Key Points
First evidence of phenotypic correction in FA hematopoietic repopulating cells by optimized collection and short transduction of CD34+ cells.
Optimized ex vivo gene therapy of FA CD34+ cells confers proliferation advantage to phenotypically corrected repopulating cells.
Abstract
Previous Fanconi anemia (FA) gene therapy studies have failed to demonstrate engraftment of gene-corrected hematopoietic stem and progenitor cells (HSPCs) from FA patients, either after autologous transplantation or infusion into immunodeficient mice. In this study, we demonstrate that a validated short transduction protocol of G-CSF plus plerixafor-mobilized CD34+ cells from FA-A patients with a therapeutic FANCA-lentiviral vector corrects the phenotype of in vitro cultured hematopoietic progenitor cells. Transplantation of transduced FA CD34+ cells into immunodeficient mice resulted in reproducible engraftment of myeloid, lymphoid, and CD34+ cells. Importantly, a marked increase in the proportion of phenotypically corrected, patient-derived hematopoietic cells was observed after transplantation with respect to the infused CD34+ graft, indicating the proliferative advantage of corrected FA-A hematopoietic repopulating cells. Our data demonstrate for the first time that optimized protocols of hematopoietic stem cell collection from FA patients, followed by the short and clinically validated transduction of these cells with a therapeutic lentiviral vector, results in the generation of phenotypically corrected HSPCs capable of repopulating and developing proliferation advantage in immunodeficient mice. Our results suggest that clinical approaches for FA gene therapy similar to those used in this study will facilitate hematopoietic repopulation in FA patients with gene corrected HSPCs, opening new prospects for gene therapy of FA patients.
Introduction
Recent clinical trials have demonstrated that hematopoietic gene therapy with self-inactivated retroviral and lentiviral vectors constitutes an efficient and safe therapeutic approach for several monogenic diseases affecting the hematopoietic system, including primary immunodeficiencies1,2 and hemoglobinopathies.3,4 Despite results from these clinical trials, concerns persist regarding the applicability of gene therapy in Fanconi anemia (FA) because no evidence has been reported to date demonstrating reproducible engraftment of gene-corrected FA hematopoietic stem and progenitor cells (HSPCs), either in clinical trials5,6 or in xenogeneic transplantation models.
Several factors could account for these observations, including the reduced number and quality of collected FA HSPCs. Previous studies have shown the low number and accelerated decrease of HSPCs in the bone marrow (BM) of FA patients,7-9 suggesting the convenience of collecting hematopoietic grafts in early stages of the disease (see reviews in Adair et al,10 Muller and Williams,11 Tolar et al,12 and Tolar et al13 ).
Another key element that may have limited the engraftment of gene-corrected FA HSPCs is the detrimental effect that transduction protocols could have upon the repopulating properties of these sensitive cells. In fact, previous studies in FA mouse models have shown decreases in the repopulating potential of in vitro cultured or transduced HSPCs, with correlation to both FA genotype and the time of in vitro cell culture.14-16 Consequently, short transduction protocols with lentiviral or foamy viral vectors have facilitated the engraftment of gene-corrected HSPCs in FA mouse models.15,16 More recently, similar conclusions have also been obtained in xenogeneic transplants of transduced human CD34+ cells from healthy donors (HDs).17 These data suggest that a shortened transduction process may enhance the efficacy of human hematopoietic gene therapy, most particularly in FA gene therapy.
Despite the obstacles in conducting FA gene therapy, one of the main advantages of this therapeutic approach can be deduced from studies in FA mosaic patients in whom evidence of proliferative advantage has been demonstrated in reverted HSPCs.18-20 Based on these observations, it has been proposed that the infusion of a relatively low number of gene-corrected autologous FA HSPCs could be sufficient to replace FA patients’ hematopoiesis with functionally competent cells. Despite the appeal of this hypothesis, there has been no evidence to date showing that such an in vivo proliferative advantage can occur after the ex vivo gene correction of human FA HSPCs.
Because of these challenges for FA gene therapy, in the current study we developed a clinically applicable short lentiviral transduction of FA CD34+ cells mobilized to peripheral blood (PB) with 2 potent mobilizing agents: granulocyte colony-stimulating factor (G-CSF) and plerixafor.21,22 Data obtained under these optimized conditions allowed us to demonstrate for the first time the repopulating potential and in vivo proliferative advantage of gene-corrected FA patient-derived CD34+ cells in immunodeficient mice.
