Test applied, sensitivity of the assay, and cutoff values used must be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
| Category . | Definition . | Comment . |
|---|---|---|
| Response | ||
| CR without minimal residual disease (CRMRD−) | If studied pretreatment, CR with negativity for a genetic marker by RT-qPCR, or CR with negativity by MFC | Sensitivities vary by marker tested, and by method used; therefore, test used and sensitivity of the assay should be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
| Complete remission (CR) | Bone marrow blasts <5%; absence of circulating blasts and blasts with Auer rods; absence of extramedullary disease; ANC ≥1.0 × 109/L (1000/µL); platelet count ≥100 × 109/L (100 000/µL) | MRD+ or unknown |
| CR with incomplete hematologic recovery (CRi) | All CR criteria except for residual neutropenia (<1.0 × 109/L [1000/µL]) or thrombocytopenia (<100 × 109/L [100 000/µL]) | |
| Morphologic leukemia-free state (MLFS) | Bone marrow blasts <5%; absence of blasts with Auer rods; absence of extramedullary disease; no hematologic recovery required | Marrow should not merely be “aplastic”; at least 200 cells should be enumerated or cellularity should be at least 10% |
| Partial remission (PR) | All hematologic criteria of CR; decrease of bone marrow blast percentage to 5% to 25%; and decrease of pretreatment bone marrow blast percentage by at least 50% | Especially important in the context of phase 1-2 clinical trials |
| Treatment failure | ||
| Primary refractory disease | No CR or CRi after 2 courses of intensive induction treatment; excluding patients with death in aplasia or death due to indeterminate cause | Regimens containing higher doses of cytarabine (see Table 8) are generally considered as the best option for patients not responding to a first cycle of 7+3; the likelihood of responding to such regimens is lower after failure of a first |
| Death in aplasia | Deaths occurring ≥7 d following completion of initial treatment while cytopenic; with an aplastic or hypoplastic bone marrow obtained within 7 d of death, without evidence of persistent leukemia | |
| Death from indeterminate cause | Deaths occurring before completion of therapy, or <7 d following its completion; or deaths occurring ≥7 d following completion of initial therapy with no blasts in the blood, but no bone marrow examination available | |
| Response criteria for clinical trials only | ||
| Stable disease | Absence of CRMRD−, CR, CRi, PR, MLFS; and criteria for PD not met | Period of stable disease should last at least 3 mo |
| Progressive disease (PD)*,† | Evidence for an increase in bone marrow blast percentage and/or increase of absolute blast counts in the blood: | Category mainly applies for older patient given low-intensity or single-agent “targeted therapies” in clinical trials |
| • >50% increase in marrow blasts over baseline (a minimum 15% point increase is required in cases with <30% blasts at baseline; or persistent marrow blast percentage of >70% over at least 3 mo; without at least a 100% improvement in ANC to an absolute level (>0.5 × 109/L [500/µL], and/or platelet count to >50 × 109/L [50 000/µL] nontransfused); or | In general, at least 2 cycles of a novel agent should be administered | |
| • >50% increase in peripheral blasts (WBC × % blasts) to >25 × 109/L (>25 000/μL) (in the absence of differentiation syndrome)†; or | Some protocols may require blast increase in 2 consecutive marrow assessments at least 4 wk apart; the date of progression should then be defined as of the first observation date | |
| • New extramedullary disease | Some protocols may allow transient addition of hydroxyurea to lower blast counts | |
| “Progressive disease” is usually accompanied by a decline in ANC and platelets and increased transfusion requirement and decline in performance status or increase in symptoms | ||
| Relapse | ||
| Hematologic relapse (after CRMRD−, CR, CRi) | Bone marrow blasts ≥5%; or reappearance of blasts in the blood; or development of extramedullary disease | |
| Molecular relapse (after CRMRD−) | If studied pretreatment, reoccurrence of MRD as assessed by RT-qPCR or by MFC | Test applied, sensitivity of the assay, and cutoff values used must be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
ANC, absolute neutrophil count; IDH, isocitrate dehydrogenase; MLFS, morphologic leukemia-free state; WBC, white blood cell.
The authors acknowledge that this new provisional category is arbitrarily defined; the category aims at harmonizing the various definitions used in different clinical trials.
Certain targeted therapies, for example, those inhibiting mutant IDH proteins, may cause a differentiation syndrome, that is, a transient increase in the percentage of bone marrow blasts and an absolute increase in blood blasts; in the setting of therapy with such compounds, an increase in blasts may not necessarily indicate PD.
In ∼60% of younger adults, the leukemia cells are informative for a molecular marker that can be tracked by RNA-based RT-qPCR assays. Assay sensitivity depends upon the relative expression of the target in leukemic blasts compared with standard housekeeping genes (eg, ABL1) and varies according to the target, as well as between patients with the same target.91 Assays for MLLT3-KMT2A are typically associated with the lowest sensitivity (∼1 in 103) due to relatively low-level fusion gene expression,100 whereas assays for NPM1 mutations achieve sensitivities of up to 1 in 106-7 due to the high-level mutant allele expression.101-106 Many studies have shown that kinetics of MRD response to frontline therapy differs by molecular marker analyzed.85,101-109 For example, reduction in RUNX1-RUNX1T1 is slower than in NPM1 transcript levels. Importantly, MRD status has been found to be a better predictor of relapse risk than presence of cooperating mutations involving KIT and FLT3-ITD in CBF-AML,85 or FLT3-ITD, DNMT3A, and WT1 in NPM1-mutated AML.106 These data support inclusion of molecular MRD assessment into routine care to help inform transplant decisions in first remission.
Sequential MRD-monitoring studies have shown that persistent high-level PCR positivity, or a rising level of leukemic transcripts after an initial molecular response, invariably predict relapse.91 Whether the opportunity thus provided for early intervention to prevent overt relapse will be useful is under investigation. Preemptive therapy may be particularly relevant with allogeneic hematopoietic cell transplantation (HCT) where MRD status may inform conditioning strategy, or post-HCT measures aiming to avoid frank relapse.
Molecular markers can now be identified in virtually all cases. This has opened the way to detection of MRD using next-generation sequencing or digital PCR.91 Although currently investigational, studies have already shown that mutational assessment at early time points can distinguish patients at differing probability of relapse.110,111 Studies are needed to define which mutations are reliable indicators of leukemic clones associated with clinical relapse from mutations that are associated with preleukemic clones (eg, DNMT3A, IDH1/2) poorly predictive of relapse, although persistent at high levels after chemotherapy and during remission.106,112,113
2017 ELN genetic risk stratification
The original intention of the ELN genetic categories was to standardize reporting of genetic abnormalities particularly for correlations with clinical characteristics and outcome. The distinction between the intermediate I and intermediate II categories was based on genetic characteristics, rather than on prognostic stratification. Although a subsequent study demonstrated longer OS in the intermediate I group than the intermediate II group, the 2 groups were prognostically indistinguishable in older patients, who constitute the majority of cases of AML.114
Given these findings, the panel decided to simplify the ELN system by using a 3-group classification (favorable, intermediate, adverse) rather than the previous 4-group system (Table 5). A few other changes have been made. Recent studies have shown that in AML with NPM1 or biallelic CEBPA mutations, the presence of coexisting chromosomal abnormalities does not appear to modify the prognostic effect of the mutations16,25,115 ; prognosis may be more influenced by concurrent gene mutations.37 Accordingly, and as in CBF-AML, the categorization of these cases is now based on the primary leukemia-defining genetic subsets irrespective of the karyotype. The higher relapse rate and poorer OS associated with FLT3-ITD largely depends on the ITD allelic ratio. Most recent studies suggest that patients with NPM1 mutation and FLT3-ITD with a low (<0.5) allelic ratio (FLT3-ITDlow) have a similar (favorable) outcome as patients with a NPM1 mutation but no FLT3-ITD; thus, both groups are now considered favorable.57-60 In contrast, AML with wild-type NPM1 and FLT3-ITD with a high (≥0.5) allelic ratio (FLT3-ITDhigh) has a poor prognosis and is placed in the adverse-risk group,57 although the panel acknowledges that the natural course of AML with FLT3 mutation may change by use of FLT3 inhibitors.
RUNX1, ASXL1, and TP53 mutations (see “Pretreatment factors”), and monosomal karyotype116-120 have also been added to the adverse-risk group in recognition of their independent association with adverse risk. Although numerous studies have dealt with mutations in other genes, for example, DNMT3A, IDH1, IDH2, or genes in the chromatin/spliceosome group other than ASXL1 and RUNX1, the panel did not feel enough evidence has as yet accumulated to warrant their assignment to an ELN prognostic group.
