Key Points
Fc-engineered CD19 antibody cures MRD in ∼50% of mice xenografted with ALL cells and is highly synergistic in combination with chemotherapy.
Macrophages are important effector cells for this antibody in vitro and in vivo.
Abstract
Antibody therapy constitutes a major advance in the treatment of B-cell precursor acute lymphoblastic leukemia (BCP-ALL). To evaluate the efficacy and the mechanisms of action of CD19 monoclonal antibody therapy in pediatric BCP-ALL, we tested an Fc-engineered CD19 antibody carrying the S239D/I332E mutation for improved effector cell recruitment (CD19-DE). Patient-derived xenografts (PDX) of pediatric mixed-lineage leukemia gene (MLL)–rearranged ALL were established in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. Antibody CD19-DE was efficient in prolonging the survival of NSG mice in a minimal residual disease (MRD) model. The majority of surviving mice remained polymerase chain reaction (PCR)–MRD negative after treatment. When antibody therapy was initiated in overt leukemia, antibody CD19-DE was still efficient in prolonging survival of xenografted mice in comparison with nontreated control animals, but the effects were less pronounced than in the MRD setting. Importantly, the combination of antibody CD19-DE and cytoreduction by chemotherapy (dexamethasone, vincristine, PEG-asparaginase) resulted in significantly improved survival rates in xenografted mice. Antibody CD19-DE treatment was also efficient in a randomized phase 2–like PDX trial using 13 MLL-rearranged BCP-ALL samples. Macrophage depletion by liposomal clodronate resulted in a reversal of the beneficial effects of CD19-DE, suggesting an important role for macrophages as effector cells. In support of this finding, CD19-DE was found to enhance phagocytosis of patient-derived ALL blasts by human macrophages in vitro. Thus, Fc-engineered CD19 antibodies may represent a promising treatment option for infants and children with MLL-rearranged BCP-ALL who have a poor outcome when treated with chemotherapy only.
Introduction
Despite considerable improvements in patient outcomes, B-cell precursor acute lymphoblastic leukemia (BCP-ALL) relapse remains a major problem and a leading cause of cancer-related mortality in children.1 Also, certain patient subgroups such as infants have a particularly poor prognosis.2 Patient outcome may be improved by the inclusion of therapeutic antibodies, for example, the CD20 antibody rituximab, which has proven highly efficient in B-cell lymphomas and has also been used in BCP-ALL.3-5 However, in contrast to lymphomas or mature B-cell ALL, significant expression of CD20 is found in fewer than 50% of pediatric BCP-ALL cases.6 In particular, CD20 is usually absent in BCP-ALL with rearrangements of the mixed-lineage leukemia gene (MLL),6,7 which represents the most frequent cytogenetic subtype in infants. Infants with BCP-ALL have an extremely poor outcome, with 4-year event-free survival rates below 50%.8,9 Thus, other antigens have been evaluated as candidate target structures for antibody therapy.5,10 CD19 is displayed throughout B-cell differentiation and is highly expressed by the majority of ALL cases of B-lineage.5,11 The [CD19 × CD3] bispecific T-cell engager molecule (BiTE) blinatumomab has already been applied clinically in the relapsed/refractory setting, although with considerable toxicity.12-15
Novel CD19 targeting approaches include CD19-specific chimeric antigen receptor (CAR) T cells or antibody drug conjugates.16 Native unconjugated CD19 IgG1 antibodies poorly mediated effector functions,17 so that designer antibodies harboring modified fragment crystallizable (Fc) domains have been developed. These antibodies were engineered to increase the affinity to activating Fcγ receptors (FcγR), which can be achieved, for example, by introducing amino acid mutations into the constant heavy chain region 2 (CH2) or by altering the antibody glycosylation pattern.17 This approach was based on observations suggesting a pivotal role for efficient FcγR engagement by therapeutic antibodies in patients. For example, patients with homozygous expression of the FCGRIIIA158-V allelic variant, which exhibits a higher affinity to the Fc domain, showed improved responses to rituximab in comparison with patients with the lower-affinity FCGRIIIA 158-F allele.18-20 The introduction of both Fc mutations and glycosylation changes has been applied to CD19 antibodies, and the resulting Fc-engineered variants were more effective in triggering antibody-dependent cell-mediated cytotoxicity (ADCC) or phagocytosis (ADCP).21-23 For example, preclinical in vitro and in vivo studies suggested that the Fc-optimized and humanized CD19 antibody MOR208 (formerly XmAb5574) may be beneficial in B-cell malignancies, including BCP-ALL.21,24-26 MOR208 carries the amino acid mutations S239D/I332E in the CH2 region of its Fc domain and is currently tested in clinical studies in non-Hodgkin lymphoma, chronic lymphocytic leukemia,27-29 and adult ALL (ClinicalTrials.gov identifier: NCT01685021). These trials have shown that the antibody is well tolerated and efficient. Notably, unconjugated native CD19 antibodies are convenient to apply, and side effects of T-cell activation are mostly absent.
We have shown that MOR208 is efficient against BCP-ALL cells in vitro30 ; however, the in vivo efficacy in pediatric ALL remains unknown. We thus analyzed the therapeutic effects of an Fc-engineered CD19 antibody carrying the MOR208 V-regions and S239D/I332E mutations in its Fc domain (referred to as CD19-DE) in preclinical minimal residual disease (MRD) xenograft models of aggressive MLL-rearranged pediatric ALL and suggest that the recruitment of macrophages contributes to its efficacy. CD19-DE was also tested in a randomized phase 2–like study in xenografts, increasing the degree of evidence derived from our preclinical studies.
Methods
Cell lines and patient cells
We purchased 697 (EU-3) BCP-ALL cells from DSMZ. Patients were treated according to the Interfant-06 and ALL-Berlin-Frankfurt-Münster (BFM) 2000 and 2009 protocols (Table 1). Informed consent was obtained according to institutional regulations, in accordance with the Declaration of Helsinki.