Materials and methods
Patients
Studies conducted with samples from HD and from FA patients, complementation group A (FA-A patients) were approved by the authors' institutional review board and conducted under the Declaration of Helsinki. Patient and HD identities were encoded to protect confidentiality. In all instances, written informed consent was obtained. FA patients 3 to 7 years of age with no evidence of myelodysplasia or acute myeloid leukemia (AML) and without evidence of BM failure as defined in the FANCOSTEM clinical trial (NCT02931071) were included in our study. None of these patients had been previously transfused or treated with androgens or steroids. The age of patients included in the study, as well as the complementation group, percentage of CD34+ cells in BM, and other characteristics of their BM and PB cells analyzed before HSPC mobilization are shown in supplemental Table 1, available on the Blood Web site.
Human hematopoietic cells
Purified CD34+ cells from FA-A patients were obtained either from BM aspirates or from small aliquots of mobilized PB (mPB) from patients recruited in the Clinical Phase II Trial to Evaluate CD34+ Cells Mobilization and Collection in Patients With Fanconi Anemia for Subsequent Transduction With a Lentiviral Vector Carrying the FANCA Gene (NCT02931071), according to procedures described in the clinical protocol. HSPC mobilization in FA-A patients was conducted using G-CSF (Neupogen, AMGEN; 12 µg/kg/12 h × 6 to 7 days) and plerixafor (Mozobil, Genzyme; 240 µg/d × 2 to 3 days). Mobilized HSPCs were collected by apheresis, and purified CD34+ cells remaining in cell collection bags and tubes from the CliniMACS System (Miltenyi Biotec) were used in these experiments. In 1 case, mPB CD34+ cells from a HD were also obtained using a similar protocol as described for FA patients. In this case, however, 10 µg/kg/12 h of G-CSF was administered for 6 days, followed by a 1-day treatment with plerixafor. Small aliquots of mPB CD34+ cells from HDs were immunoselected using the MACS CD34 Micro-Bead kit (Miltenyi Biotec).
Immunophenotypic analyses of HSPCs were performed using antibodies CD45 APC and CD34 PE (both from Becton Dickinson). Fluorochrome-matched isotypes were used as controls. 4′,6-diamidino-2-phenylindole (Roche)-positive cells were excluded from the analysis. Analysis was performed using FlowJo software, v7.6.5.
Lentiviral vectors and transduction protocol
The therapeutic PGK-FANCA.Wpre* LV23 carrying the FANCA gene under the control of the phosphoglycerate kinase promoter (PGK) was produced by Genethon under pre–good manufacturing practices (pre-GMP) and GMP conditions for gene therapy of FA-A patients (NCT03157804). We have previously shown in FA mouse models the safety and efficacy of this vector,24 which received Orphan Drug designation both in Europe and in the United States (European Union 10/822, 2010 and US 16-5193). In some experiments, a PGK-enhanced green fluorescent protein (EGFP) reporter LV was also used as a negative control. CD34+ cells from HDs and FA-A patients were prestimulated for 8 to 10 hours in X-Vivo-20 medium (StemCell Technologies) supplemented with 100 ng/mL SCF, TPO and Flt3-ligand, and 20 ng/mL IL3 (all of these from Peprotech), 10 µg/mL anti-tumor necrosis factor-α (TNF-α; Enbrel-Etanercept), and 1 mM N-acetylcysteine (Pharmazam) under hypoxic conditions (5% of O2) in plates precoated with 5 µg/cm2 of Retronectin (Takara). After prestimulation, cells were transduced with 3 × 108 IU/mL of pre-GMP or GMP PGK-FANCA.Wpre* LVs for 12 to 14 hours in the presence of 8 µg/mL protamine sulfate. Thereafter, cells were collected, washed, and cultured in vitro and/or transplanted into nonobese diabetic severe combined immunodeficiency-Il2rg−/− (NSG) mice.
Colony forming cell assays
To assess the number of colony-forming cells (CFCs) from transduced HD and FA CD34+ cells, samples were cultured for 14 days at 37°C, 5% CO2, and 5% O2 in methylcellulose medium (Methocult #H4434), which is specific for the growth of human hematopoietic progenitor cells (CFU-GMs, BFU-Es and CFU-Mix colonies), and supplemented with 10 µg/mL anti-TNF-α and 1 mM N-acetylcysteine. To determine the proportion of mitomycin C (MMC)-resistant CFCs, samples were cultured both in the absence and the presence of 10 nM MMC (Sigma Chemicals), as previously described.23 Because BM cells from nontransplanted NSG mice do not generate colonies in this medium (not shown), this methylcellulose culture medium was also used to determine the proportion of MMC-resistant FA patient–derived CFCs after transplantation.