Response criteria and outcome measures
The panel proposes a few new response categories. Although recognizing these are arbitrarily defined, they reflect recent data and aim at harmonizing definitions used in different trials (Tables 6 and 7).
Outcome measures for clinical trials in AML
| Category . | Definition . |
|---|---|
| Overall survival | Defined for all patients of a trial; measured from the date of entry into a clinical trial or from the date of diagnosis (eg, for correlative science studies) to the date of death from any cause; patients not known to have died at last follow-up are censored on the date they were last known to be alive |
| Relapse-free survival (RFS)*,† | Defined only for patients achieving CR, or CRi; measured from the date of achievement of a remission until the date of relapse or death from any cause; patients not known to have relapsed or died at last follow-up are censored on the date they were last examined |
| Event-free survival (EFS)† | Defined for all patients of a trial; measured from the date of entry into a study to the date of primary refractory disease, or relapse from CR, or CRi, or death from any cause; patients not known to have any of these events are censored on the date they were last examined |
| Cumulative incidence of relapse (CIR)†,‡ | Defined for all patients achieving CR, CRi; measured from the date of achievement of a remission until the date of relapse; patients not known to have relapsed are censored on the date they were last examined; patients who died without relapse are counted as a competing cause of failure |
| Category . | Definition . |
|---|---|
| Overall survival | Defined for all patients of a trial; measured from the date of entry into a clinical trial or from the date of diagnosis (eg, for correlative science studies) to the date of death from any cause; patients not known to have died at last follow-up are censored on the date they were last known to be alive |
| Relapse-free survival (RFS)*,† | Defined only for patients achieving CR, or CRi; measured from the date of achievement of a remission until the date of relapse or death from any cause; patients not known to have relapsed or died at last follow-up are censored on the date they were last examined |
| Event-free survival (EFS)† | Defined for all patients of a trial; measured from the date of entry into a study to the date of primary refractory disease, or relapse from CR, or CRi, or death from any cause; patients not known to have any of these events are censored on the date they were last examined |
| Cumulative incidence of relapse (CIR)†,‡ | Defined for all patients achieving CR, CRi; measured from the date of achievement of a remission until the date of relapse; patients not known to have relapsed are censored on the date they were last examined; patients who died without relapse are counted as a competing cause of failure |
CID, cumulative incidence of death; CIR, cumulative incidence of relapse.
RFS and disease-free survival have been used with the same definition.
In clinical trials in which the response criterion CRMRD− is used, consideration should be given to include molecular relapse as assessed by RT-qPCR or MFC as a criterion for relapse; similarly, for analysis of EFS, no achievement of CRMRD− may be regarded as an event. The definitions of RFS, EFS, and CIR must be clearly defined within each protocol.
It is important to provide estimates of CID as well because just considering the results of CIR may be misleading if, for instance, CIR is lower for 1 group but CID is actually higher for that same group.
CRMRD−
The category CRMRD− is proposed because relapse is more likely in patients in CR or CR with incomplete hematologic recovery (CRi) with detectable residual disease.91,92 The best time to test for MRD in patients in CR by conventional criteria is not settled. Assessment of MRD after cycle 2 or even cycle 1 of induction allows earlier identification of poor responders.91,92,97,106 However, MRD can disappear after consolidation therapy. The frequency with which this occurs may differ in different molecular subsets and future assessment of these frequencies will likely inform therapeutic decisions.
Primary refractory AML
The panel proposes criteria for “primary refractory disease” (also commonly termed “induction failure”) because the definition of refractory disease currently differs in clinical practice and clinical trials. Failure to attain CR following exposure to at least 2 courses of intensive induction therapy defines patients to be “primary refractory.” Although possibly influenced by selection bias, CR rates from a second course of 7+3 can be 40% to 45%, which is often higher than the rate targeted by newer therapies.121 Regimens containing higher doses of cytarabine are generally considered as the best option for patients not responding to a first cycle of 7+3. The likelihood of CR with a second course of a higher dose cytarabine-based regimen after failure of a first of the 2 cycles may be relatively lower than is the case with a second 7+3 after failure of a first.122,123
Progressive disease
This proposed new category primarily applies to patients given less intense or single-agent targeted therapies. A uniformly accepted definition of progressive disease (PD) should facilitate a standardized interpretation of new drug trials. Because criteria for PD are arbitrary, it is unknown whether PD augurs a poorer prognosis than stable disease and warrants investigation. In the interim, observation of PD does not necessarily imply a patient should be removed from a given therapy.
MDS-AML overlap/secondary AML
Genetic basis
The related and partially overlapping clinical phenotypes of MDS and AML are reflected in the genetic bases of the 2 diseases.31,37,78-80,124 A subset of mutations are highly specific for de novo AML, whereas another set of mutations is specific for secondary AML and are found commonly in MDS. Genetic analyses of a panel of genes mutated in myeloid malignancies, and perhaps the addition of gene expression and DNA-methylation profiling, have the potential to inform the distinction between MDS and AML, and to determine which cases of AML arose from an antecedent MDS.37,80,81 The prognoses of patients with clinically diagnosed de novo AML whose gene mutation profile resembles those of patients with clinically diagnosed secondary AML is more like secondary than de novo AML.81
Mutations associated with secondary AML occur in genes encoding SRSF2, SF3B1, U2AF1, and ZRSR2 (splicing factors); ASXL1, EZH2, and BCOR (epigenetic regulators); and STAG2 (a member of the cohesin complex).81 In such cases, these mutations likely occur during an MDS phase, remain in the clone that progresses to acute leukemia, and often persist in clonal remission following chemotherapy. Similarly, mutations in ASXL1, EZH2, and SRSF2 genes have been shown to identify patients with primary myelofibrosis who are at risk for leukemic transformation and who have particularly poor outcomes.82,125 In contrast, NPM1 mutations, and CBF and KMT2A rearrangements are highly specific for de novo AML.81
Blast count
Given the biologic overlap between secondary AML and MDS any minimum blast percentage used to distinguish AML from MDS with higher blast counts (ie, MDS with excess blasts-2 [MDS-EB2]) must be arbitrary. Thus, this minimum has decreased from 30% in the FAB system to 20% in the WHO system with many AML clinical trial groups allowing entry of patients with >10% blasts. Bone marrow failure is the usual cause of death in both AML and MDS-EB2, and most of the latter die without “progression to AML,” with data suggesting the natural history of MDS-EB2 is more similar to AML than to lower risk MDS.128,129
These observations suggest that it is best to determine eligibility for an “AML” or “MDS” study based on disease- and patient-specific factors rather than on a fixed blast percentage. Integration of data from molecular genetics into future classification systems will be useful to refine current diagnostic algorithms and support a more biologically precise disease classification.
Current therapy
The general approach to current therapy has not changed substantially in recent years. Initial assessment evaluates whether a patient is considered a candidate for intensive induction chemotherapy. Although assessment of risk of treatment-related mortality (TRM) after intensive therapy is usually most relevant in older patients (commonly above the age of 65 years), age is merely one, and not the most important, predictor of TRM.130-135 Furthermore, TRM rates are declining due to improved supportive care and to better health status in older patients.136,137
Therefore, age alone should not be the decisive determinant to guide therapy. Although few randomized trials have addressed the question and these trials have been small, there are suggestions that older, medically fit patients may benefit more from “intensive” than “nonintensive” induction therapy, subject to the constraints of selection bias.137 Hence, although recognizing that firm criteria to consider older patients (or any patients) unfit for intensive induction therapy cannot be provided, the panel feels these should include only factors such as poor performance status and significant comorbidities and, in the case of conventional regimens such as 7+3, adverse ELN cytogenetics/molecular genetics (Table 5) because in these instances the benefit may not outweigh the risk. Results from cytogenetics should be obtained preferably within 5 to 7 days. Results from NPM1 and FLT3 mutational screening should be available within 48 to 72 hours (at least in patients eligible for intensive chemotherapy), and results from additional molecular genetics within the first treatment cycle. Abnormal renal or liver function should not be considered solely but in the context of other comorbidities and, although dose reduction may be called for, should not per se exclude patients from administration of intensive therapy. Several systems to quantify comorbidities and/or risk of TRM after intensive induction therapy have been proposed (see “Older patients not considered candidates for intensive chemotherapy”).