Patient characteristics
| Parameter . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . | Patient 10 . | Patient 11 . | Patient 12 . | Patient 13 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 0.4 | 0.3 | 0.6 | 5.2 | 1.0 | 0.5 | 0.4 | 0.4 | 17.5 | 1.7 | 0.4 | 0.2 | 0.1 |
| Sex | F | F | F | F | M | F | F | F | F | M | M | F | M |
| WBC (initial)/µL | 103.000 | 334.000 | 416.500 | 284.430 | 42.400 | 210.800 | 166.450 | 73.960 | 27.010 | 24.630 | 1023.000 | 733.500 | 221.000 |
| Blasts (PB) initial (%) | 91 | 97 | 97 | 92 | 82 | 88 | 80 | 88 | 80 | 30 | 96 | 94 | 90 |
| Blasts (BM) initial (%) | 97 | 98 | 99 | 95 | 96 | 88 | 95 | 93 | 89 | 72 | 93 | 88 | 95 |
| Central nervous system status | 1 | n.d. | 3 | 2c | n.d. | n.d. | n.d. | 3 | 1 | 2a | 2a | n.d. | n.d. |
| Immunophenotype | Pro-B | Pro-B | Pro-B | Pro-B | c-ALL | Pro-B | c-ALL | c-ALL | Pro-B | Pro-B | Pro-B | Pro-B | Pro-B |
| MLL-fusion partner | ENL | ENL | ENL | AF4 | AF10 | AF9 | AF9 | AF9 | AF4 | AF4 | ENL | AF4 | AF4 |
| Pred-response | Good | Poor | Good | Poor | Good | Good | Poor | Poor | Good | Good | Poor | Poor | Good |
| Blasts (BM), day 15 (%) | 1 | 32 | 3 | 15 | 0.5 | 8 | 1 | 0 | 0 | 1 | 75 | 35 | 6 |
| MRD treatment, day 33 | Neg | Neg | n.d. | n.d. | Neg | n.d. | Neg | n.d. | 10−6 | 10−3 | n.d. | n.d. | 10−6 |
| MRD, second time point* | 10−6† | Neg | n.d. | 10−3 | 10−5 | n.d. | Neg | n.d. | Neg | 10−4 | n.d. | n.d. | 10−6 |
| Relapse | No | Yes | No | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes |
| Death | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Study | BFM 2000 | BFM 2000 | IFAN 2006 | BFM 2009 | BFM 2000 | IFAN 2006 | IFAN 2006 | IFAN 2006 | BFM 2009 | BFM 2009 | IFAN 1999 | IFAN 1999 | IFAN 2006 |
| Parameter . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . | Patient 10 . | Patient 11 . | Patient 12 . | Patient 13 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 0.4 | 0.3 | 0.6 | 5.2 | 1.0 | 0.5 | 0.4 | 0.4 | 17.5 | 1.7 | 0.4 | 0.2 | 0.1 |
| Sex | F | F | F | F | M | F | F | F | F | M | M | F | M |
| WBC (initial)/µL | 103.000 | 334.000 | 416.500 | 284.430 | 42.400 | 210.800 | 166.450 | 73.960 | 27.010 | 24.630 | 1023.000 | 733.500 | 221.000 |
| Blasts (PB) initial (%) | 91 | 97 | 97 | 92 | 82 | 88 | 80 | 88 | 80 | 30 | 96 | 94 | 90 |
| Blasts (BM) initial (%) | 97 | 98 | 99 | 95 | 96 | 88 | 95 | 93 | 89 | 72 | 93 | 88 | 95 |
| Central nervous system status | 1 | n.d. | 3 | 2c | n.d. | n.d. | n.d. | 3 | 1 | 2a | 2a | n.d. | n.d. |
| Immunophenotype | Pro-B | Pro-B | Pro-B | Pro-B | c-ALL | Pro-B | c-ALL | c-ALL | Pro-B | Pro-B | Pro-B | Pro-B | Pro-B |
| MLL-fusion partner | ENL | ENL | ENL | AF4 | AF10 | AF9 | AF9 | AF9 | AF4 | AF4 | ENL | AF4 | AF4 |
| Pred-response | Good | Poor | Good | Poor | Good | Good | Poor | Poor | Good | Good | Poor | Poor | Good |
| Blasts (BM), day 15 (%) | 1 | 32 | 3 | 15 | 0.5 | 8 | 1 | 0 | 0 | 1 | 75 | 35 | 6 |
| MRD treatment, day 33 | Neg | Neg | n.d. | n.d. | Neg | n.d. | Neg | n.d. | 10−6 | 10−3 | n.d. | n.d. | 10−6 |
| MRD, second time point* | 10−6† | Neg | n.d. | 10−3 | 10−5 | n.d. | Neg | n.d. | Neg | 10−4 | n.d. | n.d. | 10−6 |
| Relapse | No | Yes | No | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes |
| Death | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Study | BFM 2000 | BFM 2000 | IFAN 2006 | BFM 2009 | BFM 2000 | IFAN 2006 | IFAN 2006 | IFAN 2006 | BFM 2009 | BFM 2009 | IFAN 1999 | IFAN 1999 | IFAN 2006 |
Central nervous system status is as described previously.33
BM, bone marrow; c-ALL, common acute lymphoblastic leukemia; F, female; IFAN, Interfant trial; M, male; n.d., not determined; Neg, negative; PB, peripheral blood; Pro-B, pro-B cell.
Day 78 for BFM patients; before MARMA for IFAN patients.
Low positive, not quantifiable.39
Antibody generation
For generation of CD19-DE and trastuzumab-DE with S239D/I332E Fc modifications, variable light and variable heavy (VH) chain sequences of MOR208 or the anti-HER2 antibody trastuzumab were synthesized de novo using published sequences31,32 and ligated in frame into antibody light chain (LC) or heavy chain (HC) expression vectors pSectag2-LC and pSectag2-HC-DE (unpublished data). An expression vector for the HC of a native CD19 IgG1 antibody was generated by cloning MOR208 VH regions into pSectag2-HC (unpublished data). Antibodies were produced in CHO cells by stable transfection of HC and LC expression vectors according to published procedures and purified using Capture Select IgG-CH1 affinity matrix (ThermoFisher), following the manufacturer’s recommendations. Antibody concentration and integrity were determined by quantitative capillary electrophoresis by using Experion Pro260 technology (BioRad).