Analysis of the lentiviral vector copy number
DNA from transduced cells that were expanded for 14 days in liquid culture was extracted using the DNeasy blood and tissue kit (Qiagen). Genomic DNA from hematopoietic colonies was extracted using proteinase K lysis by adding 20 µL of lysis buffer (0.3 mM Tris HCl, pH 7.5; 0.6 mM CaCl2; 1.5% Glycerol; 0.675% Tween-20; and 0.3 mg/mL Proteinase K). Lysis was performed at 65°C for 30 minutes, 90°C for 10 minutes, and 4°C for 10 minutes. Finally, 30 µL of water was added to each sample. The vector copy number per cell (VCN/cell) was analyzed by duplex detection of Ψ sequence normalized to Albumin, using primers specific for Ψ sequence: Ψ forward (Ψ.F): 5′ CAGGACTCGGCTTGCTGAAG 3′ and Ψ reverse (Ψ.R): 5′ TCCCCCGCTTAATACTGACG 3′ and detected with the Taqman probe Ψ.P FAM: 5′CGCACGGCAAGAGGCGAGG3′. To normalize to endogenous Albumin, specific primers for Albumin were used: Alb forward (Alb.F): 5′ GCTGTCATCTCTTGTGGGCTG 3′ and Alb reverse (Alb.R.): 5′ ACTCATGGGAGCTGCTGGTTC 3′ with a Taqman probe Alb.P VIC: 5′ CCTGTCATGCCCACACAAATCTCTCC 3′. Quantitative polymerase chain reaction was conducted in an Applied 7500 Fast Real Time PCR system (Thermo Fisher Scientific), as previously described.23
Transplantation of transduced HD and FA-A CD34+ cells
Transduced CD34+ cells from HD and FA-A patients were injected, either intravenously or intrabone, into NSG mice previously irradiated with 1.5 Gy (MG324 Philips). To evaluate the level of human hematopoietic engraftment, cells were collected by femoral BM aspiration at 4 to 12 weeks posttransplant and stained with hCD45 APC-Cy7 (eBioscience) and CD34 APC (BD Biosciences) antibodies according to the manufacturer’s instructions. Analyses of multilineage engraftment were performed using CD45 APC-Cy7 (BioLegend), CD34 APC (BD Biosciences), CD33 PE (BD Biosciences), CD19 PE-Cy7 and CD3 FITC (BioLegend), according to the manufacturer’s instructions. Fluorochrome-matched isotypes were used as controls. 4′,6-Diamidino-2-phenylindole–positive cells were excluded from the analysis. All flow cytometric analyses were performed on the LSR Fortessa (BD Biosciences) and analyzed with FlowJo software v7.6.5.
Statistical analysis
Statistical analysis was performed using GraphPad Prism, version 7.0 for Windows. Results are reported as mean ± standard error. Statistical differences between means were evaluated by Student t test. Differences were considered significant at P ≤ .05. Linear regression analysis was applied to determine the correlation between mean VCNs per cell in nonselected colonies and their resistance to 10 nM MMC (Figure 1C).
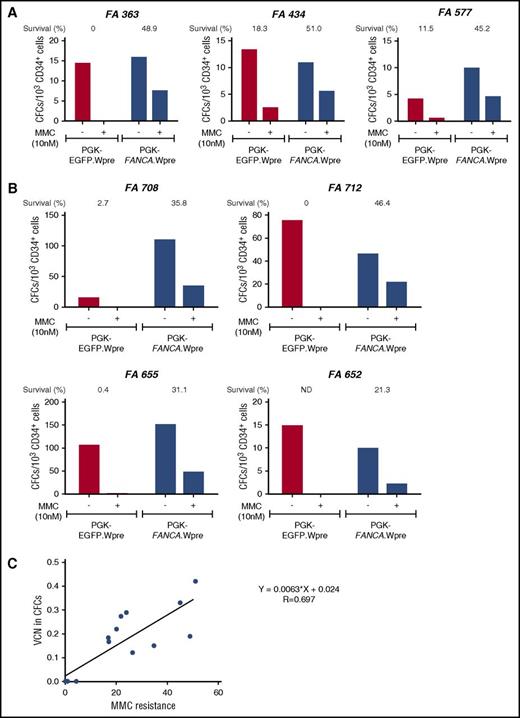
Correction of the MMC hypersensitivity of hematopoietic progenitors from FA-A patients using a short transduction protocol with a therapeutic lentiviral vector. (A) Bone marrow CD34+ cells from 3 FA-A patients were prestimulated for 8 to 10 hours and then transduced for another 12 to 14 hours with a nontherapeutic (PGK-EGFP.Wpre*; red bars) or a therapeutic (PGK-FANCA.Wpre*; blue bars) lentiviral vector. Transduced samples were then grown in methyl-cellulose, in the absence or the presence of 10 nM MMC, to test their sensitivity to MMC. (B) Similar experiments were conducted using G-CSF plus plerixafor-mobilized PB CD34+ cells from FA-A patients. (C) Correlation analysis between the proportion of colonies resistant to 10 nM MMC and the mean VCN/colony in pooled colonies grown in the absence of MMC. (A-B) CD34+ cells were transduced with pre-GMP and GMP lots of the therapeutic vector, respectively.