Intensive induction therapy
With 3 days of an anthracycline and 7 days of cytarabine (commonly referred to as “7+3” regimens), CR is achieved in 60% to 80% of younger adults and in 40% to 60% of older adults (60 years or above) (Table 8).1,2,138
Selected conventional care regimens for patients with AML
| Selected conventional care regimens . | |
|---|---|
| Patients eligible for intensive chemotherapy | |
| Induction therapy (all ages) (“7+3”)*,†,‡ | • 3 d of an IV anthracycline: daunorubicin at least 60 mg/m2; idarubicin 12 mg/m2; or mitoxantrone 12 mg/m2, and 7 d of continuous infusion cytarabine (100-200 mg/m2) |
| Consolidation therapy‡,§ | |
| Younger patients (18-60/65 y) | |
| • Favorable-risk genetics | • 2-4 cycles of IDAC (1000-1500 mg/m2 IV over 3 h q12h, d1-3; or 1000-1500 mg/m2 IV over 3 h d1-5 or 6) |
| • Intermediate-risk genetics | • Allogeneic HCT from matched-related or unrelated donor |
| • 2-4 cycles of IDAC (1000-1500 mg/m2 IV over 3 h q12h, d1-3; or 1000-1500 mg/m2 IV over 3 h d1-5 or 6), or | |
| • High-dose therapy and autologous HCT | |
| • Adverse-risk genetics | • Allogeneic HCT from matched-related or unrelated donor |
| Older patients (>60/65 y) | |
| • Favorable-risk genetics | • 2-3 cycles of IDAC (500-1000 mg/m2 IV over 3 h q12h, d1-3; or 500-1000 mg/m2 IV over 3 h d1-5 or 6) |
| • Intermediate/adverse-risk genetics | • No established value of intensive consolidation therapy; consider allogeneic HCT in patients with low HCT-Comorbidity Index, or investigational therapy |
| Patients considered not candidates for intensive chemotherapy|| | |
| Azacitidine¶ | 75 mg/m2, SC, d1-7, q4 wk, until progression |
| Decitabine# | 20 mg/m2, IV, d1-5, q4 wk, until progression |
| Low-dose cytarabine** | Low-dose cytarabine (20 mg q12h, SC, d1-10, q4 wk; until progression); not recommended in patients with adverse-risk genetics |
| Best supportive care | Including hydroxyurea; for patients who cannot tolerate any antileukemic therapy, or who do not wish any therapy |
| Common salvage regimens in patients not responding to a first induction cycle or with relapsed disease who are candidates for intensive therapy | |
| IDAC†† (with or without anthracycline) | IDAC (1000-1500 mg/m2 IV over 3 h q12 h, d1-3 [500-1000 mg/m2 in patients >60 y]; or 1000-1500 mg/m2 IV over 3 h d1-5 or 6 [500-1000 mg/m2 in patients >60 y]); with or without daunorubicin 45-60 mg/m2, IV, d1-3; idarubicin 8-10 mg/m2, IV, d3-5, or mitoxantrone 8-10 mg/m2, IV, d1-3 |
| FLAG-IDA‡‡ | Fludarabine 30 mg/m2 IV, d2-6; cytarabine 1500-2000 mg/m2 IV over 3 h, starting 4 h after fludarabine infusion, d2-6; idarubicin 10 mg/m2 IV, d2-4; G-CSF 5 µg/kg, SC, d1-5; additional G-CSF may be administered starting 7 d after end of chemotherapy until WBC count >500/uL |
| Consider dose reduction in patients >60 y: fludarabine 20 mg/m2; cytarabine 500-1000 mg/m2; idarubicin 8 mg/m2 | |
| MEC | Mitoxantrone 8 mg/m2, d1-5; etoposide 100 mg/m2, d1-5; cytarabine 1000 mg/m2, d1-5 |
| Allogeneic HCT | Consider transplantation for patients with primary refractory disease, for patients in second CR or with major cytoreduction but still active disease following salvage therapy |
| Consider second transplantation under certain conditions (see “Salvage treatment”) | |
| Perform early HLA typing | |
| Selected conventional care regimens . | |
|---|---|
| Patients eligible for intensive chemotherapy | |
| Induction therapy (all ages) (“7+3”)*,†,‡ | • 3 d of an IV anthracycline: daunorubicin at least 60 mg/m2; idarubicin 12 mg/m2; or mitoxantrone 12 mg/m2, and 7 d of continuous infusion cytarabine (100-200 mg/m2) |
| Consolidation therapy‡,§ | |
| Younger patients (18-60/65 y) | |
| • Favorable-risk genetics | • 2-4 cycles of IDAC (1000-1500 mg/m2 IV over 3 h q12h, d1-3; or 1000-1500 mg/m2 IV over 3 h d1-5 or 6) |
| • Intermediate-risk genetics | • Allogeneic HCT from matched-related or unrelated donor |
| • 2-4 cycles of IDAC (1000-1500 mg/m2 IV over 3 h q12h, d1-3; or 1000-1500 mg/m2 IV over 3 h d1-5 or 6), or | |
| • High-dose therapy and autologous HCT | |
| • Adverse-risk genetics | • Allogeneic HCT from matched-related or unrelated donor |
| Older patients (>60/65 y) | |
| • Favorable-risk genetics | • 2-3 cycles of IDAC (500-1000 mg/m2 IV over 3 h q12h, d1-3; or 500-1000 mg/m2 IV over 3 h d1-5 or 6) |
| • Intermediate/adverse-risk genetics | • No established value of intensive consolidation therapy; consider allogeneic HCT in patients with low HCT-Comorbidity Index, or investigational therapy |
| Patients considered not candidates for intensive chemotherapy|| | |
| Azacitidine¶ | 75 mg/m2, SC, d1-7, q4 wk, until progression |
| Decitabine# | 20 mg/m2, IV, d1-5, q4 wk, until progression |
| Low-dose cytarabine** | Low-dose cytarabine (20 mg q12h, SC, d1-10, q4 wk; until progression); not recommended in patients with adverse-risk genetics |
| Best supportive care | Including hydroxyurea; for patients who cannot tolerate any antileukemic therapy, or who do not wish any therapy |
| Common salvage regimens in patients not responding to a first induction cycle or with relapsed disease who are candidates for intensive therapy | |
| IDAC†† (with or without anthracycline) | IDAC (1000-1500 mg/m2 IV over 3 h q12 h, d1-3 [500-1000 mg/m2 in patients >60 y]; or 1000-1500 mg/m2 IV over 3 h d1-5 or 6 [500-1000 mg/m2 in patients >60 y]); with or without daunorubicin 45-60 mg/m2, IV, d1-3; idarubicin 8-10 mg/m2, IV, d3-5, or mitoxantrone 8-10 mg/m2, IV, d1-3 |
| FLAG-IDA‡‡ | Fludarabine 30 mg/m2 IV, d2-6; cytarabine 1500-2000 mg/m2 IV over 3 h, starting 4 h after fludarabine infusion, d2-6; idarubicin 10 mg/m2 IV, d2-4; G-CSF 5 µg/kg, SC, d1-5; additional G-CSF may be administered starting 7 d after end of chemotherapy until WBC count >500/uL |
| Consider dose reduction in patients >60 y: fludarabine 20 mg/m2; cytarabine 500-1000 mg/m2; idarubicin 8 mg/m2 | |
| MEC | Mitoxantrone 8 mg/m2, d1-5; etoposide 100 mg/m2, d1-5; cytarabine 1000 mg/m2, d1-5 |
| Allogeneic HCT | Consider transplantation for patients with primary refractory disease, for patients in second CR or with major cytoreduction but still active disease following salvage therapy |
| Consider second transplantation under certain conditions (see “Salvage treatment”) | |
| Perform early HLA typing | |
Patients should go on clinical trials if possible.
EMA, European Medicines Agency; FLAG-AMSA, FLAG + amsacrine; FLAG-MITO, FLAG + mitoxantrone; q, every; SC, subcutaneously.
Regimens containing higher doses of cytarabine are generally considered as the best option for patients not responding to a first cycle of “7+3” (see common salvage regimens).
Older patients (in general >65 y) and patients with adverse genetics are less likely to respond to conventional induction therapy and may receive hypomethylating agents, or, preferably, investigational therapy.
Patients, at least those aged 18 to 60 y, with newly diagnosed AML and activating FLT3 mutations may be considered to receive additional therapy with midostaurin (administered after the chemotherapy).61
Results from assessment of MRD should be taken into account for selecting the appropriate consolidation therapy.