Xenografts
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Charles River) were maintained as approved by the governmental animal care and use committees. PDX were established by intrafemoral injection of 100 ALL cells per animal (day 0), unless otherwise indicated.33,34 A number of 100 cells was the lowest cell number causing 100% leukemia in a representative limiting dilution assay (Figure 1A). CD19-DE and control antibodies trastuzumab-DE or trastuzumab (Roche) were applied by intraperitoneal injection of 1 mg/kg body weight on days +1, +3, +6, +10, +13, and every 21 days thereafter (MRD model). In some experiments, development of overt leukemia (>1% human blasts in the peripheral blood) was awaited (day 0) and antibody therapy initiated alone according to the same schedule or in combination with chemotherapy, mimicking an ALL induction regimen (dexamethasone 70 µg orally, days 1-5; vincristine 10 µg intravenously, day 1; and PEG-asparaginase 100 IU intravenously, day 1). This cycle was repeated every 28 days (overt leukemia model). For depletion of macrophages, 100 μL of liposomal clodronate (ClodronateLiposomes.com) was applied by intraperitoneal injection on day −1 and then once per week. Macrophage depletion was assessed by staining murine bone marrow samples with phycoerythrin-conjugated CD11b and APC-coupled anti-F4/80 antibodies (BD, Miltenyi) and fluorescence-activated cell sorter analyses. Polymerase chain reaction (PCR)–MRD was determined by analyses of bone marrow DNA for patient-specific immunoglobulin (Ig)-rearrangements and the respective MLL fusion, as has been previously published.35-37
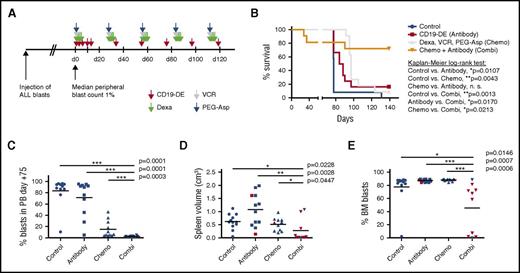
CD19-DE is efficient in an MRD-model of infant BCP-ALL in NSG mice. (A) Establishment of the MRD model: 6 NSG mice per group were injected with 10, 50, 100, or 500 cells from patient 1 in Table 1. Mice were sacrificed upon leukemia development or after 1 year at the latest. The percentage of engrafted animals was determined. (B) Experimental scheme. Patient-derived ALL xenografts were established by orthotopic intrafemoral injection of 100 ALL cells per animal in NSG mice (day 0). The antibody was injected intraperitoneally (1 mg/kg) on days +1, +3, +6, +10, +13, and every 21 days thereafter. (C) Reduction of human ALL blast counts in the peripheral blood of mice xenografted with leukemic cells from patients 1 and 2 by treatment with antibody CD19-DE (Mann-Whitney U test); control mice were left untreated; n = 8 in the control group, and n = 7 in the treatment group. Human blast percentages were determined by 2-color flow cytometry using antibodies specific for human CD19 or CD45 antigens. (D) Reduction of human ALL blast counts in the peripheral blood of mice xenografted with leukemic cells from patients 3 and 4 by CD19-DE (Mann-Whitney U test); control mice received trastuzumab or trastuzumab-DE, as indicated; n = 7 in all groups. (E, F) Survival prolongation in xenografted mice by CD19-DE in patients 1 and 2 (E) and patients 3 and 4 (F) (Kaplan-Meier log-rank test). FACS, fluorescence-activated cell sorting; n.s., not significant.
CD19-DE is efficient in an MRD-model of infant BCP-ALL in NSG mice. (A) Establishment of the MRD model: 6 NSG mice per group were injected with 10, 50, 100, or 500 cells from patient 1 in Table 1. Mice were sacrificed upon leukemia development or after 1 year at the latest. The percentage of engrafted animals was determined. (B) Experimental scheme. Patient-derived ALL xenografts were established by orthotopic intrafemoral injection of 100 ALL cells per animal in NSG mice (day 0). The antibody was injected intraperitoneally (1 mg/kg) on days +1, +3, +6, +10, +13, and every 21 days thereafter. (C) Reduction of human ALL blast counts in the peripheral blood of mice xenografted with leukemic cells from patients 1 and 2 by treatment with antibody CD19-DE (Mann-Whitney U test); control mice were left untreated; n = 8 in the control group, and n = 7 in the treatment group. Human blast percentages were determined by 2-color flow cytometry using antibodies specific for human CD19 or CD45 antigens. (D) Reduction of human ALL blast counts in the peripheral blood of mice xenografted with leukemic cells from patients 3 and 4 by CD19-DE (Mann-Whitney U test); control mice received trastuzumab or trastuzumab-DE, as indicated; n = 7 in all groups. (E, F) Survival prolongation in xenografted mice by CD19-DE in patients 1 and 2 (E) and patients 3 and 4 (F) (Kaplan-Meier log-rank test). FACS, fluorescence-activated cell sorting; n.s., not significant.
In vitro phagocytosis assay
Mononuclear cells (MNC) were isolated from residual cells in leukoreduction chambers after routine platelet collection by Percoll centrifugation.38 Human monocytes were enriched from MNC by magnetic activated cell sorting and a pan monocyte isolation kit (Miltenyi). Monocytes were differentiated into macrophages in RPMI 1640 medium containing 10% fetal calf serum, 1% penicillin/streptomycin, and 50 ng/mL recombinant macrophage colony-stimulating factor (R&D Systems) for 5 to 6 days. ALL cells were labeled with PKH67 Green Fluorescent Cell Linker Kit (Sigma Aldrich). For microscopy, 104 macrophages were seeded in chambered coverslips (ibidi) and allowed to adhere overnight. Thirty thousand ALL cells were added, and antibodies were applied to a final concentration of 10 µg/mL. Cells were incubated for 2 h at 37°C and analyzed using fluorescence microscopy (Nikon). Phagocytosis was determined as the percentage of macrophages with ingested PKH67 green-positive ALL cells or fragments per 100 macrophages by at least 2 independent observers. Trastuzumab (Roche) was used as a control antibody.
Data processing and statistical analyses
Statistical analyses were performed using GraphPad Prism (La Jolla, CA). Statistical significance was assessed using the Mann-Whitney U test and repeated-measures analysis of variance (ANOVA) with Bonferroni posttest. Survival was analyzed using the Kaplan-Meier method and log-rank statistics. A P value of <.05 was considered statistically significant.