Correction of the MMC hypersensitivity of hematopoietic progenitors from FA-A patients using a short transduction protocol with a therapeutic lentiviral vector. (A) Bone marrow CD34+ cells from 3 FA-A patients were prestimulated for 8 to 10 hours and then transduced for another 12 to 14 hours with a nontherapeutic (PGK-EGFP.Wpre*; red bars) or a therapeutic (PGK-FANCA.Wpre*; blue bars) lentiviral vector. Transduced samples were then grown in methyl-cellulose, in the absence or the presence of 10 nM MMC, to test their sensitivity to MMC. (B) Similar experiments were conducted using G-CSF plus plerixafor-mobilized PB CD34+ cells from FA-A patients. (C) Correlation analysis between the proportion of colonies resistant to 10 nM MMC and the mean VCN/colony in pooled colonies grown in the absence of MMC. (A-B) CD34+ cells were transduced with pre-GMP and GMP lots of the therapeutic vector, respectively.
Results
Efficient correction of FA-A hematopoietic progenitors after short transduction with a therapeutic lentiviral vector
Because of the detrimental effects associated with prolonged ex vivo transduction protocols of HSPCs, we first investigated whether a 24-hour transduction protocol of HD mPB CD34+ cells with a high concentration (3 × 108 IU/mL) of the PGK-FANCA.Wpre* therapeutic LV23 efficiently transduced these progenitor cells (see our Materials and methods section). As shown in Table 1, the cellularity and CFC yields obtained in the transduced product were around 40% of input values, whereas the viability and proportion of colonies/103 cells was essentially not affected (103.1% and 83.8% of input values, respectively), indicating that no significant toxicity was induced in the hematopoietic progenitors by the transduction process. With respect to the transduction efficacy of the protocol, a mean value of 0.40 ± 0.1 copies/cell was obtained in cells that were ex vivo expanded for 14 days after transduction of CD34+ cells, showing the efficiency of the proposed protocol in transducing HD mPB CD34+ cells.
Transduction efficacy of mobilized peripheral blood CD34+ cells from healthy donors exposed to a short transduction with a GMP-produced PGK-FANCA.Wpre* lentiviral vector
| . | . | Total cells (×106) . | Viability . | Total colonies (×105) . | Colonies/1000 CD34+ cells . | VCN/cell (14-d liquid culture) . |
|---|---|---|---|---|---|---|
| Validation I | Input | 3.00 | 90.96 | 4.73 | 158 | — |
| Output | 1.10 (36.7%) | 83.60 (91.91) | 1.30 (27.4%) | 118 (74.7%) | 0.39 | |
| Validation II | Input | 3.00 | 88.76 | 5.63 | 188 | — |
| Output | 1.56 (51.0%) | 94.03 (105.94) | 2.30 (47.4%) | 150 (79.9%) | 0.33 | |
| Validation III | Input | 3.00 | 86.4 | 7.63 | 254 | — |
| Output | 1.39 (46.3%) | 96.3 (111.46) | 3.45 (44.9%) | 247 (96.9%) | 0.67 | |
| Mean ± SE | Input | 3.00 ± 0 | 88.71 ± 1.32 | 6.00 ± 0.86 | 200 ± 28.35 | — |
| Output | 1.34 ± 0.13 (44.7% ± 1.92%) | 91.31 ± 3.91 (103.10 ± 5.82) | 2.34 ± 0.62 (37.7% ± 6.29%) | 171 ± 38.78 (83.8% ± 6.94%) | 0.40 ± 0.10 |
| . | . | Total cells (×106) . | Viability . | Total colonies (×105) . | Colonies/1000 CD34+ cells . | VCN/cell (14-d liquid culture) . |
|---|---|---|---|---|---|---|
| Validation I | Input | 3.00 | 90.96 | 4.73 | 158 | — |
| Output | 1.10 (36.7%) | 83.60 (91.91) | 1.30 (27.4%) | 118 (74.7%) | 0.39 | |
| Validation II | Input | 3.00 | 88.76 | 5.63 | 188 | — |
| Output | 1.56 (51.0%) | 94.03 (105.94) | 2.30 (47.4%) | 150 (79.9%) | 0.33 | |
| Validation III | Input | 3.00 | 86.4 | 7.63 | 254 | — |
| Output | 1.39 (46.3%) | 96.3 (111.46) | 3.45 (44.9%) | 247 (96.9%) | 0.67 | |
| Mean ± SE | Input | 3.00 ± 0 | 88.71 ± 1.32 | 6.00 ± 0.86 | 200 ± 28.35 | — |
| Output | 1.34 ± 0.13 (44.7% ± 1.92%) | 91.31 ± 3.91 (103.10 ± 5.82) | 2.34 ± 0.62 (37.7% ± 6.29%) | 171 ± 38.78 (83.8% ± 6.94%) | 0.40 ± 0.10 |
A total of 3 × 106 CD34+ cells were prestimulated in 3 mL of supplemented medium for 8 to 10 hours, followed by another 12 to 14 hours of transduction with the therapeutic lentiviral vector. Analyses of VCN/cell was conducted in cells maintained in liquid culture for 14 days, according to a validated GMP protocol. Percentages (in parentheses) determined after transduction (output) with respect to values determined before transduction (input) are shown. In these experiments, CD34+ cells were mobilized with G-CSF.