For discussion of patients not considered candidates for intensive chemotherapy see first 2 paragraphs of “Current therapy.”
Approved by FDA and EMA for adult patients who are not eligible for HCT with AML with 20% to 30% blasts and multilineage dysplasia; in addition, approved by EMA for patients who are not eligible for allogeneic HCT with AML with >30% marrow blasts.
Approved by EMA (not by FDA) for patients with newly diagnosed de novo or secondary AML, who are not candidates for standard induction chemotherapy.
In some countries used in a dosage of 20 mg/m2 SC once daily.
Evidence from pharmacologic studies and clinical trials in first-line treatment indicate that doses higher than 1500 mg/m2 are above the plateau of the maximal therapeutic effect;147 single-agent IDAC should not be used in patients relapsing within 6 mo following consolidation with higher doses of cytarabine.
Idarubicin may be replaced by mitoxantrone 10 mg/m2, IV, days 2 to 4 (FLAG-MITO); or by amsacrine 100 mg/m2, days 2 to 4 (FLAG-AMSA).
Anthracycline dose level.
Randomized studies have indicated that daunorubicin at 45 mg/m2 daily ×3 is associated with a lower CR rate and a higher relapse rate than 90 mg/m2 daily ×3 when daunorubicin is used in a single induction cycle.139-141 This clear dose-effect relation seems much less prominent in patients >65 years of age. However, another comparison found that 90 mg/m2 daunorubicin daily ×3 in a first induction cycle was not superior to daunorubicin at 60 mg/m2 daily ×3.142 In this study, both groups received additional daunorubicin at 50 mg/m2 for 3 days once in CR which added significant toxicities to the high-dose schedule and may have obscured or counteracted the benefit of the 90 mg/m2 during the first cycle. A recent exploratory analysis from this study suggests the potential for improved outcomes among patients with FLT3-ITD with anthracycline intensification, although this finding requires further validation.143 Current evidence suggests that the dose of daunorubicin should not be <60 mg/m2.
In patients 50 to 70 years of age, daunorubicin (80 mg/m2 for 3 days) or idarubicin (12 mg/m2 for 4 days) were compared with the usual idarubicin schedule (12 mg/m2 for 3 days). Although the CR rate was slightly higher with 4 days of idarubicin, there were no differences between the 3 arms in rates of relapse, EFS, or OS.144
Cytarabine dose.
Recent studies123,145 confirm earlier ones demonstrating increased toxicity without improvement in efficacy with higher dose cytarabine (2000-3000 mg/m2). A randomized trial found that fludarabine + high-dose cytarabine + granulocyte colony-stimulating factor (G-CSF; FLAG) + idarubicin (FLAG-IDA) not only produced a lower relapse rate than daunorubicin-cytarabine with or without etoposide, but was also associated with more deaths in remission resulting in similar OS.123 Only 1 randomized study has shown prolonged OS (52% vs 43% at 6 years) with cytarabine at 3000 mg/m2 (every 12 hours, days 1, 3, 5, 7) compared with 100 mg/m2 (daily ×7) in cycle I, but only in patients <46 and not 46 to 60 years of age.146 The bulk of evidence indicates that cytarabine at doses >1000 mg/m2 should not be included in induction regimens.147 Furthermore, neither this study nor any others have shown that particular cytogenetic subsets benefit from such high cytarabine doses (see also “Conventional postremission therapy”).
Role of other drugs.
FLT3 inhibitors.
The RATIFY trial evaluated intensive induction and consolidation chemotherapy plus midostaurin or placebo followed by a 1-year midostaurin/placebo maintenance phase in 717 patients aged 18 to 60 years with FLT3-mutated AML.61 Use of midostaurin increased the CR rate when all CRs reported within 30 days of ending protocol therapy were considered (68% vs 59%; P = .04). The trial met its primary end point in improving OS (hazard ratio 0.78; P = .009), regardless of whether patients received allogeneic HCT. Thus, patients with FLT3-mutated AML may be considered to receive intensive chemotherapy in combination with midostaurin.
Gemtuzumab ozogamicin.
The role of gemtuzumab ozogamicin (GO), an antibody-toxin (calicheamicin) conjugate that targets CD33+ AML, is complicated. Two randomized studies using a single GO dose during chemotherapy in patients primarily age <60 years failed to show a survival advantage,148,149 although the first used a suboptimal daunorubicin dose (45 mg/m2) in the GO arm vs 60 mg/m2 in the control arm.148 Both studies suggested the addition of GO was associated with longer relapse-free survival (RFS) in the favorable-risk subset of CBF-AML. The second study149 extended this finding to survival in some patients with intermediate-risk cytogenetics. Two studies in older patients (median age, 61 and 67 years), 1 using a single 3 mg/m2 GO dose and the other using 3 mg/m2 GO on days 1, 4, and 7 of induction found survival benefit with GO, largely attributable to fewer relapses in patients with favorable- or intermediate-risk cytogenetics.150,151 An individual patient data meta-analysis of these 4 studies and a fifth published in abstract form reinforced these conclusions.152 In contrast, 1 large study in patients age 61 to 75 years found shorter survival (P = .071) in the GO arm largely reflecting higher early mortality in patients age 70 to 75 years.153 The dose and schedule of GO may be critical for the benefit-toxicity ratio. GO is currently only available in clinical trials and through a compassionate use program sponsored by the US Food and Drug Administration (FDA).
CPX-351.
CPX-351 is an encapsulation in nanoscale liposomes of cytarabine and daunorubicin at a synergistic 5:1 molar ratio.154-157 Phase 2 studies suggested a beneficial effect of the agent in first-line treatment of secondary and therapy-related AML,155 and in the poor-risk stratum (by the European Prognostic Index [EPI])158 of relapsed AML.156 A subsequent phase 3 trial randomized 309 patients age 60 to 75 years with high-risk AML, defined as AML with myelodysplasia-related changes or therapy-related AML, to CPX-351 or “7+3”.157 CPX-351 produced a higher response rate (CR/CRi, 47.7% vs 33.3%; P = .016), and longer OS (hazard ratio, 0.69; P = .005 with medians of 9.6 vs 6 months and 2-year survival rates of 31% and 12%). Results were similar after accounting for allogeneic HCT. Thus, CPX-351 may improve therapy of older patients with high-risk features.
Purine analogs.
In 1 study, cladribine (at 5 mg/m2 days 1-5) added to “7+3” in adults up to age 60 years produced a higher CR rate and better OS than 7+3, particularly in patients age 50 to 60 years and in those with adverse-risk cytogenetics.159 However, the relatively low CR rate (56%) and median OS (14 months) in the control arm have raised questions, and we await independent confirmation. In the intensive arm of their AML16 trial in older patients (median age, 67 years), the National Cancer Research Institute (NCRI) Cooperative Group randomized 806 patients between daunorubicin (50 mg/m2 days 1-3) and either cytarabine (100 mg/m2 days 1-10) or clofarabine (20 mg/m2 days 1-5). Rates of CR (66%-71%), relapse (68%-74% at 3 years) and OS (22%-23% at 3 years) were essentially identical.160
Intensive postremission therapy
Conventional postremission therapy.
Postremission strategies comprise intensive chemotherapy and high-dose therapy followed by autologous or allogeneic HCT (Table 8). Assessment of residual disease by RT-qPCR or MFC is critical in monitoring patients in morphological remission to inform further therapy (see “Factors after diagnosis”).
Conventional intensive consolidation.
Consolidation regimens include single-agent cytarabine at high doses and multiagent chemotherapy which lead to similar outcomes. Administration of up to 4 cycles of high-dose cytarabine (2000-3000 mg/m2, commonly 6 doses per cycle) has been widely used. Recent trials have questioned the need for such high doses. One study randomized 933 patients, 15 to 60 years of age, between consolidation with mitoxantrone and cytarabine at 3000 mg/m2 (every 12 hours for 6 days) vs a similar chemotherapy program, but with intermediate-dose cytarabine (IDAC) at 1000 mg/m2 for consolidation with no differences in outcome.161 Similarly, in a study with multiple randomizations in induction, the postremission comparison between cytarabine 3000 mg/m2 and 1500 mg/m2 (n = 657) showed no difference in survival.123 A third study in 781 complete responders (15-64 years of age) failed to show a benefit for 3 cycles of cytarabine at 2000 mg/m2 (every 12 hours for 5 days) compared with 4 cycles of a multiagent chemotherapy consolidation that contained 200 mg/m2 cytarabine by 24-hour continuous infusion for 5 days.162 None of these studies have identified a benefit of the high-dose cytarabine regimens in cytogenetically favorable-risk AML. In a smaller study in patients 15 to 50 years of age, no difference in survival was noted between 4 cycles of cytarabine at 3000 mg/m2 and a combination of multiple cytotoxic agents.163
Altogether, there is no convincing evidence that cytarabine regimens at 3000 mg/m2 are more effective than regimens at intermediate-dose levels at 1000 to 1500 mg/m2, with or without the addition of an anthracycline.147 Open questions remain regarding the optimal number of cycles of consolidation therapy. In most studies, 2 to 4 cycles have been given after attainment of CR. In 1 randomized study, 2 cycles of postremission treatment following 2 induction cycles was not inferior to 3 postremission cycles.123 Intensified postremission chemotherapy in high-risk patients, especially older patients is without clear benefit.164
Intensive chemotherapy followed by autologous HCT.