Results
CD19-DE prolongs the survival of xenografts in an MRD model of infant BCP-ALL
For an evaluation of the efficacy of Fc-engineered CD19 antibodies, antibody CD19-DE was first tested in patient-derived xenografts (PDX) from infants with MLL-rearranged ALL. Xenografts of 4 patients (patients 1-4; Table 1) were established in NSG mice by intrafemoral injection of 100 leukemia cells (MRD model, based on limiting dilution assays; Figure 1A). For patients 1 and 2, control animals were left untreated. For patient 3, trastuzumab was used as control IgG1, and for patient 4, both trastuzumab and a trastuzumab version bearing the same Fc modification as CD19-DE (trastuzumab-DE) were applied. Antibody treatment was started on day +1 posttransplantation (Figure 1B). Untreated mice and trastuzumab controls showed leukemia engraftment in fewer than 100 days, as was evidenced by high amounts of human leukemic cells in the peripheral blood of the animals (Figure 1C-D). Intraperitoneal application of antibody CD19-DE led to a reduction of human blasts in the peripheral blood, with statistical significance being reached for patients 1, 3, and 4 at different time points (Mann-Whitney U test; Figure 1C-D). However, in all patient-derived models, therapy with antibody CD19-DE significantly prolonged the survival of NSG mice in the MRD setting (Kaplan-Meier log-rank test; Figure 1E-F). Importantly, neither trastuzumab nor trastuzumab-DE was able to prevent leukemic engraftment (Figure 1F). When the experiment was terminated (day +160 for patients 1 and 2, day +110 for patients 3 and 4), 5 out of 7 treated mice from patient 1, 4 of 7 mice from patient 2, and all treated mice from both patients 3 and 4 showed no leukemic symptoms. Three of 5 surviving mice from patient 1, 4 of 4 surviving mice from patient 2, and 0 of 7, but 5 of 7, mice from patients 3 and 4, respectively, had ≤0.5% human leukemic blasts in the bone marrow, as was evidenced by flow cytometry for human CD45 and human CD19, whom we considered leukemia-free with this method (supplemental Figure 1A-B, found on the Blood Web site). Two of 5 mice from patient 1, 7 of 7 mice from patient 3, and 2 of 7 mice from patient 4 had different degrees of leukemia in the bone marrow but did not show clinical symptoms. To determine the PCR-MRD status of leukemia-free surviving mice, we prepared DNA from bone marrow aspirates from patient-1 and -2 PDX mice. Analyses for patient-specific Ig rearrangements and the MLL fusion revealed that all mice but 1 were either MRD-negative or showed a low-positive, nonquantifiable signal according to published definitions39 (Table 2). One mouse from patient 2 had an MRD load of 10−3 by PCR for the MLL fusion; however, this particular mouse was MRD-negative for 2 specific Ig rearrangements. We next performed serial transplantations with MRD-negative bone marrow from 5 CD19-DE treated primary mice into 2 secondary recipients each. At day +100, we found no evidence for leukemic engraftment in the bone marrow in these animals (supplemental Figure 2). Our data suggest that CD19-DE is efficient in reducing peripheral and bone marrow blast counts and significantly prolonging survival of xenografted mice in an in vivo MRD model of infant BCP-ALL. Furthermore, our data suggest that obtaining PCR-MRD negativity in vivo is possible.
PCR-MRD status of surviving leukemia-free mice, patients 1 and 2
| . | MRD (MLL) . | MRD (Marker 1) . | MRD (Marker 2) . |
|---|---|---|---|
| Patient 1, M389 | Negative | Negative | Negative |
| Patient 1, M391 | 10−6* | Negative | 10−6* |
| Patient 1, M392 | Negative | Negative | 10−6* |
| Patient 2, M404 | 10−3 | Negative | Negative |
| Patient 2, M407 | 10−6* | Negative | Negative |
| Patient 2, M408 | 10−6* | Negative | Negative |
| Patient 2, M409 | Negative | Negative | Negative |
| . | MRD (MLL) . | MRD (Marker 1) . | MRD (Marker 2) . |
|---|---|---|---|
| Patient 1, M389 | Negative | Negative | Negative |
| Patient 1, M391 | 10−6* | Negative | 10−6* |
| Patient 1, M392 | Negative | Negative | 10−6* |
| Patient 2, M404 | 10−3 | Negative | Negative |
| Patient 2, M407 | 10−6* | Negative | Negative |
| Patient 2, M408 | 10−6* | Negative | Negative |
| Patient 2, M409 | Negative | Negative | Negative |
Low positive, not quantifiable.39
CD19-DE is efficient in combination with chemotherapy in an overt leukemia model of infant BCP-ALL
To analyze the efficacy of CD19-DE in overt leukemia, we prepared 46 patient-1 PDX mice by intrafemoral injection of 100 human leukemic cells per animal. We thereby hypothesized that cytoreductive chemotherapy in combination with antibody treatment would be more efficient in prolonging survival of xenografted mice. In contrast to the previous setting, leukemia development (defined as a median percentage of 1% human leukemic blasts in the peripheral blood in a randomly selected subcohort of at least 30% of the mice) was awaited before therapy was initiated. This was achieved after 21 days (data not shown). Four groups of mice were left untreated (control), treated with CD19-DE alone (antibody), treated with a regimen mimicking ALL induction chemotherapy (chemotherapy), or treated with both CD19-DE and chemotherapy (combination). The experiment ended on day +139, and all surviving mice were sacrificed (Figure 2A). In this overt leukemia model, CD19-DE was still efficient in prolonging survival, in comparison with untreated control animals (P = .0107, Kaplan-Meier log-rank test; Figure 2B); however, the effects were less pronounced than in the MRD model. Importantly, chemotherapy was not superior to CD19-DE (Figure 2B) in this setting (P = .0043; Figure 2B). Notably, unlike that in the MRD model, all surviving animals in the antibody (n = 2) and chemotherapy (n = 1) groups had asymptomatic overt leukemia (>90% human leukemic blasts in the bone marrow; data not shown). Importantly, 8 of 11 animals (72.7%) treated with a combination of CD19-DE and cytoreductive chemotherapy showed prolonged survival in comparison with no treatment (P = .0013), antibody alone (P = .0170), and chemotherapy alone (P = .0213; Figure 2B). In the combination therapy group, 2 of 3 deaths occurred at early time points shortly after chemotherapy had been administered. Both of these animals were leukemia-free by flow cytometry (data not shown). Thus, we suspected that these mice succumbed to chemotherapy complications. The percentages of human leukemic blasts in the peripheral blood on day +75 were similar between control and antibody-treated groups. In contrast, chemotherapy and combination treated animals displayed reduced peripheral blast counts, and this reduction was statistically significant for the combination in comparison with all other groups (P = .0001, P = .0001, P = .0003, respectively; Figure 2C). Finally, we analyzed leukemic engraftment in all animals sacrificed in this experiment. Spleen volume as a surrogate parameter for leukemic burden33 and bone marrow infiltration by human leukemic blasts were not significantly different among the control, the antibody, and the chemotherapy groups (Figure 2D-E; red triangles and squares represent the surviving animals). However, the combination treatment caused a reduction in both spleen volume and bone marrow infiltration in comparison with the other conditions (Figure 2D-E). In contrast, neither spleen sizes nor levels of bone marrow infiltration were significantly different between the control and the antibody groups (Figure 2D-E), suggesting that despite the prolongation in survival, leukemic burden in these mice was not reduced by CD19-DE alone in the overt leukemia model. Escape variants with CD19 loss were not observed (data not shown). Altogether, our data suggest that cytoreductive capacity is augmented by a combination of standard induction chemotherapy with CD19-DE in PDX mice. Moreover, a combined immunochemotherapy outperforms both antibody therapy and chemotherapy alone, prolonging survival in this model.