In a second set of experiments, we investigated whether the proposed 24-hour transduction protocol corrected the phenotype of hematopoietic progenitors from FA patients. BM CD34+ cells from 3 FA-A patients were transduced with the therapeutic PGK-FANCA.Wpre* LV and a nontherapeutic PGK-EGFP.Wpre* LV. We then tested their survival to cytotoxic doses (10 nM) of MMC. As shown in Figure 1A, higher survival rates were always observed after transduction of FA-A CD34+ cells with the therapeutic vector compared with the negative control vector (mean values, 48.37 ± 1.70% vs 9.93 ± 5.34%, respectively; P = .018), demonstrating phenotypic correction of these FA progenitor cells.
Similar experiments were then conducted with G-CSF plus plerixafor-mobilized PB CD34+ (G/P-mPB CD34+) cells from 4 FA-A patients. Once again, the therapeutic vector conferred a significant increase in the survival of FA CFCs compared with the negative control PGK-EGFP.Wpre* LV (33.65 ± 5.21% vs 1.03 ± 0.84%, respectively; P = .017; Figure 1B). This confirms that the proposed 24-hour transduction of FA-A CD34+ cells with the therapeutic vector efficiently corrects the characteristic hypersensitivity of FA-A CFCs to DNA crosslinking drugs.
To examine if the acquired resistance to MMC was a good indicator of the transduction efficiency of FA progenitor cells, mean VCN per cell were first determined in colonies generated by CD34+ cells that had been transduced with the therapeutic vector and grown in the absence of MMC. Subsequently, these VCN/cell values were correlated with the survival of colonies exposed to MMC (Figure 1C). As shown in the figure, a good correlation was observed between these 2 parameters, indicating that survival to 10 nM of MMC constitutes a reliable indicator of the transduction rate of FA CFCs with the therapeutic LV.
In vivo repopulating properties of gene corrected FA-A CD34+ cells transduced under clinically applicable conditions
To investigate if 24-hour transduced G/P-mPB FA-A CD34+ cells had in vivo repopulating ability, aliquots consisting of 3 × 105 to 2 × 106 transduced cells from 4 FA-A patients were transplanted into NSG mice. In these experiments, the average proportion of MMC-resistant colonies was 23.0 ± 3.0%. As shown in Figure 2 and supplemental Table 2, moderate but reproducible engraftment of human hematopoietic cells, including myeloid, lymphoid, and hCD34+ cells, were observed in these animals, regardless of whether transduced CD34+ cells were transplanted intravenously or intrabone.