One cycle of intensive chemotherapy followed by autologous HCT using peripheral blood CD34+ cells offers condensed treatment. In 1 randomized study, autologous HCT provided better RFS and similar OS as conventional consolidation chemotherapy.165 Recent data addressing the value of autologous HCT come from retrospective analyses accounting for the “lead time bias” consequent to the need for transplanted patients to live a minimum amount of time in order to receive a transplant. In these studies, autologous HCT leads to better EFS and RFS than chemotherapy.16,166,167 This effect is mainly apparent in favorable- and intermediate-risk disease (mainly by 2010 ELN criteria) where outcome after autologous HCT approaches results after allogeneic HCT if OS is the end point. Limiting autologous HCT to patients who are MRD− might improve results.
Maintenance therapy.
Allogeneic HCT.
AML is the most frequent indication for allogeneic HCT with a 10% annual increase in transplants performed worldwide.170-172 Expanded use of mismatched and unrelated donors as well as cord blood means a donor can be found for most patients. Furthermore, nonmyeloablative or reduced-intensity conditioning (RIC) regimens allow allogeneic HCT in patients aged up to 75 years. Nonetheless, in reality, only a minority of AML patients undergo transplantation because of older age, comorbidities, toxicity of prior therapy, inability to achieve a remission, and early relapse or refractory leukemia.173
Indications.
The decision to perform allogeneic HCT depends on the assessment of the risk-benefit ratio (ie, nonrelapse mortality [NRM]/morbidity vs reduction of relapse risk) based on cytogenetic and molecular genetic features as well as patient, donor, and transplant factors.174-177 AML with favorable-risk genetics are not a priori assigned to allogeneic HCT in first CR.57-59,77,174,177 Allogeneic HCT is generally recommended when the relapse incidence without the procedure is expected to be >35% to 40%. The higher the expected relapse risk, the more risk of NRM may be accepted. Especially in the adverse genetic group, it is generally assumed, although not unambiguously demonstrated, that the transplant should be performed as soon as CR has been achieved. Allogeneic HCT is the only curative option for patients with primary refractory disease.
Sequential MRD monitoring by RT-qPCR or MFC provides a reliable guide to management. Patients with persistent MRD or with early MRD reoccurrence can receive salvage therapy and proceed to transplant before hematologic relapse, or may proceed directly to transplant depending on the likelihood of success with salvage therapy. Although allogeneic HCT often produces superior outcomes to chemotherapy it does not abrogate the negative effect of unfavorable genetics or pretransplant MRD.99,119 Patients without MRD or adverse genetics but with high risk of NRM could receive chemotherapy only or autologous transplantation in CR1.167,178
Myeloablative conditioning vs RIC.
RIC potentially extends the curative graft-versus-leukemia effect to patients of older age or to young patients with significant comorbidities.179-182 Conditioning intensity varies. For instance, busulfan/fludarabine is more dose-intense than fludarabine/low-dose total-body irradiation.183 Currently, >30% of allogeneic transplants are performed using RIC and have yielded encouraging results.184 Although RIC and ablative conditioning have produced similar survival in patients aged 40 to 60 years in first CR,167 a trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0901) randomizing 218 patients (+54 with MDS) aged 18 to 65 years and with HCT comorbidity index (HCT-CI) scores associated with <20% to 30% NRM between RIC (typically fludarabine/busulfan) and more ablative (typically busulfan/cyclophosphamide) regimens suggests an advantage for more ablative regimens.185 This emphasizes the importance of randomized trials in transplantion with broad eligibility criteria to avoid selection bias. Currently, myeloablative regimens are generally recommended for healthy younger patients and RIC in elderly patients or in younger patients with severe comorbidities. Outcomes after myeloablative conditioning using busulfan/cyclophosphamide appear to be equivalent, if not superior, to outcomes after cyclophosphamide/total-body irradiation.186-188
Comorbidities and risk scores.
Several transplant-related models have been developed to optimize decision-making about suitable candidates for allogeneic HCT.189 The HCT-CI is a validated tool that sums a patient’s comorbidities into a single score that predicts the likelihood of NRM given a myeloablative or RIC regimen.190 A Disease Risk Index based on disease stage and cytogenetics has been developed that predicts the likelihood of disease recurrence following myeloablative or RIC regimens, independent of age, conditioning intensity, graft source, and donor type.191 The modified European Society for Blood and Marrow Transplantation (EBMT) risk score was designed to predict OS rather than just NRM or relapse, and includes age, disease stage, donor source, gender mismatch, and time from diagnosis.192 Recent reports suggest that a combination of the HCT-CI and the EBMT score may provide improved prediction of NRM and OS.193,194
New modalities.
Partial or complete T-cell depletion and posttransplant cyclophosphamide may reduce the risks of acute and chronic graft-versus-host disease (GVHD).195-198 The biggest challenge remains prevention of posttransplant relapse.199 Preparative regimens including novel agents or radiolabeled monoclonal antibodies,200 or therapy during the early posttransplant period with tyrosine kinase inhibitors or hypomethylating agents (HMAs) are being tested.201-203 Furthermore, cell-based therapies are being developed to enhance the graft-versus-leukemia effect, such as natural killer cell enrichment or adoptive transfer, and the use of genetically engineered antigen-specific T cells that target AML-specific antigens.204-209
Older patients not considered candidates for intensive chemotherapy
Some AML patients will not tolerate intensive chemotherapy. Several risk scoring systems are available that use patient-specific and disease-specific factors to make the choice of intensive or alternative treatment.74,132,133,210 The relevance of systems193,194 originally designed to forecast NRM after allogeneic HCT is under investigation.211
Treatment alternatives for unfit patients are limited to best supportive care, low-intensity treatment, or clinical trials with investigational drugs. Low-intensity options are either low-dose cytarabine (LDAC) or therapy with HMA (Table 8). LDAC is generally well-tolerated and produces CR rates in the order of 15% to 25%; however, OS (median, 5-6 months) is unsatisfactory.212
Therapy with HMA has been evaluated in randomized trials. An increase in median OS with decitabine vs mostly LDAC (7.7 vs 5.0 months) was observed.213 The AZA-AML-001 trial compared azacitidine with 3 conventional care regimens in patients aged ≥65 years with >30% blasts: LDAC (158 patients), 7+3 (44 patients), or best supportive care only (45 patients)214 ; azacitidine increased the median survival (10.4 vs 6.5 months). Azacitidine may be particularly advantageous in AML with adverse cytogenetics.215 Superiority of azacitidine over conventional care regimens was previously shown in AML with 20% to 30% blasts.216 Up to 6 courses may be needed to observe maximal response with azacitidine or decitabine, although patients without response after 3 courses are unlikely to respond with further therapy.217 HMA seem to alter the natural course of AML in some patients who do not achieve CR. Thus, hematologic improvement can also yield clinical benefit, that is, a reduction in transfusions and improved quality of life (QoL).
Treatment of unfit and most older patients with AML is currently unsatisfactory. We strongly recommend enrolling these patients in clinical trials.
Relapsed disease and primary refractory disease
Treatment of patients with relapsed or primary refractory disease requires a balanced assessment of the likely benefit of further therapy vs the potential complications associated with salvage chemotherapy.218
Prognostic markers
Factors influencing survival were incorporated in the EPI score applicable to adults between 15 and 60 years of age.158 Poor outcome is associated with shorter CR1 duration, increasing age at the time of relapse, nonfavorable karyotype at initial diagnosis, or history of prior allogeneic HCT.