CD19-DE is efficient in combination with chemotherapy in an overt leukemia model of infant BCP-ALL in NSG mice. (A) Experimental scheme. Patient-derived ALL xenografts were established by orthotopic intrafemoral transplantation of 100 ALL cells per animal in NSG mice (patient 1), and engraftment was awaited for 21 days (day 0). Mice were then left untreated (control, n = 12), treated with antibody CD19-DE following the same scheme as depicted in Figure 1B (antibody, n = 12); treated with a chemotherapy regimen consisting of dexamethasone days 1-5, vincristine on day 1, and PEG-asparaginase day 1 every 28 days (chemotherapy, n = 11); or a combination of chemotherapy and antibody (combination, n = 11). (B) Marked prolongation of survival of xenografted mice by treatment with the combination of chemotherapy and antibody in comparison with all other groups (Kaplan-Meier log-rank test). (C) Percentage of human blasts in the peripheral blood of mice by FACS analysis on day +75 (Mann-Whitney U test). (D, E) Postmortem analyses of the mice depicted in panel B. Spleen volume measured by the formula: longest length × broadest width × highest height as a marker of leukemic burden (D); human leukemic blasts in the bone marrow (E) (Mann-Whitney U test). Note that there is 1 missing value in the combination group because this mouse was found dead and in decay after >24 h and could not be analyzed. Red triangles and squares represent the surviving animals. combi, combination; d, day; Dexa, dexamethasone; chemo, chemothrapy; PEG-Asp, PEG-asparaginase; VCR, vincristine.
CD19-DE is efficient in combination with chemotherapy in an overt leukemia model of infant BCP-ALL in NSG mice. (A) Experimental scheme. Patient-derived ALL xenografts were established by orthotopic intrafemoral transplantation of 100 ALL cells per animal in NSG mice (patient 1), and engraftment was awaited for 21 days (day 0). Mice were then left untreated (control, n = 12), treated with antibody CD19-DE following the same scheme as depicted in Figure 1B (antibody, n = 12); treated with a chemotherapy regimen consisting of dexamethasone days 1-5, vincristine on day 1, and PEG-asparaginase day 1 every 28 days (chemotherapy, n = 11); or a combination of chemotherapy and antibody (combination, n = 11). (B) Marked prolongation of survival of xenografted mice by treatment with the combination of chemotherapy and antibody in comparison with all other groups (Kaplan-Meier log-rank test). (C) Percentage of human blasts in the peripheral blood of mice by FACS analysis on day +75 (Mann-Whitney U test). (D, E) Postmortem analyses of the mice depicted in panel B. Spleen volume measured by the formula: longest length × broadest width × highest height as a marker of leukemic burden (D); human leukemic blasts in the bone marrow (E) (Mann-Whitney U test). Note that there is 1 missing value in the combination group because this mouse was found dead and in decay after >24 h and could not be analyzed. Red triangles and squares represent the surviving animals. combi, combination; d, day; Dexa, dexamethasone; chemo, chemothrapy; PEG-Asp, PEG-asparaginase; VCR, vincristine.
CD19-DE is efficient in a preclinical phase 2–like xenograft trial of MLL-rearranged BCP-ALL
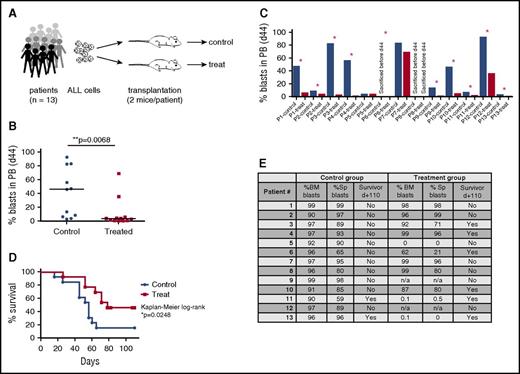
Next, we aimed to determine the efficacy of CD19-DE in a randomized preclinical phase 2–like study in MRD models of MLL-rearranged BCP-ALL. We therefore prepared xenografts of 13 patients with MLL-rearranged ALL, as described above. The predefined end point of the study was overall survival in treated versus nontreated animals. On the basis of the data depicted in Figure 1E-F, our trial was designed to detect a 50% survival difference at 120 days (90% dead in the nontreated group and 40% dead in the treated group). A 1-sided Fisher’s exact test at α = 0.05 and 80% power yielded a number of 13 patient-derived leukemias needed per group. Subsequently, each of the 13 patient samples (Table 1) were transplanted into 2 mice, and xenografts were then randomized into control or treatment groups (Figure 3A). Treatment with CD19-DE was initiated according to Figure 1B (MRD model). Measurement of human leukemic blasts in the peripheral blood on day 44 revealed that leukemia engraftment in control mice occurred earlier than that in treatment animals (Figure 3B). We thereby also observed a >2-fold difference in the percentages of peripheral blasts in control versus treated animals in 10 out of 13 patient samples (Figure 3C). Most important, therapy with antibody CD19-DE significantly prolonged survival of NSG mice in this randomized preclinical phase 2–like setting (P = .0248, Kaplan-Meier log-rank test; Figure 3D), proving its efficacy in a stringent study design. On day +110, the experiment ended. Eleven of 13 (84.6%) control mice had to be sacrificed before day +110, and 2 of 13 (15.4%) mice were sacrificed on day +110. All control animals had high human blast counts in the bone marrow and the spleen (Figure 3E). Of the treatment mice, 7 of 13 (53.8%) died earlier than day +110. One of these animals (animal 5) died for reasons other than leukemia and 2 of these animals (animals 9 and 12) could not be analyzed because of postmortem changes. Six of 13 (46.2%) treatment mice survived until day +110. Of these animals, 4 (66.7%) had asymptomatic leukemia (animals 3, 4, 6, 10), whereas 2 (33.3%) showed ≤0.5% human blasts in bone marrow and spleen (animals 11 and 13) (Figure 3E). Taken together, the efficacy of CD19-DE in a randomized experiment with xenografts from 13 patients suggests clinical activity in human MLL-rearranged leukemia.