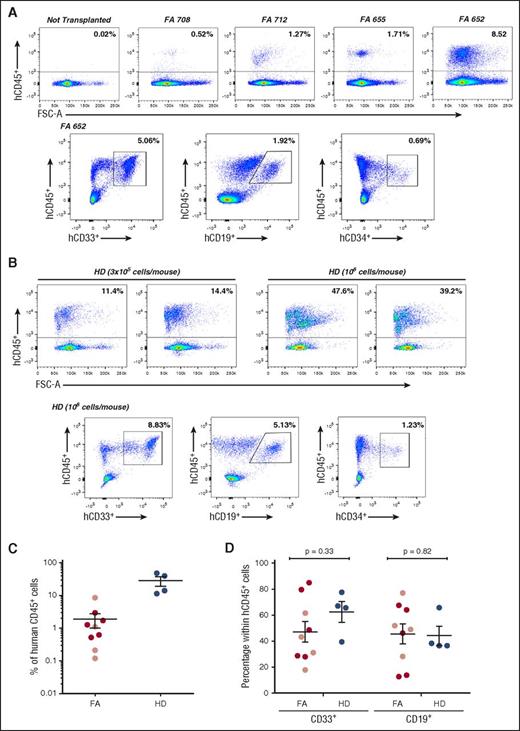
Analysis of the repopulating properties of mobilized peripheral blood CD34+cells from FA-A patients and healthy donors after transduction for 24 hours with a GMP-produced therapeutic lentiviral vector. The figure shows representative analyses of donor hematopoietic reconstitution in the BM of recipient NSG mice 4 weeks after infusion of FA-A (A) and HD (B) mPB CD34+ cells transduced for 24 hours with the therapeutic PGK-FANCA.Wpre* lentiviral vector. (C) Analysis of engraftment measured as percentage of hCD45+ cells in individual NSG mice after intravenous (dark red circles) or intrabone (light red circles) injection of FA or the intravenous injection (blue circles) of HD mPB CD34+ cells transduced under the established conditions. (D) Distribution of donor myeloid and lymphoid cell engraftment in the BM of recipient mice transplanted with transduced FA-A and HD CD34+ cells (symbols as in panel C). CD34+ cells from 4 different FA-A patients and from 1 HD, all of them treated with G-CSF and plerixafor.
Analysis of the repopulating properties of mobilized peripheral blood CD34+cells from FA-A patients and healthy donors after transduction for 24 hours with a GMP-produced therapeutic lentiviral vector. The figure shows representative analyses of donor hematopoietic reconstitution in the BM of recipient NSG mice 4 weeks after infusion of FA-A (A) and HD (B) mPB CD34+ cells transduced for 24 hours with the therapeutic PGK-FANCA.Wpre* lentiviral vector. (C) Analysis of engraftment measured as percentage of hCD45+ cells in individual NSG mice after intravenous (dark red circles) or intrabone (light red circles) injection of FA or the intravenous injection (blue circles) of HD mPB CD34+ cells transduced under the established conditions. (D) Distribution of donor myeloid and lymphoid cell engraftment in the BM of recipient mice transplanted with transduced FA-A and HD CD34+ cells (symbols as in panel C). CD34+ cells from 4 different FA-A patients and from 1 HD, all of them treated with G-CSF and plerixafor.
Following the same experimental procedure used with FA cells, G/P mPB CD34+ cells from a HD were also transduced with the FANCA-LV. A similar transduction efficacy was obtained in CFCs from this donor compared with CFCs from FA patients (VCN/cell was always in the range of 0.10 to 0.20; not shown). Aliquots of 3 × 105 and 1 × 106 transduced cells/mouse were transplanted (an equivalent cell range was used with FA cells). As expected, higher levels of engraftment were observed in mice transplanted with transduced HD CD34+ cells compared with transplants of similar numbers of transduced FA cells (Figure 2C). Despite differences in the repopulation potential of HD and FA CD34+ cells, a similar distribution of myeloid and lymphoid cells was observed in recipients transplanted with either group of transplanted cells (Figure 2D).
We next explored whether FA patients’ engrafted cells consisted of phenotypically corrected FA cells. With this aim, the proportion of MMC-resistant human CFCs was assessed before and 4 weeks after the infusion of transduced FA samples. As shown in Figure 3A, a moderate proportion of transduced progenitors from patient FA-655 was resistant to MMC before infusion into NSG mice (17.1% of the colonies were resistant to 10 nM MMC). Strikingly, when the same analysis was conducted after transplantation, all human-derived CFCs present in mouse BM were resistant to MMC, indicating a 6.4-fold increase in the proportion of MMC-resistant progenitors during the 4 weeks of repopulation (Figure 3A). As expected, when clonogenic assays were conducted with BM cells from nontransplanted NSG mice, no colonies were generated, consistent with the absence of human CD45+ cells in the flow cytometry analyses of Figure 2A, and with the human specificity of the methylcellulose used in these experiments (see our Materials and methods section).
Increased proportion of MMC-resistant colonies 4 weeks after transplantation of transduced CD34+cells from 2 FA-A patients. The figure illustrates the number of colonies and the proportion of colonies resistant to FA cytotoxic doses of MMC (% of survival to 10 nM MMC) before and 4 weeks after transplantation of FA CD34+ cells transduced with the therapeutic PGK-FANCA.Wpre* lentiviral vector into NSG mice (blue bars). As controls, colony numbers generated by samples transduced with the nontherapeutic PGK-EGFP.Wpre* LV or samples from nontransplanted mice (red bars) are also shown. Control cultures showed that no hematopoietic colonies were generated when BM cells from nontransplanted mice were tested.