Salvage treatment
No specific salvage regimen has emerged as the standard for treating primary refractory or relapsed AML.122,219-226 Enrollment in a clinical trial should therefore be the priority for such patients whenever possible. Table 8 provides recommendations for salvage regimens in patients considered fit for intensive therapy.
In younger adults (16-49 years), a second CR can be achieved with intensive salvage therapy in about 55% in the absence of prior allogeneic HCT.227 Two-thirds are able to proceed to allogeneic HCT in CR2, resulting in a 40% 5-year OS. Response rates are lower (∼20%-30%) in more unselected adult patients with relapsed/refractory disease.222 Benefit may also be derived from allogeneic HCT in the presence of active disease, with CR2 achieved in 42% and long-term survival observed in 9% to 22%.228-231
Another approach for patients with refractory or active disease is to use a short course of chemotherapy such as fludarabine, cytarabine, and amsacrine immediately prior to RIC and allogeneic HCT. With this approach, CR rates after allogeneic HCT of 70% to 90% are achieved, with expected 4-year survival ranging between 32% and 45%.231,232 The possible constraint of selection bias should again be noted; nonetheless, at least 20% of patients with primary refractory disease can still be cured with allogeneic HCT.233
Outcome for patients relapsing after allogeneic HCT during first or second CR is particularly poor.234,235 The Center for International Blood and Marrow Transplant Research (CIBMTR) recently found234 3-year OS was 4%, 12%, 26%, and 38% for relapses within 1 to 6 months, 6 months to 2 years, 2 to 3 years, and ≥3 years after allogeneic HCT, respectively. Lower mortality was independently associated with longer time from HCT to relapse and a first HCT using RIC; and inferior outcome associated with age >40 years, active GVHD, adverse cytogenetics, mismatched unrelated donor, and use of cord blood for first HCT.234 Outcomes may be better if patients receive chemotherapy to reduce disease burden followed by donor lymphocyte infusion, rather than chemotherapy alone.236,237 Use of HMA has modest efficacy in AML relapsing post-HCT, producing CR rates of ∼15%238 ; responses may be higher when combining donor lymphocyte infusion and azacitidine.239 Responses have been observed in relapses after HCT, including extramedullary manifestations, using CTLA-4 blockade with ipilimumab.240 The value of using a different donor for the second transplant remains unproven.235
In patients not fit for intensive salvage chemotherapy, effective treatment options are lacking. Azacitidine and decitabine induce CR rates of 16% to 21% and median survival times of 6 to 9 months in older patients with relapsed/refractory AML223-225 ; median postrelapse survival after therapy with LDAC is 5 to 6 months.226 For patients in second or third relapse, various therapeutic options are associated with CR rates of ∼20% and median OS outcomes of ∼3 months,221 stressing the need for enrollment into clinical trials.
Therapy-related AML
Biology of t-AML
Therapy-related myeloid neoplasms (t-MNs) are a distinct category within the WHO classification including cases of t-MDS and t-AML. t-AML is a well recognized clinical syndrome occurring as a late complication following cytotoxic therapy for a primary neoplasm or a nonneoplastic disorder.241,242 Currently comprising ∼7% of all newly diagnosed AML, the incidence of t-AML is rising due to increasing numbers of cancer survivors at risk and changes in treatment.125,243,244
These neoplasms have been thought to be the direct consequence of mutational events induced by cytotoxic therapy. Association between type of prior exposure and phenotype of t-AML support a direct role of prior cytotoxic therapy. The more common subtype, seen in ∼75% of patients, typically occurs 5 to 7 years after first exposure to alkylating agents or radiation, is often preceded by MDS, and is frequently accompanied by chromosomes 5 and/or 7 abnormalities, complex karyotype, and TP53 mutation. In general, t-AML is associated with more adverse genetic lesions.245-248 In a study analyzing mutation hotspots of 53 genes in 70 t-MNs (28 t-MDS, 42 t-AML), TP53 was the most commonly mutated gene in t-MDS (35.7%) and t-AML (33.3%).248 Some individuals develop t-AML after treatment with topoisomerase II inhibitors; their latency period is often only 1 to 3 years, antecedent MDS is rare, and balanced rearrangements involving KMT2A (MLL) at 11q23, RUNX1 at 21q22, or PML/RARA are common. The distinction between these 2 subtypes has become less evident due to the use of multiagent chemotherapy, often in combination with radiotherapy.
An alternative mechanism is suggested by cases with a preexisting myeloid clone that is resistant to chemotherapy.249 Cases of t-AML were identified in which the exact TP53 mutation found at diagnosis was already present at low frequency in blood or bone marrow many years before t-AML development.249 Similarly, somatic mutations in PPM1D, a serine/threonine phosphatase that negatively regulates p53,250 have been found in blood of patients with breast, ovarian, and lung cancer.251-254 In ovarian cancer, the frequency of PPM1D mutations in blood was significantly associated with prior chemotherapy, and the variant allele frequency increased during chemotherapy.251 These data suggest a model in which hematopoietic progenitor cells carrying mutations in the TP53 pathway undergo selective pressure by cytotoxic therapy, ultimately leading to t-AML.
Some cases of t-MNs have been shown to be associated with germ line mutations in cancer susceptibility genes.255,256 In a recent study of survivors of breast cancer developing t-AML, many patients had personal or family histories suggestive of inherited cancer susceptibility; 10 of 41 patients studied (21%) carried germ line mutations in BRCA1, BRCA2, TP53, or CHEK2 genes.256 The identification of such preexisting conditions will facilitate screening and counseling of patients prior to treatment of their primary disease.
Treatment of t-AML
The survival of patients with t-MNs has remained poor mainly due to sequelae of prior therapy, and to adverse disease-related features.257-261 Therapy may be compromised by a higher treatment-related morbidity and mortality.259 There is still little prospective treatment data because these patients have often been excluded from frontline clinical trials. Clinical trials should allow enrollment of patients with t-MN. Allogeneic HCT should be considered, due to the poor results with conventional chemotherapy.
Clinical trials
Necessity for biobanking
We strongly recommend storage of biosamples (see “Biobanking”) be done in all clinical trials. Such biobanking can be performed as part of an interventional trial, or within a noninterventional biobanking or registry study.
Trial design
Trials of new therapies have traditionally been disease-specific, proceeding through phase 1 (determination of maximum tolerated dose [MTD]), phase 2 (determination of efficacy), and phase 3 (randomized comparison of new and standard therapies). Recent challenges to this paradigm have arisen.
Early drug development.
“Basket trials.”
MTD vs “optimal biologic dose.”
When a drug’s ability to modulate its target appears fundamental to its clinical activity, phase 1 studies might seek to identify the optimal biologic dose (OBD) rather than the MTD. Randomization between OBD and MTD might be considered in phase 2 to shed light on which approach is preferable.262
Combined phase 1-2 designs.
To accelerate drug development, many phase 1 protocols now include an expansion phase which focuses on efficacy.264 On the assumption of a relation between efficacy and toxicity, multiple outcome designs simultaneously base dose finding on toxicity and efficacy, with a dose declared admissible for further study if associated with relatively low probabilities of toxicity and high probabilities of efficacy.265
“Pick-a-winner designs” to accelerate drug development.
The conventional distinction between the single-arm phase 2 trial and the larger (randomized) phase 3 study has been questioned. The frequent failure of therapies found “promising” in single-arm phase 2 trials to translate into truly successful treatments because of various biases in phase 2 is well known.266 Because these biases can only be addressed by randomization, there has been increasing interest in randomized phase 2 designs, also known as “selection” or “pick-a-winner” designs.267,268 Here, randomization between a standard and a new treatment begins sooner than currently. A first stage enrolls a relatively small number of patients, thus allowing more agents to be investigated in a given time. Treatments that meet a particular efficacy criterion are carried forward against the standard into a larger second stage, analogous to standard phase 3 studies, whereas treatments not meeting these criteria drop out. One limitation of the design is that small sample sizes may preclude the identification of patients with biologically defined subsets of the disease that may benefit from a particular new agent.
Adaptive designs.
Adaptive designs use incoming information from the early stages of a trial to affect conduct of later stages.269,270 Although designs such as the 3+3 and the Simon 2-stage are technically “adaptive,” newer designs make more frequent use of incoming information. An example is “adaptive randomization” in which patients are initially randomized 1:1 after which randomization probabilities change at various intervals, to reflect incoming results.269 An advantage is that fewer patients may receive an ultimately unsatisfactory therapy, whereas a disadvantage is a loss of power. Another example is the continuous reassessment method, which in phase 1 trials permits more account to be made of covariates other than dose than does the standard 3+3 design.271
End points
OS and EFS.
Table 7 lists outcome measures, and Table 9 recommended reporting criteria for phase 3 clinical trials. OS is the end point most commonly used for approval of new therapies. However, OS may be an imperfect indicator of a new drug’s efficacy because advances in rescue therapies and supportive care have made it possible to keep patients alive after AML has relapsed or failed to enter CR.262,272 In contrast, EFS includes relapse and failure to enter CR as well as death and thus may better reflect a single treatment’s efficacy.272-275 Furthermore, less time is required to assess EFS, and use of EFS facilitates crossover designs, that is, patients are randomly assigned to a sequence of treatments.
Recommended minimum reporting criteria for phase 3 clinical trials
| Reporting objective . | Reporting end point . |
|---|---|
| Response rate | • CR/CRi achieved at completion of induction cycle 1 (%) |
| • CR/CRi rate after completion of all induction cycles (%) | |
| Treatment failure | • Primary refractory disease (%) as indicated by failure to achieve CR/CRi after completing induction therapy (2 cycles) |
| • % Death from any cause within 30 d | |
| • % Death from any cause within 60 d | |
| RFS | • Median RFS from date of CR to relapse (mo) |
| • 1-y/3-y/5-y RFS (%) | |
| EFS | • Median EFS (mo) |
| • 1-y/3-y/5-y EFS (%) | |
| OS* | • Median OS (mo) |
| • 1-y/3-y/5-y OS (%) | |
| Time to neutrophil recovery | • No. of days from day 1 of commencing induction therapy to first day neutrophils 0.5 × 109/L |
| • No. of days from day 1 of commencing induction therapy to first day neutrophils 1.0 × 109/L | |
| Time to platelet recovery | • No. of days from day 1 of commencing induction therapy to first day platelets 50 × 109/L |
| • No. of days from day 1 of commencing induction therapy to first day platelets 100 × 109/L |
| Reporting objective . | Reporting end point . |
|---|---|
| Response rate | • CR/CRi achieved at completion of induction cycle 1 (%) |
| • CR/CRi rate after completion of all induction cycles (%) | |
| Treatment failure | • Primary refractory disease (%) as indicated by failure to achieve CR/CRi after completing induction therapy (2 cycles) |
| • % Death from any cause within 30 d | |
| • % Death from any cause within 60 d | |
| RFS | • Median RFS from date of CR to relapse (mo) |
| • 1-y/3-y/5-y RFS (%) | |
| EFS | • Median EFS (mo) |
| • 1-y/3-y/5-y EFS (%) | |
| OS* | • Median OS (mo) |
| • 1-y/3-y/5-y OS (%) | |
| Time to neutrophil recovery | • No. of days from day 1 of commencing induction therapy to first day neutrophils 0.5 × 109/L |
| • No. of days from day 1 of commencing induction therapy to first day neutrophils 1.0 × 109/L | |
| Time to platelet recovery | • No. of days from day 1 of commencing induction therapy to first day platelets 50 × 109/L |
| • No. of days from day 1 of commencing induction therapy to first day platelets 100 × 109/L |
OS should also be reported with patients censored on day 0 of allogeneic HCT.
Incorporation of MRD.
The utility of CR as a surrogate for OS has been questioned.276,277 Likewise, if CRs are short-lived, a higher CR rate may not result in meaningful improvements in EFS. Considerable evidence indicates that patients in CR by conventional criteria who have MRD as assessed by RT-qPCR or MFC are at higher risk of relapse and death than patients without MRD (see “Monitoring of minimal residual disease”). This suggests the potential utility of CRMRD− as a rapidly assessable end point that may serve as a surrogate for EFS or long-term survival provided these relationships can be confirmed and means to measure MRD can be harmonized.91,92
QoL.
Regulatory drug approval agencies accept improvement in QoL as well as in quantity of life as a criterion for new drug approval. Although QoL has received little attention, clinical observation suggests that patients who achieve CR may have improved QoL, for example, due to receipt of fewer transfusions and spending less time in medical facilities than patients who do not achieve CR, even if survival is not improved; the same may apply with CRi.278
Novel therapies
AML is an important field for new drug investigation.2,262,279 Novel therapies are usually first evaluated in patients with relapsed/refractory disease or in older patients not considered candidates for standard intensive chemotherapy. Novel therapies in preclinical or clinical development may be categorized as protein kinase inhibitors, epigenetic modulators, new cytotoxic agents, mitochondrial inhibitors including apoptosis therapies, therapies targeting specific oncogenic proteins, therapeutic and immune checkpoint antibodies and cellular immunotherapies, and therapies targeting the AML microenvironment (Table 10).
Novel therapies in clinical development in AML
| Novel therapies in clinical development . | |
|---|---|
| Protein kinase inhibitors | • FLT3 inhibitors (midostaurin, quizartinib, gilteritinib, crenolanib) |
| • KIT inhibitors | |
| • PI3K/AKT/mTOR inhibitors | |
| • Aurora and polo-like kinase inhibitors, CDK4/6 inhibitors, CHK1, WEE1, and MPS1 inhibitors | |
| • SRC and HCK inhibitors | |
| Epigenetic modulators | • New DNA methyltransferase inhibitors (SGI-110) |
| • HDAC inhibitors | |
| • IDH1 and IDH2 inhibitors | |
| • DOT1L inhibitors | |
| • BET-bromodomain inhibitors | |
| Chemotherapeutic agents | • CPX-351 |
| • Vosaroxin | |
| • Nucleoside analogs | |
| Mitochondrial inhibitors | • Bcl-2, Bcl-xL, and Mcl-1 inhibitors |
| • Caseinolytic protease inhibitors | |
| Therapies targeting oncogenic proteins | • Fusion transcripts targeting |
| • EVI1 targeting | |
| • NPM1 targeting | |
| • Hedgehog inhibitors | |
| Antibodies and immunotherapies | • Monoclonal antibodies against CD33, CD44, CD47, CD123, CLEC12A |
| • Immunoconjugates (eg, GO, SGN33A) | |
| • BiTEs and DARTs | |
| • CAR T cells or genetically engineered TCR T cells | |
| • Immune checkpoint inhibitors (PD-1/PD-L1, CTLA-4) | |
| • Anti-KIR antibody | |
| • Vaccines (eg, WT1) | |
| Therapies targeting AML environment | • CXCR4 and CXCL12 antagonists |
| • Antiangiogenic therapies | |
| Novel therapies in clinical development . | |
|---|---|
| Protein kinase inhibitors | • FLT3 inhibitors (midostaurin, quizartinib, gilteritinib, crenolanib) |
| • KIT inhibitors | |
| • PI3K/AKT/mTOR inhibitors | |
| • Aurora and polo-like kinase inhibitors, CDK4/6 inhibitors, CHK1, WEE1, and MPS1 inhibitors | |
| • SRC and HCK inhibitors | |
| Epigenetic modulators | • New DNA methyltransferase inhibitors (SGI-110) |
| • HDAC inhibitors | |
| • IDH1 and IDH2 inhibitors | |
| • DOT1L inhibitors | |
| • BET-bromodomain inhibitors | |
| Chemotherapeutic agents | • CPX-351 |
| • Vosaroxin | |
| • Nucleoside analogs | |
| Mitochondrial inhibitors | • Bcl-2, Bcl-xL, and Mcl-1 inhibitors |
| • Caseinolytic protease inhibitors | |
| Therapies targeting oncogenic proteins | • Fusion transcripts targeting |
| • EVI1 targeting | |
| • NPM1 targeting | |
| • Hedgehog inhibitors | |
| Antibodies and immunotherapies | • Monoclonal antibodies against CD33, CD44, CD47, CD123, CLEC12A |
| • Immunoconjugates (eg, GO, SGN33A) | |
| • BiTEs and DARTs | |
| • CAR T cells or genetically engineered TCR T cells | |
| • Immune checkpoint inhibitors (PD-1/PD-L1, CTLA-4) | |
| • Anti-KIR antibody | |
| • Vaccines (eg, WT1) | |
| Therapies targeting AML environment | • CXCR4 and CXCL12 antagonists |
| • Antiangiogenic therapies | |
BiTE, bispecific T-cell engager; CAR, chimeric antigen receptor; DART, dual-affinity retargeting molecule; HDAC, histone deacetylase; KIR, killer-cell immunoglobulin-like receptor; mTOR, mechanistic target of rapamycin; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; PI3K, phosphatidylinositol 3-kinase; TCR, T-cell receptor.
Efforts to develop protein kinase inhibitors, inhibiting mutated forms of the FLT3 receptor have led to successive generations of FLT3 inhibitors.280 The first generation comprised tandutinib, sunitinib, lestaurtinib, sorafenib, and midostaurin, and the next generation quizartinib, crenolanib, and gilteritinib. These compounds differ not only in their ability to inhibit FLT3-ITD or tyrosine kinase domain or even the wild-type receptor, but also in their selectivity for FLT3 as well as their toxicity profiles. As discussed in “Intensive induction therapy,” the phase 3 trial evaluating midostaurin in younger adult patients with FLT3 mutations reached its primary end point, improvement of OS.61 Randomized trials evaluating intensive chemotherapy with other FLT3 inhibitors, such as lestaurtinib and sorafenib, failed to show an improvement in response rate and in OS.281-284 The trial with sorafenib in younger patients (not restricted to AML with FLT3 mutations) showed an improvement in EFS, mainly reflecting results in patients without FLT3-ITD, that did not translate into a significant OS benefit.284 Randomized trials evaluating next-generation FLT3 inhibitors are ongoing.
Another rapidly expanding area is development of novel epigenetic therapies.285,286 Guadecitabine (SGI-110) is a second-generation HMA currently in phase 3 development.287 Guadecitabine is a dinucleotide of decitabine and deoxyguanosine that increases the in vivo exposure of decitabine by protecting it from inactivation by cytidine deaminase. One novel targeted approach is the inhibition of the metabolic enzymes IDH1 and IDH2 that are frequently mutated in AML.288 Early trial results with these inhibitors show durable responses and appear promising.289,290 Other examples are targeting of BRD4, a member of the BET family of bromodomain epigenetic readers,291 or of KMT2A (MLL)–rearranged leukemias.292,293
In a randomized trial conducted in patients with relapsed and refractory AML, the topoisomerase II inhibitor vosaroxin in combination with IDAC demonstrated a small survival benefit in patients older than 60 years (7.1 vs 5.0 months); a benefit was not shown in younger patients, potentially due to the higher transplant rate (45.8% <60 years vs 20.2% ≥60 years).222
Finally, targeted immunotherapy is an important novel approach.294 A variety of therapeutic antibodies directed against AML antigenic targets (eg, CD33, CD123, CLEC12A), bispecific T-cell engagers, or dual-affinity retargeting molecules as well as engineered chimeric antigen receptor T cells targeting the CD33 and CD123 antigens are currently in early clinical trial.
Management of special situations
Hyperleukocytosis
A recent systematic review assessed early mortality in patients with an initial white blood cell count ≥100 × 109/L and found neither leukapheresis nor hydroxyurea/low-dose chemotherapy influenced the early death rate.295 Hyperleukocytosis reflects a medical emergency. After immediate diagnostic testing, patients should begin cytoreductive treatment without delay preferably with the planned induction regimen.
Others
There have been no new developments in management of central nervous system (CNS) AML, myeloid sarcoma, or pregnancy in AML since the 2010 ELN publication and readers are referred there for information.1
Supportive care
Prophylactic anti-infectious treatment
For prophylaxis and treatment of infections, prevailing institutional infectious organisms and their drug-resistance pattern should primarily be considered. As noted in the 2010 ELN recommendations, prophylaxis with a quinolone should be given.1
A systematic survey of randomized trials in AML found “high-level evidence” supporting use of posaconazole to prevent invasive fungal infections during remission induction therapy and in patients with GVHD after allogeneic HCT. Micafungin can be used when azoles are strictly prohibited, although fluconazole is generally acceptable because it has a very low interaction with CYP3A4. There was insufficient evidence to guide antifungal prophylaxis in patients undergoing allogeneic HCT without GVHD or other high-risk factors.296
Other issues
There have been few new developments regarding use of myeloid growth factors or transfusion support since the 2010 ELN recommendations to which the reader is referred.1 Neither growth factors nor granulocyte transfusions can be recommended outside of the individual patient setting. In 2 randomized trials comparing prophylactic (at a count <10 × 109/L) vs therapeutic (only if bleeding) platelet transfusion, more grade 2-4 bleeding occurred in the therapeutic arms together with a slight excess in fatal (CNS) hemorrhage.297,298 Thus, prophylactic platelet transfusion at a count <10 × 109/L remains the standard for patients with AML.
Acknowledgments
The authors gratefully acknowledge Rüdiger Hehlmann for his continuous generous support of these recommendations on behalf of the European LeukemiaNet; Adam Ivey and Elli Papaemmanuil for their support in generating Figure 1; and Lucy Godley and Rafael Bejar for reviewing the section on myeloid neoplasms with germ line predisposition.
H. Döhner was supported by SFB 1074 “Experimental models and clinical translation in leukemia” funded by the Deutsche Forschungsgemeinschaft (DFG). D.G. was grateful for support from the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference RP-PG-0108-10093). J.S. was supported by grants from the Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR) 2014SGR-1281, Instituto de Salud Carlos III (ISCIII) RD12/0036/0071 and Fondo de Investigación en Salud (FIS) PI14/00450. F.L.-C. was supported by Associazione Italiana per la Ricerca sul Cancro grant AIRC I.G. 5916. C.D.B. was supported by National Institutes of Health National Cancer Institute grants CA180861, CA016058.
Authorship
Contribution: All authors reviewed the literature and wrote first drafts of specific sections; H. Döhner and C.D.B. assembled the sections and wrote the final version of the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: H. Döhner provided consultancy services to Agios, Amgen, Astex Pharmaceuticals, Celator, Celgene, Novartis, Roche, Seattle Genetics, Sunesis, and Tolero, and received research funding from Boehringer Ingelheim, Celgene, Novartis, Bristol-Myers Squibb, and Arog Pharmaceuticals. S.A. provided consultancy services to Amgen and Daiichi Sankyo. H. Dombret provided consultancy services to Roche/Genentech, Amgen, Pfizer, Novartis, Celgene, Jazz Pharmaceuticals, Agios, Sunesis, Ambit, Daiichi Sankyo, Karyopharm, Kite Pharma, Menarini, Astellas, Janssen, Servier, and Seattle Genetics, and received research funding from Roche/Genentech, Amgen, Ariad, Jazz Pharmaceuticals, and Kite Pharma. B.L.E. provided consultancy services to Celgene, Genoptix, and H3 Biomedicine, and received research funding from Celgene. P.F. provided consultancy services to Celgene, Novartis, and Teva, and received research funding from Celgene, Janssen, Novartis, Astex, and Teva. R.A.L. provided consultancy services to Novartis, Ariad, CVS/Caremark, Erytech, Pfizer, Celgene, and Bristol-Myers Squibb, and received research funding from Astellas, Erytech, Novartis, and Daiichi Sankyo. R.L.L. provided consultancy services to Novartis and served on the supervisory board for Qiagen. F.L.-C. provided consultancy services to Teva and Novartis, received honoraria from Teva and Lundbeck, and received research support from Teva and Novartis. D.N. received research funding from Novartis and Amgen, and served on the speakers’ bureaus for Novartis and Amgen. G.J.O. provided consultancy services to Novartis, Pfizer, Bristol-Myers Squibb, Johnson & Johnson, Sunesis, Celgene, Karypharm, and Amgen, and received research support from Novartis, Johnson & Johnson, Celgene, Immunogene, and Becton Dickinson. J.S. provided consultancy services to Celgene, Novartis, Sunesis, Karyopharm, Pfizer, Janssen, and Meda Pharmaceuticals; received research support from Celgene, Novartis, and Amgen; was a speaker for Celgene, Pfizer, and Janssen; and was a member of the Board of Directors for the European Haematology Association, the Spanish Society of Hematology, and the José Carreras International Leukemia Foundation. A.H.W. provided consultancy services to Novartis, Celgene, Servier, Abbvie, Roche, Amgen, and CTI, and received research funding from Abbvie, Novartis, Celgene, Servier, Ariad, and Amgen. B.L. provided consultancy services to Celgene, Agios, AstraZeneca, and Astex, and was a Section Editor for Leukemia. The remaining authors declare no competing financial interests.
Thomas Büchner died on 5 August 2016. David Grimwade died on 17 October 2016.
Correspondence: Hartmut Döhner, Department of Internal Medicine III, University of Ulm, Albert-Einstein-Allee 23, 89081, Ulm, Germany; e-mail: hartmut.doehner@uniklinik-ulm.de.