CD19-DE is efficient in a preclinical phase 2–like xenograft trial of MLL-rearranged BCP-ALL. (A) Experimental scheme. Patient-derived ALL xenografts were established by orthotopic intrafemoral transplantation of 100 ALL cells per animal from 13 patients (Table 1) into 2 NSG mice each (day 0). Mice were randomized into control or CD19-DE (treatment) groups. The antibody was injected intraperitoneally (1 mg/kg) on days +1, +3, +6, +10, +13, and every 21 days thereafter. (B, C) Reduction of human ALL blast counts in the peripheral blood of xenografted mice by treatment with antibody CD19-DE: pooled analysis, Mann-Whitney U test (B); individual analysis (C). Note that in case of patient 6, the control mouse had already been sacrificed before day +44, and the treatment mouse was still alive. (D) Survival prolongation of mice xenografted with ALL blasts from 13 different patients by treatment with antibody CD19-DE in the preclinical phase 2–like study (Kaplan-Meier log-rank test). (E) Percentage of human blasts in the bone marrow and spleens of all animals at the time of euthanasia. n/a, not applicable (postmortem changes precluded this analysis); Sp, spleen. *Patients with a >2-fold difference in peripheral blasts in control versus treatment mice.
CD19-DE is efficient in a preclinical phase 2–like xenograft trial of MLL-rearranged BCP-ALL. (A) Experimental scheme. Patient-derived ALL xenografts were established by orthotopic intrafemoral transplantation of 100 ALL cells per animal from 13 patients (Table 1) into 2 NSG mice each (day 0). Mice were randomized into control or CD19-DE (treatment) groups. The antibody was injected intraperitoneally (1 mg/kg) on days +1, +3, +6, +10, +13, and every 21 days thereafter. (B, C) Reduction of human ALL blast counts in the peripheral blood of xenografted mice by treatment with antibody CD19-DE: pooled analysis, Mann-Whitney U test (B); individual analysis (C). Note that in case of patient 6, the control mouse had already been sacrificed before day +44, and the treatment mouse was still alive. (D) Survival prolongation of mice xenografted with ALL blasts from 13 different patients by treatment with antibody CD19-DE in the preclinical phase 2–like study (Kaplan-Meier log-rank test). (E) Percentage of human blasts in the bone marrow and spleens of all animals at the time of euthanasia. n/a, not applicable (postmortem changes precluded this analysis); Sp, spleen. *Patients with a >2-fold difference in peripheral blasts in control versus treatment mice.
CD19-DE is dependent on the presence of macrophages in vivo and effectively mediates phagocytosis of ALL cells in vitro
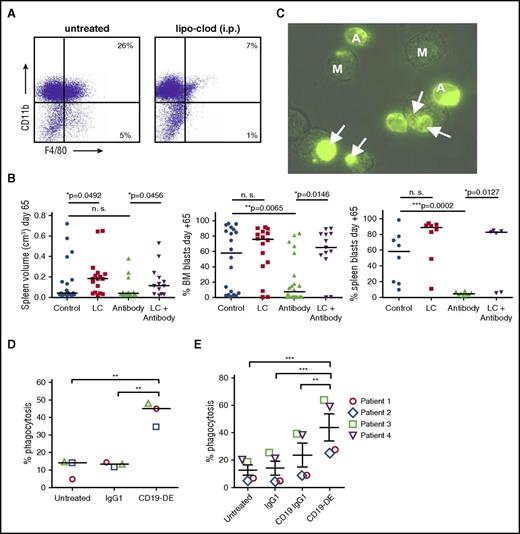
We next hypothesized that in human leukemia xenografted in NSG mice, macrophages played a key role in mediating CD19-DE efficiency. We therefore initiated a xenograft experiment with cells from patient 1 testing CD19-DE with and without the depletion of the macrophage compartment using liposomal clodronate (LC)40 (20-22 animals per group, as indicated). A total of 84 mice were transplanted with human leukemic cells in 2 independent experiments, and antibody therapy was initiated according to Figure 1B. Intraperitoneal treatment with LC was initiated on day −1 of the experiment and repeated every 7 days thereafter. As was expected, this led to a depletion of macrophages in the bone marrow (Figure 4A). Four out of 20 (20%) mice treated with LC and 9 out of 22 (40.9%) animals treated with the combination of antibody and LC died early during the experiment. Because none of the 20 untreated mice and 22 mice treated with CD19-DE died, this phenomenon was attributed to a substantial toxicity of repeated LC injections, and the experiment was finalized on day +65. Nevertheless, macrophage depletion resulted in a reversal of the beneficial effects of antibody therapy as measured by increases in splenic volumes and percentage of human blasts in the bone marrow of the xenografts in the group treated with antibody and LC, in comparison with antibody treatment alone (P = .0456 and P = .0146; Figure 4B, left and middle panels). A subset of mice was evaluated for human blasts in the spleen of the xenografts, and as observed before, the group treated with an antibody/LC combination displayed a reduced percentage of infiltrating blasts in comparison with antibody treatment alone (P = .0127; Figure 4B, right panel). CD19-DE was efficient in reducing bone marrow and spleen infiltration in comparison with control animals (P = .0065 and P = .0002; Figure 4B, middle and right panels). Mice treated with LC only showed increased splenic volumes in comparison with untreated control animals (P = .0492; Figure 4B, left panel). However, LC treatment alone did not lead to a statistically significant increase in bone marrow and spleen infiltration (Figure 4B, middle and right panels). These data suggest an important role for macrophages in CD19-DE antibody therapy in this model. CD19-DE was therefore analyzed for its ability to engage human macrophages in vitro. Human macrophages were differentiated from peripheral blood monocytes and analyzed as effector cells for CD19-DE in in vitro phagocytosis assays using the 697 ALL cell line (Figure 4C). In this assay, CD19-DE efficiently mediated phagocytosis of 697 ALL cells in comparison with untreated cells and cells treated with a control antibody (Figure 4D). CD19-DE was moreover able to trigger phagocytosis of human ALL blasts derived from xenografted spleens bearing ALL cells from patients 1-4 (Table 1; Figure 4E; Supplemental Figure 3). An unmodified CD19 IgG1 antibody containing the MOR208 V-regions but a native human IgG1 Fc domain was included in these experiments and found to be inefficient (Figure 4E). These data further highlight the importance of Fc engineering for the efficacy of CD19 antibodies and suggest that macrophages are a powerful effector cell population for this type of therapy.
CD19-DE efficacy is dependent on the presence of macrophages in vivo and effectively mediates phagocytosis of ALL cells in vitro. (A) NSG mice were treated with a single injection of liposomal clodronate intraperitoneally, and their bone marrow was analyzed for macrophage depletion by flow cytometry 6 days later. (B) Patient-derived ALL xenografts were established by orthotopic intrafemoral transplantation of 1000 ALL cells per animal in NSG mice (patient 1; day 0). Mice were treated with CD19-DE, as is depicted in Figure 1B (“antibody only”), with LC alone on day −1 and every 7 days thereafter (“LC only”) or with a combination of CD19-DE and LC (“antibody + LC”). Postmortem analyses of the mice that did not succumb to LC toxicity: splenic volume (left) as a marker of leukemic burden; human leukemic blasts in the bone marrow (middle); and human leukemic blasts in the spleen in a subset of mice (right) (Mann-Whitney U test). Mice that could be evaluated for spleen volume and bone marrow infiltration on day +65: control n = 20; LC only n = 16; antibody n = 22; and LC + antibody n = 13. Mice that could be evaluated for spleen blasts on day +65: control n = 8; LC only n = 8; antibody n = 8; and LC + antibody n = 6. (C) Microscopy analyses of antibody-mediated phagocytosis of green-fluorescent 697 ALL cells by human macrophages. Phagocytosis of a leukemic cell by a macrophage (arrows). (D) Enhancement of antibody-mediated phagocytosis of 697 ALL cells by treatment with antibody CD19-DE in vitro (each dot represents an independent experiment, ANOVA, and Bonferroni’s multiple-comparison test). (E) Enhancement of antibody-mediated phagocytosis of 4 primary xenograft-derived patient ALL cells (patients 1-4 in Table 1) by treatment with antibody CD19-DE in vitro (each symbol represents an independent patient, ANOVA, and Bonferroni’s multiple comparison test). Replicate analyses of the individual patients are depicted in Supplemental Figure 3. A, ALL cell; CD19 IgG1, CD19-specific control antibody not containing the CD19-DE Fc modification; IgG1, nontargeting control antibody; i.p., intraperitoneal; lipo-clod, liposomal clodronate; M, macrophage. *P < .05. **P < .01. ***P < .001.
CD19-DE efficacy is dependent on the presence of macrophages in vivo and effectively mediates phagocytosis of ALL cells in vitro. (A) NSG mice were treated with a single injection of liposomal clodronate intraperitoneally, and their bone marrow was analyzed for macrophage depletion by flow cytometry 6 days later. (B) Patient-derived ALL xenografts were established by orthotopic intrafemoral transplantation of 1000 ALL cells per animal in NSG mice (patient 1; day 0). Mice were treated with CD19-DE, as is depicted in Figure 1B (“antibody only”), with LC alone on day −1 and every 7 days thereafter (“LC only”) or with a combination of CD19-DE and LC (“antibody + LC”). Postmortem analyses of the mice that did not succumb to LC toxicity: splenic volume (left) as a marker of leukemic burden; human leukemic blasts in the bone marrow (middle); and human leukemic blasts in the spleen in a subset of mice (right) (Mann-Whitney U test). Mice that could be evaluated for spleen volume and bone marrow infiltration on day +65: control n = 20; LC only n = 16; antibody n = 22; and LC + antibody n = 13. Mice that could be evaluated for spleen blasts on day +65: control n = 8; LC only n = 8; antibody n = 8; and LC + antibody n = 6. (C) Microscopy analyses of antibody-mediated phagocytosis of green-fluorescent 697 ALL cells by human macrophages. Phagocytosis of a leukemic cell by a macrophage (arrows). (D) Enhancement of antibody-mediated phagocytosis of 697 ALL cells by treatment with antibody CD19-DE in vitro (each dot represents an independent experiment, ANOVA, and Bonferroni’s multiple-comparison test). (E) Enhancement of antibody-mediated phagocytosis of 4 primary xenograft-derived patient ALL cells (patients 1-4 in Table 1) by treatment with antibody CD19-DE in vitro (each symbol represents an independent patient, ANOVA, and Bonferroni’s multiple comparison test). Replicate analyses of the individual patients are depicted in Supplemental Figure 3. A, ALL cell; CD19 IgG1, CD19-specific control antibody not containing the CD19-DE Fc modification; IgG1, nontargeting control antibody; i.p., intraperitoneal; lipo-clod, liposomal clodronate; M, macrophage. *P < .05. **P < .01. ***P < .001.
Discussion
Our preclinical in vivo data suggest therapeutic potential of Fc-engineered CD19 antibodies in the therapy of MLL-rearranged BCP-ALL and demonstrate an important function for macrophages. The most remarkable results were achieved when CD19 antibodies were administered in a situation of MRD or with concomitant chemotherapy. Importantly, this Fc-engineered CD19 antibody achieved MRD-negativity in aggressive MLL-rearranged BCP-ALL PDX models in NSG mice lacking functional lymphocyte compartments.
CD19-targeted approaches are on the rise in the treatment of relapsed/refractory childhood ALL and include CD19-directed CAR T cells and the BiTE blinatumomab.5,12-15,41,42 Our data suggest that Fc-engineered CD19 antibodies may provide additional treatment options as well. However, it remains to be clarified which of the Fc-engineering approaches may be the most appropriate for CD19 targeting. The S239D/I332E modification increases affinity to all activating FcγR (FcγRI, FcγRIIa, and FcγRIIIa).21 It can thus be assumed that such variants are advantageous over glycoengineered, afucosylated antibodies, which predominantly bind FcγRIIIa with higher affinity.43 However, these strategies have not been compared in the background of CD19 antibodies.
Enhanced efficacy was achieved by combining CD19-DE with chemotherapy. The improvements may be due to cytoreduction and thus an increase in the effector-to-target-cell ratio, which is crucial for antibodies with functions largely dependent on effector cell activity. However, other mechanisms such as chemotherapy-mediated infiltration of macrophages into the bone marrow and subsequently enhanced ADCP44 cannot be excluded. Our results clearly encourage further evaluation of Fc-engineered CD19 antibodies in combination with different chemotherapy regimens.
Here, CD19-DE was found to be effective in a randomized preclinical phase 2–like study, providing stringent in vivo evidence for a broad activity of this antibody in MLL-rearranged ALL. Preclinical testing in xenograft models is the gold standard in hematological malignancies. This, however, may not always fully reflect the diversity of a patient population owing to the fact that usually every xenograft in every treatment group bears the same patient sample. A strategy to address this limitation is the performance of randomized preclinical phase 2–like studies, as recently suggested by Townsend et al.45 Our data show that this approach is also appropriate for testing immunotherapeutic strategies.
The therapeutic successes of blinatumomab and CAR T-cell therapies are associated with toxicities. Common toxicities of T-cell recruitment include cytokine release syndrome (CRS) and encephalopathy, even though the incidence and severity of these toxicities were dramatically lower with blinatumomab than with CAR T cells.14,15 Also, toxicity is dependent on the tumor burden, and for instance, no CRS has so far been reported in the MRD situation with any of the 2 strategies. Applying CD19 antibodies like MOR208 in a phase 1 clinical trial had almost negligible side effects. Except for profound neutropenia in 1 of 12 patients, grade 3/4 side effects were exclusively observed at application doses by far exceeding the doses in this study.27 The safety profile was similar in case studies using this type of therapy in children46 and adults.28,29 Prolonged B-cell depletion as with CAR T cells47 was not observed with this type of CD19 antibody, and no CRS has been reported.46 Additional advantages of this CD19 antibody may be the ease of application and a long half-life. It waits to be seen whether antigen escape by loss of CD19 expression or outgrowth of CD19-negative leukemic subclones may occur, similar to what has previously been observed with blinatumomab41,48 or CD19 CAR T cells.48-50 Our data suggest that CD19-DE is highly efficient in MLL-rearranged leukemia, even though CD19 expression in that entity is comparatively low, at least in cell lines.26 MLL-rearranged leukemia may have a propensity to show lineage switches to AML under the pressure of CD19-directed immunotherapy,51,52 which similarly to the emergence of CD19 loss variants was not observed here. The efficacy of CD19-DE and other immunotherapies53 in MLL-rearranged ALL is also important considering the poor prognosis of these patients, who often require allogeneic stem cell transplantation. The addition of CD19 antibodies to chemotherapy may be a strategy sparing some patients the acute and long-term sequelae of this procedure.
Clearly, our study has limitations regarding its applicability to the clinical situation. Although we demonstrated that macrophages exerted important effector functions with CD19-DE, it is likely that additional effector functions are elicited in patients. Thus, in vitro studies have previously suggested that ADCC by natural killer (NK) cells may represent another important effector function of engineered CD19 antibodies such as MOR20830 and CD19-DE (unpublished data). However, this is difficult to verify in vivo because NSG mice lack functional lymphocytes. It has been shown that functional NK cells in nonobese diabetic/severe combined immunodeficiency mice prevent or delay the engraftment of some BCP-ALL samples in hematopoietic organs.54 However, this does not imply that murine NK cells exert the same effector functions as do their human counterparts. In fact, differences in the expression profile and function of FcγR may preclude definite conclusions.55 In particular, mouse NK cells express solely FcγRIII and, in contrast to murine macrophages, lack expression of FcγRIV. FcγRIV is the ortholog of the clinically relevant human FcγRIIIA, which in humans is expressed by NK cells, macrophages, and a subset of monocytes.
In conclusion, our preclinical in vivo model suggests a therapeutic potential of Fc-engineered CD19 antibodies in MLL-rearranged BCP-ALL and supports a critical role of macrophage activation. Thus, further development of naked Fc-engineered CD19 antibodies appears promising. This type of antibody is easy to administer and may avoid toxicities of T-cell activation, making it particularly attractive for application in infants and children.
Presented orally in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Katrin Timm-Richert, Katrin Neumann, Anja Muskulus, and Britta von Below for excellent technical assistance and Claus Meyer (Institute of Pharmaceutical Biology, Goethe University, Frankfurt, Germany) for providing the MLL-breakpoint sequences for the MRD analysis.
This study was supported by the Max-Eder Group Leader pro-gram of the Deutsche Krebshilfe (D.M.S.); the Mildred-Scheel Professorship program of the Deutsche Krebshilfe (M.P.); research grants from the Wilhelm Sander Foundation (C.K., M.P., and D.M.S.); and a research grant from the Deutsche Forschungsgemeinschaft (Va124/9-1; T.V.).
Authorship
Contribution: D.M.S., M.P., and C.K. initiated the study, designed and supervised the research, performed experiments, analyzed data, and wrote the manuscript; A.A. designed and performed the experiments, analyzed data, and edited the manuscript; J.A., S.R., C.S., L.L., F.V., and F.M. designed and performed the experiments and analyzed data; G.C., S.V., T.V., M.S., and M.G. commented on and discussed the research direction and edited the manuscript. All authors discussed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denis M. Schewe, Pediatric Hematology/Oncology, ALL-BFM Study Group, Kiel University and University Hospital Schleswig-Holstein, Kiel, Germany; e-mail: denis.schewe@uksh.de.
References
Author notes
M.P. and C.K. contributed equally as senior authors to this study.