Increased proportion of MMC-resistant colonies 4 weeks after transplantation of transduced CD34+cells from 2 FA-A patients. The figure illustrates the number of colonies and the proportion of colonies resistant to FA cytotoxic doses of MMC (% of survival to 10 nM MMC) before and 4 weeks after transplantation of FA CD34+ cells transduced with the therapeutic PGK-FANCA.Wpre* lentiviral vector into NSG mice (blue bars). As controls, colony numbers generated by samples transduced with the nontherapeutic PGK-EGFP.Wpre* LV or samples from nontransplanted mice (red bars) are also shown. Control cultures showed that no hematopoietic colonies were generated when BM cells from nontransplanted mice were tested.
A similar experiment was also performed with transduced G/P-mPB CD34+ cells from another FA patient (FA-652). Although the number of CFCs present in the CD34+ population of this patient was about 3 times lower than in the previous one (FA-655), the proportion of MMC-resistant progenitors was again increased (2.4-fold in this case) after transplantation as compared with data obtained before infusion (Figure 3B).
Discussion
Despite the difficulties involved in the gene therapy in FA,5,6 improvements in the collection and genetic modification of HSPCs and the increased understanding in the molecular and cellular basis of FA have opened new possibilities in this field (see reviews in Adair et al,10 Muller and Williams,11 Tolar et al,12 and Tolar et al13 ).
Owing to the limited number of CD34+ cells that could be collected from BM or PB of G-CSF–treated FA patients,5-7,25 a clinical trial evaluating the safety and efficacy of HSPC mobilization with both G-CSF and plerixafor in FA patients is currently under way at our institutions (NCT02931071). This combination of mobilizing drugs was shown to be highly efficient in patients who mobilize HSPCs poorly21,22 as well as in FA mouse models.26
Based on the detrimental effects associated with prolonged ex vivo transduction of FA mouse HSPCs,14-16 recently confirmed in human HSPCs from HDs,17 we first aimed at the validation of a short lentiviral transduction protocol in BM and mPB CD34+ cells from HD and FA patients. Thereafter, we evaluated the phenotypic correction of these cells and investigated whether our optimized transduction conditions facilitated the engraftment of phenotypically corrected FA CD34+ cells in immunodeficient mice.
Similar short transduction protocols have already been used by our laboratory23 and by Becker et al27 for in vitro FA gene therapy studies, although none of these studies has validated or tested the in vivo repopulating ability of transduced FA CD34+ cells. Because of the hypersensitivity of FA cells to oxygen28 and TNF-α,29 N-acetylcysteine and etanercept, efficient antioxidant and anti-TNF-α drugs, respectively, were included throughout the transduction process. Additionally, a low-oxygen atmosphere was used during prestimulation and cell transduction to minimize cell damage. Under these conditions, reproducible transductions rendering VCN/cell in the range of 0.33 to 0.67 were obtained in the validation studies with HD CD34+ cells. Similar transduction and phenotypic correction efficacies were seen when either BM or mPB CD34+ cells from FA patients were used.
Remarkably, our data in Figure 2 showed that transduced CD34+ cells from FA patients were capable of repopulating the hematopoiesis of immunodeficient mice, although as expected, at lower levels than in mice transplanted with HD CD34+ cells. It is important to note that although all transplanted HD CD34+ cells are functional regardless of their transduction with the LV, only gene-complemented FA cells (23% on average in these experiments; supplemental Table 2) would acquire normalized functionality. Additionally, it is not expected that gene complementation will normalize the low number of repopulating cells characteristic of FA patients, but should rather restore their function, as our analysis of the myeloid and lymphoid repopulation in transplanted NSG mice suggests (Figure 2D).
Although 2 studies have also reported engraftment of human FA hematopoietic cells in immunodeficient mice, marked differences distinguish these studies from our own. In the study of Du et al,30 the authors focused on the engraftment of FA leukemic cells, demonstrating that only IL3-Rα+ CD34+ cells from FA patients with AML were able to repopulate immunodeficient mice. In a second study, Cohen-Hangenauer et al proposed a transduction protocol of FA CD34+ cells with very limited clinical applicability and efficacy (only 1 recipient mouse was considered positive after transplantation of samples from 3 patients). FA BM cells were exposed to 3 cycles of retroviral transduction during 3 consecutive days and then maintained in long-term culture for 9 weeks before infusion into immunodeficient mice.31 This differs from our gene therapy approach, which is based on a shortened duration of ex vivo FA CD34+ cell manipulation, both to minimize risks of apoptosis and genetic instability14,32 and to improve the repopulating ability of corrected HSPCs.15-17
As shown in supplemental Table 1, our repopulation studies were conducted with samples from FA patients with normal BM cytogenetics, a characteristic low percentage of CD34+ cells in BM (0.30%, 0.41%, 1.44%, and 0.48%, respectively), and the expected hypersensitivity of FA lymphoid T cells and myeloid CFCs to DNA crosslinking drugs. These hematopoietic analyses indicate that FA patients considered in our experimental gene therapy approach correspond to patients in early stages of BM failure, with no evidence of myelodysplasia or BM cell transformation and with no signs of somatic mosaicism that could account for the repopulating properties of their CD34+ cells.
In addition to the repopulation studies presented in Figure 2, our results showing an increased proportion of MMC-resistant progenitors after transplantation of transduced CD34+ cells (Figure 3) also demonstrate for the first time the in vivo proliferative advantage of FA-A CD34+ cells after ex vivo gene therapy, consistent with the behavior of reverted HSPCs in FA mosaic patients.18-20 Although our experimental FA gene therapy approach aims at limiting risks of malignant transformation of ex vivo manipulated FA cells, either corrected or uncorrected, this possibility must be carefully evaluated. In this respect, previous FA mouse gene therapy studies conducted with the therapeutic lentiviral vector used in this study did not indicate any evidence of leukemic transformation or expansion of gene corrected hematopoietic stem cell clones from oncogene transactivation.24 Also of particular interest are studies conducted in FA-A leukemic patients, showing that FANCA reverse mutations were not present in clonal BM cells,33 indicating that myelodysplastic syndrome/AML cells from these patients arise from uncorrected FA cells. With respect to risks associated with the transformation of endogenous uncorrected cells, the presence of nonreverted aberrant clones has been only occasionally reported in the BM of FA mosaic patients.34 Moreover, to the best of our knowledge, there are no reports showing a significant incidence of leukemia in patients with reverted HSPCs, suggesting that the growth advantage of corrected FA HSPCs limits the expansion of uncorrected FA clones with mutations predisposing to myelodysplastic syndrome/AML.
Our experiments in a xenogeneic transplantation model of FA gene therapy suggest that the progressive repopulation of gene-corrected FA HSPCs confer therapeutic effects similar to those observed in mosaic patients; these effects would also be expected in FA patients receiving gene therapy. Gene therapy clinical trials currently under way in Spain (NCT03157804) and Seattle (NCT01331018) will address many important questions regarding optimal transduction and the proliferative advantage of gene corrected FA HSPCs. Results from these trials in unconditioned patients will also determine the necessity of implementing conditioning regimens in FA gene therapy, ideally using nongenotoxic or minimally genotoxic regimens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Aurora de la Cal for her assistance with the coordination in the delivery of bone marrow and peripheral blood samples from patients with Fanconi anemia (FA), Miguel A. Martin for the careful maintenance of NSG mice, Omaira Alberquilla for her technical assistance in flow cytometry, and Jonathan Schwartz for careful reading of the manuscript and helpful discussions. The authors also thank the FA patients, families, and clinicians from the FA network. We are grateful to Fundación Botín for promoting translational research at the Hematopoietic Innovative Therapies Division of the Centro de Investigaciones Energéticas Medioambientales y Tecnológicas. Centro de Investigación Biomédica en Red de Enfermedades Raras is an initiative of the Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional (FEDER).
This work was supported by grants from the 7th Framework Program European Commission (HEALTH-F5-2012-305421, EUROFANCOLEN), Ministerio de Sanidad, Servicios Sociales e Igualdad (EC11/060 and EC11/559), Ministerio de Economía, Comercio y Competitividad y FEDER (SAF2015-68073-R), and Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III (RD12/0019/0023). J. Surrallés is supported by ICREA Academia and SAF2015-64152-R.
Authorship
Contribution: P.R., S.N., and J.A.B. conceived and designed the experiments. P.R., S.N., G.G., R.S.-D., M.L.L., R.Y., J.A.C., M.R.P., and J.C.S. conducted the experiments. P.A.M., S.C., A.G., C.D.d.H., J. Sevilla, and J. Surallés provided reagents, tools, and contributed with ideas. P.R., S.N., and J.A.B. wrote the manuscript.
Conflict-of-interest disclosure: P.R., J.C.S., and J.A.B. receive funding from Rocket Pharma. J.C.S. and J.A.B. are consultants from Rocket Pharma. J. Sevilla has received honoraria for educational lectures from Novartis and Miltenyi and is a member of advisory committees for Novartis and Rocket Pharma. The remaining authors declare no competing financial interests.
Correspondence: Juan A. Bueren, Division of Hematopoietic Innovative Therapies, CIEMAT/CIBERER/II-FJD, Av Complutense 40, 28040 Madrid, Spain; e-mail: juan.bueren@ciemat.es.
References
Author notes
P.R. and S.N. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal