Key Points
Tolerance of oxidative DNA lesions ensures the genomic and functional integrity of hematopoietic stem and precursor cells.
Endogenous DNA damage–induced replication stress is associated with mitochondrial dysfunction.
Abstract
Endogenous DNA damage is causally associated with the functional decline and transformation of stem cells that characterize aging. DNA lesions that have escaped DNA repair can induce replication stress and genomic breaks that induce senescence and apoptosis. It is not clear how stem and proliferating cells cope with accumulating endogenous DNA lesions and how these ultimately affect the physiology of cells and tissues. Here we have addressed these questions by investigating the hematopoietic system of mice deficient for Rev1, a core factor in DNA translesion synthesis (TLS), the postreplicative bypass of damaged nucleotides. Rev1 hematopoietic stem and progenitor cells displayed compromised proliferation, and replication stress that could be rescued with an antioxidant. The additional disruption of Xpc, essential for global-genome nucleotide excision repair (ggNER) of helix-distorting nucleotide lesions, resulted in the perinatal loss of hematopoietic stem cells, progressive loss of bone marrow, and fatal aplastic anemia between 3 and 4 months of age. This was associated with replication stress, genomic breaks, DNA damage signaling, senescence, and apoptosis in bone marrow. Surprisingly, the collapse of the Rev1Xpc bone marrow was associated with progressive mitochondrial dysfunction and consequent exacerbation of oxidative stress. These data reveal that, to protect its genomic and functional integrity, the hematopoietic system critically depends on the combined activities of repair and replication of helix-distorting oxidative nucleotide lesions by ggNER and Rev1-dependent TLS, respectively. The error-prone nature of TLS may provide mechanistic understanding of the accumulation of mutations in the hematopoietic system upon aging.
Introduction
The aging-associated attrition of proliferating tissues is accompanied by mutagenesis and genomic rearrangements, cellular senescence, and mitochondrial dysfunction, possibly in response to the accumulation of endogenous DNA damage.1 Each cell in the body acquires 104 to 105 endogenous DNA lesions per day.2 Most DNA lesions are repaired by a network of complementary DNA repair systems, each of which deals with a specific class of lesions, as an integral part of the DNA damage response (reviewed in Mandal et al,3 Blanpain et al,4 and Vitale et al5 ). The dominant, global-genome, nucleotide excision repair (ggNER; Figure 1A) pathway specifically recognizes and removes helix-distorting endogenous and exogenous nucleotide lesions.6 ggNER deficiency, as exemplified by mice with a disruption of the Xpc gene, only causes minor phenotypes when the organism is not exposed to exogenous genotoxic agents. This result suggests that unrepaired endogenous helix-distorting DNA lesions can be tolerated at the genome of proliferating cells.6,7 Unrepaired nucleotide lesions usually arrest processive replication forks, resulting in lesion-containing single-stranded DNA (ssDNA) tracts. Persistent ssDNA tracts can collapse to cytotoxic and recombinogenic dsDNA breaks. To fill such lesion-containing ssDNA tracts and to enable termination of genomic replication, cells have evolved multiple mechanisms, collectively called DNA damage tolerance.8 Thereby, these mechanisms prevent genomic instability, senescence, and apoptosis caused by unreplicated damaged nucleotides. DNA translesion synthesis (TLS) is the major DNA damage tolerance mechanism in mammals. TLS employs specialized DNA polymerases to directly replicate across damaged nucleotides. Because these TLS polymerases frequently misincorporate opposite the lesion, cellular survival by TLS comes at the expense of nucleotide substitution mutagenesis (Figure 1A).9 The core TLS polymerase Rev1 inserts cytidines opposite abasic nucleotides and a limited spectrum of nucleotide adducts at the minor groove of the DNA helix.10,11 Additionally, Rev1 plays an important regulatory role in TLS of helix-distorting nucleotide lesions, of (nondamaged) G-quadruplex structures,12 whereas it also operates in the repair of interstrand DNA cross-links.9,13
Rev1 hematopoietic stem cell (HSC) display competitive and proliferative defects (see alsosupplemental Figure 1). The involvement of TLS in tolerance of endogenous DNA damage in the hematopoietic system was investigated by analyzing Rev1 blood and bone marrow, by competitive repopulation experiments, and by culture of hematopoietic stem and progenitor cells (HSPCs) in vitro. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± standard error of the mean (SEM). (A) Helix-distorting nucleotide lesions (blue spheres) can be repaired by ggNER, dependent on the Xpc gene. In case a lesion escapes timely repair, it arrests processive replication (black rectangle), resulting in replication stress and DNA damage signaling. The lesion can be bypassed postreplicatively by Rev1-dependent DNA TLS (zig-zag line). Thereby, TLS prevents the induction of replication stress and double-stranded DNA (dsDNA) breaks. TLS frequently misincorporates (in red) opposite the damaged nucleotide, which originates nucleotide substitution mutations. (B) Cytopenia in 26- to 30-month-old Rev1 mice (n = 11), compared with age-matched WT mice (n = 6). (C) Relative contribution of myeloid and lymphoid cells in the WT and Rev1 blood at 3 months of age (3m) and when moribund (MB). N = 10. (D) Frequencies of LSK, LSK34-, and LSK-SLAM cells in bone marrow of 5-month-old Rev1 (n = 5) and WT mice (n = 5). Frequencies are depicted as percent of mononuclear cells. (E) Impaired function of Rev1-deficient HSCs as demonstrated by competitive repopulation assays. Scheme of competitive transplantation experiments (top). Competitive transplantation of WT (n = 9) and Rev1 HSCs (n = 8) (bottom). (See also supplemental Figure 1.) (F) Impaired proliferative capacity of HSPCs as demonstrated by reduced CAFC numbers from 5-month-old WT (n = 4) and Rev1 (n = 4) mice. (G) Sizes of colonies after single-cell sorting of LSK-SLAM cells from 5-month-old Rev1 (n = 3) and WT (n = 3) mice.
Rev1 hematopoietic stem cell (HSC) display competitive and proliferative defects (see alsosupplemental Figure 1). The involvement of TLS in tolerance of endogenous DNA damage in the hematopoietic system was investigated by analyzing Rev1 blood and bone marrow, by competitive repopulation experiments, and by culture of hematopoietic stem and progenitor cells (HSPCs) in vitro. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± standard error of the mean (SEM). (A) Helix-distorting nucleotide lesions (blue spheres) can be repaired by ggNER, dependent on the Xpc gene. In case a lesion escapes timely repair, it arrests processive replication (black rectangle), resulting in replication stress and DNA damage signaling. The lesion can be bypassed postreplicatively by Rev1-dependent DNA TLS (zig-zag line). Thereby, TLS prevents the induction of replication stress and double-stranded DNA (dsDNA) breaks. TLS frequently misincorporates (in red) opposite the damaged nucleotide, which originates nucleotide substitution mutations. (B) Cytopenia in 26- to 30-month-old Rev1 mice (n = 11), compared with age-matched WT mice (n = 6). (C) Relative contribution of myeloid and lymphoid cells in the WT and Rev1 blood at 3 months of age (3m) and when moribund (MB). N = 10. (D) Frequencies of LSK, LSK34-, and LSK-SLAM cells in bone marrow of 5-month-old Rev1 (n = 5) and WT mice (n = 5). Frequencies are depicted as percent of mononuclear cells. (E) Impaired function of Rev1-deficient HSCs as demonstrated by competitive repopulation assays. Scheme of competitive transplantation experiments (top). Competitive transplantation of WT (n = 9) and Rev1 HSCs (n = 8) (bottom). (See also supplemental Figure 1.) (F) Impaired proliferative capacity of HSPCs as demonstrated by reduced CAFC numbers from 5-month-old WT (n = 4) and Rev1 (n = 4) mice. (G) Sizes of colonies after single-cell sorting of LSK-SLAM cells from 5-month-old Rev1 (n = 3) and WT (n = 3) mice.
The long-lived nature of hematopoietic stem cells (HSCs) makes these cells particularly susceptible to endogenous and exogenous genotoxic insults that can limit their functional capacity and that also induce genomic alterations that predispose to hematopoietic malignancies.14 Therefore, the hematopoietic system provides a paradigm to study the involvement of endogenous DNA damage in development, maintenance, decay, and cancer development of proliferating and differentiating tissues.14-18
Here we investigated the role of Rev1-dependent TLS in the development and maintenance of the hematopoietic system. We reveal the requirement of TLS opposite unrepaired endogenous helix-distorting DNA lesions for the maintenance of hematopoietic stem and precursor cells (HSPCs). We furthermore demonstrate that ggNER and, to a greater extent, TLS provide independent and complementary mechanisms to protect the hematopoietic system against the detrimental phenotypes caused by endogenous DNA lesions.
Methods in brief
Full methods are included in the supplemental Data (available on the Blood Web site).
Mice and cell lines
All mouse experiments were approved by the ethical review board of the Institute, and the specific pathogen-free mice were kept according to Federation of Laboratory Animal Science Associations guidelines. Wild-type (WT), Rev1,19 Xpc, and Rev1Xpc mouse cohorts were obtained by crossing FVB and C57Bl/6 parents. Equal numbers of hybrid males and females were used for most experiments. Transplantation experiments using Rev1 animals were performed in the C57Bl/6 background. Hairless albino SKH-1 mice were used for measuring sensitivity of the skin to UV light–induced nucleotide lesions.
Competitive transplantation experiments using Rev1 hematopoietic cells were performed as follows: whole bone marrow or isolated HSCs (alone or, when indicated, together with W41.SJL [c-kit receptor-mutant bone marrow cells]) from donors were introduced into lethally irradiated B6.SJL recipients. Secondary transplantation was performed as previously described.20 Chimerism of the hematopoietic system was analyzed at different time points after transplantation using multiplex polymerase chain reaction (PCR) on blood. Reconstitution of the Rev1Xpc bone marrow with Xpc bone marrow was performed at the age of 1.5 months, by injecting 5 × 106Xpc bone marrow cells. Unless stated otherwise, mice were given an intraperitoneal injection with 5-bromo-2′-deoxyuridine (BrdU) and ethynyl-2′-deoxyuridine (EdU), 1 hour before euthanization with CO2, to label replicating cells. Mouse embryonic fibroblast (MEF) lines were obtained by spontaneous immortalization of fibroblasts from 13.5-day embryos of the hybrid background. Survival after genotoxin exposure was measured using clonogenic assays.
Whole blood analysis
For whole blood analysis, peripheral blood was manually or automatically counted. For the quantification of white blood cell ratios and Howell-Jolly bodies, blood smears were stained with Giemsa.
Bone marrow and blood preparation for stainings
Bone marrow cells were extracted by flushing femurs and tibia. One intact femur was used to calculate bone marrow cellularities. Cell numbers were normalized to body weight. Paraffin-embedded sections were stained with hematoxylin and eosin. Whole blood was extracted from heart. Erythrocytes were lysed before characterization of hematopoietic and blood cells.
Analysis of cultured HSCs
Long-term-HSCs were isolated and cultured for 2 weeks with or without 100 μM N-acetylcysteine (NAC). Colony sizes were scored at day 7 and day 14. At day 14, cells were collected for immunofluorescence staining of the DNA damage marker γH2AX.
Cobblestone area–forming cell (CAFC) assay
Analysis of HSPC populations
HSPC populations were isolated by fluorescence-activated cell sorting, following labeling of HSPC population-specific surface markers with antibodies and labeling these with fluorophores. Concentrations and origin of these reagents are depicted in supplemental Table 1. To identify stromal cells, bone marrow cell suspensions were stained with the stromal cell–specific antibodies followed with fluorophore labeling and fluorescence-activated cell sorting. Fetal livers were analyzed after BrdU injection of the mother. Fetal liver cell suspensions were stained with cell surface markers for HSPCs (supplemental Table 1), followed by BrdU staining. For the analysis of proliferation and apoptosis, freshly isolated fetal liver cells were stained with HSPC cell surface markers and then for Ki67 or annexin V, respectively. 7-Aminoactinomycin D was added in the cell suspension to stain DNA prior to analysis to exclude dead cells. Data were acquired using flow cytometry.
Immunohistochemistry and immunofluorescence
Whole bone marrow cells were fixated on cytospin adhesion slides and stained for BrdU, caspase-3, Dec1,23 γ-H2AX, Ki67, p16, 8-hydroxy-2′-deoxyguanosine (8OHdG), phospho-p38, or 53BP1. Staining for incorporated EdU was performed according to the manufacturers’ instructions. Staining of bone marrow for 4-hydroxynonenal (4-HNE) was performed on deparaffinized sections after antigen retrieval. Sections were counterstained with Mayers hemalum.
Alkaline comet assays
Alkaline single-cell electrophoresis (“comet”) assays that enable us to detect ssDNA and dsDNA breaks at the genome, and staining for incorporated BrdU to identify S-phase nuclei, were performed on bone marrow cell suspensions, essentially as described.24
TLS assay
The generation of a site-specific single-stranded H-εdC lesion and the determination of the efficiency and mutagenicity of TLS were performed essentially as described for a benzo[a]pyrene-dG adduct.25
Analysis of mitochondrial function
Mitochondrial membrane potentials were measured by flow cytometry. Measurement of mitochondrial DNA (mtDNA) was performed by quantitative PCR. The relative mtDNA levels were calculated using equation 2 × 2ΔCT. Mitochondrial respiration of freshly isolated total bone marrow cells was analyzed using a Seahorse extracellular flux bioanalyzer. For western blotting of mitochondrial proteins, freshly frozen bone marrow cells were resuspended in radioimmunoprecipitation assay buffer followed by brief sonication. Electrophoresis, blotting, antibody incubations, and visualization of signals using enhanced chemiluminescence were performed using standard protocols.
Results
Rev1 contributes to HSPC maintenance
Rev1-deficient mice displayed mild cytopenia of the blood, affecting all lineages (Figure 1B-C). Analysis of short- and long-term HSC populations revealed a slight, but significant, decrease in the frequency of early hematopoietic progenitors (LSK cells), already at a young age (Figure 1D). Competitive repopulation experiments revealed that long-term Rev1 HSCs (the LSK-SLAM population) were unable to compete with simultaneously administered W41.SJL HSCs20 (which themselves display compromised repopulation capacity) in reconstituting the bone marrow of lethally irradiated WT mice (Figure 1E). Even in the absence of W41.SJL competitors, Rev1 HSCs repopulated the recipients’ hematopoietic system only inefficiently (supplemental Figure 1A). This phenotype was further aggravated in serial transplantations, indicating a persistent disadvantage of Rev1 HSCs (supplemental Figure 1B). HSCs isolated from 14-day-old fetal livers already displayed reduced repopulation capacity (supplemental Figure 1C), demonstrating that the attenuation of HSC function occurs early in development and independently of the bone marrow environment. The in vitro clonogenicity of Rev1 HSPCs was significantly reduced, compared with WT (Figure 1F), and Rev1 HSC clones were smaller than controls (Figure 1G). The combined defects in transplantability and in in vitro growth strongly suggest that Rev1-deficient HSPCs display compromised proliferative capacity, which most likely is cell intrinsic.
ggNER and TLS synergize to protect the hematopoietic system from the genotoxicity of helix-distorting DNA lesions
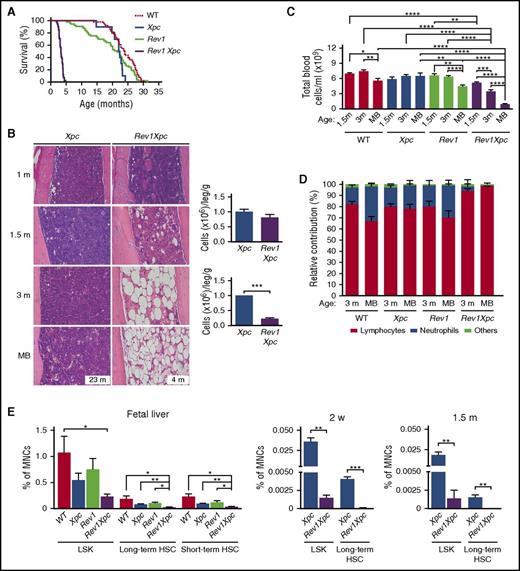
We argued that, in case the proliferative defects of Rev1 HSPCs reflect perturbed TLS of endogenous helix-distorting nucleotide lesions, inactivation of ggNER of this class of nucleotide lesions would exacerbate the hematopoietic phenotypes (Figure 1A). Indeed, disruption of Xpc synergistically increased the sensitivity of both Rev1-deficient skin and MEFs to helix-distorting photolesions induced by UV light6 (supplemental Figure 2A-B). Previously, we have shown that, upon UV exposure, Rev1Xpc MEFs display high levels of replication stress, caused by replicons arrested at unrepaired helix-distorting photolesions.26 Thus, ggNER and Rev1-dependent TLS jointly protect cells from the genotoxic effects of UV light, by repairing or tolerating photolesions, respectively (see Figure 1A). Rev1Xpc embryos were significantly smaller than WT or single-deficient littermates, and the double-deficient mice were born at sub-Mendelian ratios (supplemental Figure 2C-D), consistent with enhanced sensitivity to endogenous helix-distorting nucleotide lesions. Although Xpc mice displayed near-WT life spans, Rev1 single-deficient mice died slightly earlier than their WT littermates (20 vs 23 months of age, on the average; Figure 2A) of various causes unrelated to the hematopoietic defects. This shortened life span suggests that ggNER is not sufficient to repair all of its substrates, making proliferating cells dependent on Rev1-mediated TLS. In contrast, TLS + ggNER double-deficient, Rev1Xpc mice died at, on the average, 3.5 months of age (Figure 2A), displaying aplasia of the bone marrow and pancytopenia of bone marrow and blood that most markedly affected neutrophils (Figure 2B-D; supplemental Table 2 and supplemental Figure 2E-H). HSC counts were strongly reduced already in the liver of Rev1Xpc fetuses, and this reduction aggravated through life (Figure 2E; supplemental Figure 2I). In conclusion, the hematopoietic phenotypes of Rev1-deficient mice are exacerbated synergistically by concomitant ggNER deficiency, which provides a strong argument that failure to replicate endogenous helix-distorting nucleotide lesions results in hematopoietic attrition. We then investigated the fate of Rev1Xpc embryonic liver HSCs. All Rev1Xpc HSC subpopulations, and also long-term progenitor cells, were characterized by a reduced G0 and increased Ki67-positive S/G2/M fraction, suggesting exit from quiescence and enhanced proliferation or delay in progression through S phase (Figure 2F; supplemental Figure 2J). In support with the latter, the fraction of Rev1Xpc LSK cells and short-term HSCs that resided in S phase was increased in Rev1Xpc embryonic liver HSCs (Figure 2G; supplemental Figure 2K). The concurrent emergence of a BrdU-positive sub-G0 LKS fraction suggested increased death of proliferating Rev1Xpc HSCs (Figure 2G; supplemental Figure 2K), although we could not detect increased apoptosis (supplemental Figure 2L).
Rev1-dependent tolerance of unrepaired endogenous nucleotide lesions protects HSCs (see alsosupplemental Figures 2 and 3). Analysis of the hematopoietic system of WT, ggNER (Xpc), TLS (Rev1), and TLS + ggNER (Rev1Xpc) mice reveals that ggNER and TLS jointly protect HSPCs against cell-autonomous genotoxicity of endogenous helix-distorting DNA lesions. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. (A) Kaplan-Meier curves depicting survival of mice of all 4 genotypes: WT (n = 64), Xpc (n = 10), Rev1 (n = 53), and Rev1Xpc (n = 41). Survival of the different genotypes was compared with WT mice using the Wilcoxon test. (B) Progressive bone marrow aplasia in Rev1Xpc mice. Bar represents 50 μm. Xpc: 1 m (n = 3), 1.5 m (n = 3), 3 m (n = 3), MB (n = 6). Rev1Xpc: 1 m (n = 3), 1.5 m (n = 3), 3 m (n = 3), MB (n = 3). Right panels: Quantification of bone marrow cells at 1 (Xpc, n = 10; Rev1Xpc, n = 11) and 1.5 months (Xpc, n = 4; Rev1Xpc, n = 4) of age, respectively. (C) Rev1Xpc mice develop severe cytopenia. m, months; MB, moribund (see panel A for survival data). WT: 1.5 m (n = 8), 3 m (n = 7), MB (n = 12). Xpc: 1.5 m (n = 5), 3 m (n = 12), MB (n = 7). Rev1: 1.5 m (n = 6), 3 m (n = 17), MB (n = 33). Rev1Xpc: 1.5 m, (n = 10), 3 m (n = 11), MB (n = 10). (D) Relative contribution of myeloid and lymphoid cells in the blood of all genotypes. Note the low contribution of neutrophils specifically in Rev1Xpc blood. (E) Bone marrow aplasia in Rev1Xpc mice is caused by progressive loss of long-term HSCs (LSK-SLAM cells). MNCs, mononuclear cells. Number of mice analyzed: fetal liver: n = 4 for all genotypes. Two-weeks old: Xpc (n = 3), Rev1Xpc (n = 3); 1.5-months old: Xpc (n = 4), Rev1Xpc (n = 4). (F) Increased S/G2/M fractions in Rev1Xpc LSK cells, long-term and short term HSCs from fetal liver, suggesting increased replication, as shown by Ki67 staining (G0 cells are Ki67-negative). N = 4 for all genotypes. (G) Increased proliferation of Rev1Xpc HSPCs as demonstrated by in vivo BrdU labeling. Xpc (n = 3) and Rev1Xpc (n = 3) mice. (H) Rescue of early death, bone marrow aplasia, and cytopenia of Rev1Xpc mice by transplantation with Xpc bone marrow, indicating a hematopoietic cell-intrinsic origin of HSC exhaustion. Survival: Rev1Xpc (n = 41), transplanted Rev1Xpc (n = 10). Bone marrow: Rev1Xpc (n = 3), transplanted Rev1Xpc (n = 5). Blood cellularity: Rev1Xpc (n = 10), transplanted Rev1Xpc (n = 7). Survival of Rev1Xpc mice was compared with WT mice using the Wilcoxon test.
Rev1-dependent tolerance of unrepaired endogenous nucleotide lesions protects HSCs (see alsosupplemental Figures 2 and 3). Analysis of the hematopoietic system of WT, ggNER (Xpc), TLS (Rev1), and TLS + ggNER (Rev1Xpc) mice reveals that ggNER and TLS jointly protect HSPCs against cell-autonomous genotoxicity of endogenous helix-distorting DNA lesions. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. (A) Kaplan-Meier curves depicting survival of mice of all 4 genotypes: WT (n = 64), Xpc (n = 10), Rev1 (n = 53), and Rev1Xpc (n = 41). Survival of the different genotypes was compared with WT mice using the Wilcoxon test. (B) Progressive bone marrow aplasia in Rev1Xpc mice. Bar represents 50 μm. Xpc: 1 m (n = 3), 1.5 m (n = 3), 3 m (n = 3), MB (n = 6). Rev1Xpc: 1 m (n = 3), 1.5 m (n = 3), 3 m (n = 3), MB (n = 3). Right panels: Quantification of bone marrow cells at 1 (Xpc, n = 10; Rev1Xpc, n = 11) and 1.5 months (Xpc, n = 4; Rev1Xpc, n = 4) of age, respectively. (C) Rev1Xpc mice develop severe cytopenia. m, months; MB, moribund (see panel A for survival data). WT: 1.5 m (n = 8), 3 m (n = 7), MB (n = 12). Xpc: 1.5 m (n = 5), 3 m (n = 12), MB (n = 7). Rev1: 1.5 m (n = 6), 3 m (n = 17), MB (n = 33). Rev1Xpc: 1.5 m, (n = 10), 3 m (n = 11), MB (n = 10). (D) Relative contribution of myeloid and lymphoid cells in the blood of all genotypes. Note the low contribution of neutrophils specifically in Rev1Xpc blood. (E) Bone marrow aplasia in Rev1Xpc mice is caused by progressive loss of long-term HSCs (LSK-SLAM cells). MNCs, mononuclear cells. Number of mice analyzed: fetal liver: n = 4 for all genotypes. Two-weeks old: Xpc (n = 3), Rev1Xpc (n = 3); 1.5-months old: Xpc (n = 4), Rev1Xpc (n = 4). (F) Increased S/G2/M fractions in Rev1Xpc LSK cells, long-term and short term HSCs from fetal liver, suggesting increased replication, as shown by Ki67 staining (G0 cells are Ki67-negative). N = 4 for all genotypes. (G) Increased proliferation of Rev1Xpc HSPCs as demonstrated by in vivo BrdU labeling. Xpc (n = 3) and Rev1Xpc (n = 3) mice. (H) Rescue of early death, bone marrow aplasia, and cytopenia of Rev1Xpc mice by transplantation with Xpc bone marrow, indicating a hematopoietic cell-intrinsic origin of HSC exhaustion. Survival: Rev1Xpc (n = 41), transplanted Rev1Xpc (n = 10). Bone marrow: Rev1Xpc (n = 3), transplanted Rev1Xpc (n = 5). Blood cellularity: Rev1Xpc (n = 10), transplanted Rev1Xpc (n = 7). Survival of Rev1Xpc mice was compared with WT mice using the Wilcoxon test.
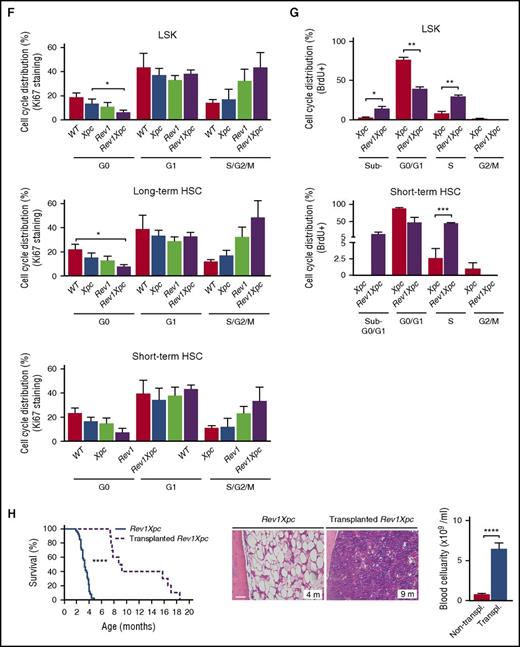
To confirm aplastic anemia as the cause of death of Rev1Xpc mice, we transplanted them with Xpc bone marrow cells. This procedure indeed significantly rescued the degenerative hematopoietic phenotypes of these mice and greatly increased their life span (Figure 2H; supplemental Figure 3A). Importantly, these results also suggest that the hematopoietic phenotypes of Rev1Xpc mice are cell autonomous. Consistently, the composition of the Rev1Xpc bone marrow stromal compartment appeared to be affected only marginally (supplemental Figure 3B-C).
Rev1-dependent TLS protects the bone marrow against replication stress, senescence, apoptosis, and DNA breaks
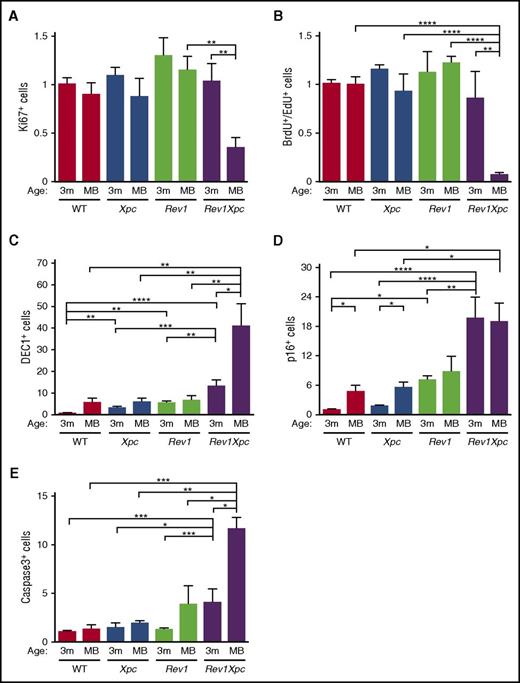
We wanted to investigate whether Rev1-mediated TLS of endogenous nucleotide lesions protects the genome of hematopoietic cells against DNA breaks. Indeed, whereas erythrocytes normally are devoid of nuclear DNA, blood-derived erythrocytes of Rev1 and Rev1Xpc mice frequently contained chromosomal fragments that presumably were derived from chromosomes broken during the preceding erythroblast stage (so-called Howell-Jolly bodies27 ) (Figure 3A). This phenotype was absent from the Xpc-reconstituted Rev1Xpc bone marrow, confirming that the DNA breakage was cell intrinsic (supplemental Figure 4A). To assess ssDNA and dsDNA breaks also in bone marrow, we used alkaline single-cell electrophoresis (“comet”) assays.24 Compared with WT HSPCs, Xpc, Rev1, and Rev1Xpc HSPCs displayed increased comet sizes, and thus increased DNA breaks, during S phase, suggesting arrested replicons, or dsDNA breaks, at endogenous helix-distorting nucleotide lesions (supplemental Figure 4B). However, these breaks persisted beyond S phase only in the absence of Rev1 (Figure 3B; supplemental Figure 4C), indicating that the strand discontinuities were protracted because of the persistent inability to complete genomic replication. In agreement, staining for the DNA breaks and replication stress markers γH2AX and 53BP128,29 was increased in bone marrow cells, not only of 3-month-old Rev1Xpc mice but also of old Rev1 mice (Figure 3C-D; supplemental Figure 4D-E). Beyond 3 months of age, proliferation and replication in Rev1Xpc bone marrow ceased (Figure 4A-B; supplemental Figure 5A-B), concomitant with the induction of senescence and apoptosis (Figures 4C-E; supplemental Figure 5C-E). Collectively, these data indicate that, in the absence of Rev1, endogenous helix-distorting DNA lesions induce replication stress and genomic breaks that compromise proliferation and viability of HSPCs.
Rev1 protects against replication stress and genomic breaks in the hematopoietic system (see alsosupplemental Figure 4). We investigated the induction of DNA breaks in the absence of Rev1-mediated TLS at endogenous helix-distorting DNA lesions in blood and bone marrow. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. (A) Chromosome fragments (Howell-Jolly bodies, arrowheads) in erythrocytes of 3-month-old Rev1 and Rev1Xpc mice. Bar represents 10 μm. Right panel: quantification. WT: 3 m (n = 5), MB (n = 6). Xpc: 3 m (n = 5), MB (n = 6). Rev1: 3 m (n = 5), MB (n = 9). Rev1Xpc: 3 m (n = 6), MB (n = 8). (B) Chromosome breaks outside of S phase, measured by single-cell alkaline comet gel electrophoresis of bone marrow of 3-month-old mice. WT (n = 4), Xpc (n = 4), Rev1 (n = 4), Rev1Xpc (n = 4). Comet intensities of BrdU-negative cells are shown. Increased DNA breaks in bone marrow hematopoietic cells of Rev1 and Rev1Xpc mice as demonstrated by γH2AX (C) and 53BP1 (D) immunostaining. The fraction of positive cells shown was normalized relative to 3-month-old WT. WT: 3 m (n = 8), MB (n = 6). Xpc: 3 m (n = 7), MB (n = 6). Rev1: 3 m (n = 6), MB (n = 6). Rev1Xpc: 3 m (n = 5-6), MB (n = 9).
Rev1 protects against replication stress and genomic breaks in the hematopoietic system (see alsosupplemental Figure 4). We investigated the induction of DNA breaks in the absence of Rev1-mediated TLS at endogenous helix-distorting DNA lesions in blood and bone marrow. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. (A) Chromosome fragments (Howell-Jolly bodies, arrowheads) in erythrocytes of 3-month-old Rev1 and Rev1Xpc mice. Bar represents 10 μm. Right panel: quantification. WT: 3 m (n = 5), MB (n = 6). Xpc: 3 m (n = 5), MB (n = 6). Rev1: 3 m (n = 5), MB (n = 9). Rev1Xpc: 3 m (n = 6), MB (n = 8). (B) Chromosome breaks outside of S phase, measured by single-cell alkaline comet gel electrophoresis of bone marrow of 3-month-old mice. WT (n = 4), Xpc (n = 4), Rev1 (n = 4), Rev1Xpc (n = 4). Comet intensities of BrdU-negative cells are shown. Increased DNA breaks in bone marrow hematopoietic cells of Rev1 and Rev1Xpc mice as demonstrated by γH2AX (C) and 53BP1 (D) immunostaining. The fraction of positive cells shown was normalized relative to 3-month-old WT. WT: 3 m (n = 8), MB (n = 6). Xpc: 3 m (n = 7), MB (n = 6). Rev1: 3 m (n = 6), MB (n = 6). Rev1Xpc: 3 m (n = 5-6), MB (n = 9).
Rev1 protects against endogenous DNA damage–induced senescence and apoptosis (see alsosupplemental Figure 5). Proliferation, replication, senescence, and apoptosis were quantified in bone marrow of all 4 genotypes. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. Reduced proliferation (Ki67 immunostaining) (A) and replication (BrdU and EdU incorporation) (B) in the bone marrow of moribund Rev1Xpc mice. WT: 3 m (n = 7-9), MB (n = 5-6). Xpc: 3 m (n = 6-8), MB (n = 6). Rev1: 3 m (n = 7), MB (n = 6). Rev1Xpc: 3 m (n = 6), MB (n = 9). Increased senescence and apoptosis in the bone marrow of Rev1Xpc mice as demonstrated by immunostaining for Dec1 (C), p16 (D), and caspase-3 (E). WT: 3 m (n = 4-8), MB (n = 5-6). Xpc: 3 m (n = 5-8), MB (n = 5-6). Rev1: 3 m (n = 3-7), MB (n = 6). Rev1Xpc: 3 m (n = 3-6), MB (n = 6-8). m, months; MB, moribund (see Figure 2A for survival data). The fraction of positive cells shown was normalized relative to 3-month-old WT bone marrow.
Rev1 protects against endogenous DNA damage–induced senescence and apoptosis (see alsosupplemental Figure 5). Proliferation, replication, senescence, and apoptosis were quantified in bone marrow of all 4 genotypes. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. Reduced proliferation (Ki67 immunostaining) (A) and replication (BrdU and EdU incorporation) (B) in the bone marrow of moribund Rev1Xpc mice. WT: 3 m (n = 7-9), MB (n = 5-6). Xpc: 3 m (n = 6-8), MB (n = 6). Rev1: 3 m (n = 7), MB (n = 6). Rev1Xpc: 3 m (n = 6), MB (n = 9). Increased senescence and apoptosis in the bone marrow of Rev1Xpc mice as demonstrated by immunostaining for Dec1 (C), p16 (D), and caspase-3 (E). WT: 3 m (n = 4-8), MB (n = 5-6). Xpc: 3 m (n = 5-8), MB (n = 5-6). Rev1: 3 m (n = 3-7), MB (n = 6). Rev1Xpc: 3 m (n = 3-6), MB (n = 6-8). m, months; MB, moribund (see Figure 2A for survival data). The fraction of positive cells shown was normalized relative to 3-month-old WT bone marrow.
Rev1-dependent TLS provides tolerance of endogenous lipid peroxidation–derived nucleotide adducts
Oxidative DNA lesions are abundant in proliferating cells.15-18 Moreover, the notion that the Rev1Xpc phenotypes are cell autonomous, combined with the specific depletion of neutrophils (see Figure 2D) that produce high levels of reactive oxygen species (ROS),30 hinted at helix-distorting oxidative DNA lesions as possible culprits for the Rev1 and Rev1Xpc HSPC phenotypes. The scarcity of long-term Rev1Xpc HSCs precluded their analysis, and therefore, we investigated responses to endogenous oxidative stress in cultured Rev1 single-deficient HSCs. Consistent with the results described previously, these cells displayed enhanced γH2AX staining indicating replication stress (Figure 5A; supplemental Figure 6A). However, culture in the presence of the ROS scavenger NAC31 rescued this γH2AX accumulation (Figure 5A; supplemental Figure 6A-B). This important result confirms that the endogenous ROS induce replication stress in HSC, in the absence of Rev1-dependent TLS.
Rev1-dependent TLS and ggNER converge on helix-distorting oxidative DNA lesions resulting from progressive mitochondrial dysfunction (see alsosupplemental Figure 6). We investigated the involvement of Rev1-dependent TLS at helix-distorting lipid peroxidation–derived nucleotide adduct by treating Rev1 HSCs with a radical scavenger, by using an in cellulo TLS assay, by investigating the sensitivity of Rev1 cells to a lipid peroxidation–derived aldehyde, to oxidative stress, and by measuring oxidative stress in bone marrow. We then characterized the quantity and functionality of Rev1Xpc mitochondria. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. (A) DNA breaks (γH2AX) in cultured HSCs (LSK-SLAM), WT (n = 3), and Rev1 (n = 3), treated or nontreated with the ROS scavenger NAC. The fraction of positive cells shown was normalized relative to WT. (B) The prototypic DNA-reactive lipid peroxidation–derived aldehyde 4-ONE and its adduction to a cytosine base (H-εdC). (C) Top: TLS assay at a site-specific H-εdC. MEFs were transfected with the substrate, followed by incubation to allow TLS, and by recovery of covalently closed progeny plasmids in Escherichia coli. The fraction of recovered substrate, compared with an undamaged internal control, is a measure of TLS activity of the MEFs. Bottom: Relative efficiency and mutation spectrum of TLS events at a site-specific H-εdC lesion. (D) Clonal survival of WT, Rev1, Xpc, and Rev1Xpc MEFs in response to the addition of the mitochondrial poison paraquat to the growth medium. (E) Clonal survival of Xpc and Rev1Xpc MEFs in response to the addition of 4-HNE to the growth medium. Oxidative stress in the bone marrow of Rev1Xpc mice as evidenced by: lipofuscin accumulation (brown inclusions) in bone marrow of moribund mice (F), 4-HNE-positive cells (G), activation of p38 signaling [phospho (γ)p38 staining] (H), and accumulation of free radical-induced oxidative DNA lesions (OHdG-positive cells) (I) in Rev1Xpc mice: 1m (n = 3-4), 3m (n = 3-5), MB (n = 5-6). The fraction of positive cells shown was normalized relative to 3-month-old WT bone marrow. (J) Relative mtDNA contents, as determined by real-time PCR, in bone marrow from Xpc (n = 5-6) and Rev1Xpc (n = 5-6) mice. All mtDNA levels were normalized to those in 3-month-old Xpc mice. (K) Western blot of mitochondrial complexes I to IV in bone marrow from Xpc and Rev1Xpc mice (4 mice per group). Lamin B1: internal standard. (L) Expression of the mitochondrial stress proteins UCP2 and PGC-1α in WT, Xpc, Rev1, and Rev1Xpc bone marrow. Lamin B: internal standard. wk, weeks; m, months; MB, moribund (see Figure 2C for survival data). (M) Mitochondrial membrane potentials in bone marrow of Xpc and Rev1Xpc mice. All potentials in Rev1Xpc bone marrow were normalized to those in Xpc bone marrow of the same age. 1 m (n = 4), 1.5 m (n = 4), 3 m (n = 4-6), MB (n = 3-4). (N) Basal oxygen consumption rates in viable cells from bone marrow from Xpc and Rev1Xpc mice. All oxygen consumption rates in Rev1Xpc bone marrow were normalized to those in Xpc bone marrow of the same age. 2wk (n = 5-6), 3 m (n = 3).
Rev1-dependent TLS and ggNER converge on helix-distorting oxidative DNA lesions resulting from progressive mitochondrial dysfunction (see alsosupplemental Figure 6). We investigated the involvement of Rev1-dependent TLS at helix-distorting lipid peroxidation–derived nucleotide adduct by treating Rev1 HSCs with a radical scavenger, by using an in cellulo TLS assay, by investigating the sensitivity of Rev1 cells to a lipid peroxidation–derived aldehyde, to oxidative stress, and by measuring oxidative stress in bone marrow. We then characterized the quantity and functionality of Rev1Xpc mitochondria. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. (A) DNA breaks (γH2AX) in cultured HSCs (LSK-SLAM), WT (n = 3), and Rev1 (n = 3), treated or nontreated with the ROS scavenger NAC. The fraction of positive cells shown was normalized relative to WT. (B) The prototypic DNA-reactive lipid peroxidation–derived aldehyde 4-ONE and its adduction to a cytosine base (H-εdC). (C) Top: TLS assay at a site-specific H-εdC. MEFs were transfected with the substrate, followed by incubation to allow TLS, and by recovery of covalently closed progeny plasmids in Escherichia coli. The fraction of recovered substrate, compared with an undamaged internal control, is a measure of TLS activity of the MEFs. Bottom: Relative efficiency and mutation spectrum of TLS events at a site-specific H-εdC lesion. (D) Clonal survival of WT, Rev1, Xpc, and Rev1Xpc MEFs in response to the addition of the mitochondrial poison paraquat to the growth medium. (E) Clonal survival of Xpc and Rev1Xpc MEFs in response to the addition of 4-HNE to the growth medium. Oxidative stress in the bone marrow of Rev1Xpc mice as evidenced by: lipofuscin accumulation (brown inclusions) in bone marrow of moribund mice (F), 4-HNE-positive cells (G), activation of p38 signaling [phospho (γ)p38 staining] (H), and accumulation of free radical-induced oxidative DNA lesions (OHdG-positive cells) (I) in Rev1Xpc mice: 1m (n = 3-4), 3m (n = 3-5), MB (n = 5-6). The fraction of positive cells shown was normalized relative to 3-month-old WT bone marrow. (J) Relative mtDNA contents, as determined by real-time PCR, in bone marrow from Xpc (n = 5-6) and Rev1Xpc (n = 5-6) mice. All mtDNA levels were normalized to those in 3-month-old Xpc mice. (K) Western blot of mitochondrial complexes I to IV in bone marrow from Xpc and Rev1Xpc mice (4 mice per group). Lamin B1: internal standard. (L) Expression of the mitochondrial stress proteins UCP2 and PGC-1α in WT, Xpc, Rev1, and Rev1Xpc bone marrow. Lamin B: internal standard. wk, weeks; m, months; MB, moribund (see Figure 2C for survival data). (M) Mitochondrial membrane potentials in bone marrow of Xpc and Rev1Xpc mice. All potentials in Rev1Xpc bone marrow were normalized to those in Xpc bone marrow of the same age. 1 m (n = 4), 1.5 m (n = 4), 3 m (n = 4-6), MB (n = 3-4). (N) Basal oxygen consumption rates in viable cells from bone marrow from Xpc and Rev1Xpc mice. All oxygen consumption rates in Rev1Xpc bone marrow were normalized to those in Xpc bone marrow of the same age. 2wk (n = 5-6), 3 m (n = 3).
Helix-distorting oxidative nucleotide lesions comprise cyclic purines and long-chain hydroxyalkenal (aldehyde) adducts, and these lesions are derived from lipid peroxidation. Notably, these lesions have been associated with human aging,32,33 and they represent endogenous substrates for ggNER.34,35 To investigate the involvement of Rev1 in tolerance of 4-oxo-2(E)-nonenal (4-ONE)-deoxycytidine, a prototypic helix-distorting hydroxyalkenal-nucleotide adduct (Figure 5B), we performed a quantitative in cellulo TLS assay.33 Indeed, mutagenic TLS at the 4-ONE-cytidine adduct largely depended on Rev1 in MEFs (Figure 5C). Consistently, Rev1 or Xpc and, to a greater extent, Rev1Xpc MEFs were hypersensitive to paraquat that induces oxidative stress by poisoning mitochondria (Figure 5D), whereas Rev1Xpc MEFs also were hypersensitive to 4-HNE, a compound closely related to 4-ONE (Figure 5E). Taken together, these data strongly suggest that a defect in Rev1-dependent TLS of persistent helix-distorting oxidative DNA lesions at the nuclear genome is responsible for the degenerative hematopoietic phenotypes of Rev1 and Rev1Xpc mice.
Although a priori one would not expect overall cellular ROS levels to be increased in the absence of ggNER and TLS, we observed significant accumulation of the oxidative stress and aging marker lipofuscin36 in bone marrow of Rev1Xpc mice (Figure 5F). Also intracellular levels of 4-HNE, as well as expression of the oxidative stress response marker phospho-p38,37 were increased in Rev1Xpc, compared with Xpc, bone marrow cells (Figure 5G-H; supplemental Figure 6C). Consequently, also levels of oxidative DNA lesions at the genome were increased in Rev1 and, to a greater extent, in Rev1Xpc, bone marrow, as demonstrated by staining for genomic 8OHdG (Figure 5I). Because 8OHdG is no substrate for ggNER, this result emphasizes that a de novo source of oxidative stress only indirectly is caused by the ggNER and TLS deficiency.
Progressive mitochondrial dysfunction and oxidative stress in Rev1Xpc HSPCs
The progressive increase of ROS levels in Rev1Xpc bone marrow suggested the emergence of a de novo source of oxidative stress in response to replication stress at helix-distorting nucleotide lesions. To investigate the origin of this phenomenon, we focused our attention to the mitochondrial compartment as the dominant source of intracellular ROS. Because mitochondrial proliferation is an early cellular stress cellular response,38 we first quantified mitochondrial DNA and protein in bone marrow. Indeed, in bone marrow of 3-month-old Rev1Xpc mice, these amounts were significantly increased (Figures 5J-K; supplemental Figure 6D). This suggests that replication stress at endogenous nucleotide lesions may lead to mitochondrial proliferation.
The mitochondrial uncoupling protein UCP2, which is induced by 4-HNE,39 and also the expression of the transcriptional coactivator PGC-1α, a key controller of mitochondrial biogenesis,40,41 participate in the mitochondrial response to oxidative stress.39,41,42 In bone marrow of Rev1Xpc mice, the expression of both mitochondrial proteins was strongly increased, consistent with the presence of chronic oxidative stress signaling (Figure 5L).
We then investigated mitochondrial function in bone marrow of all 4 genotypes. Compared with Xpc bone marrow, the mitochondrial membrane potential was attenuated in Rev1Xpc bone marrow, already at the age of 1 month (Figure 5M). Also in Rev1 single-deficient mice, the mitochondrial membrane potential appeared to be reduced slightly, although this failed to reach significance (supplemental Figure 6G). In live bone marrow cells of 3-month-old Rev1Xpc mice, the basal, ATP-dependent, and reserve respiratory capacities in viable cells had virtually vanished (Figure 5N; supplemental Figure 6E-F). Although cell counts were reduced in Rev1Xpc bone marrow, the replication and proliferation of viable cells was not significantly different between the genotypes (Figures 4A-B,E; supplemental Figure 6G). This suggests that the mitochondrial proliferation and concomitant dysfunction may be a corollary of nuclear replication stress at the nuclear genome, originating the exacerbated ROS production in proliferating Rev1Xpc bone marrow cells.
Discussion
The attrition of tissues during aging is associated with the accumulation of oxidative and other endogenous DNA lesions at the nuclear genome of long-term stem and precursor cells.5,17,18 However, the exact character of these lesions, their biological impact, the mechanistic basis of their cytotoxicity, and the pathways involved in pleiotropic responses to these damages have largely remained unexplored.3 The effect of ROS on the genomic integrity of long-term HSCs is restrained by a hypoxic environment (the stem cell niche) and by a metabolically quiescent state, employing glycolysis rather than oxidative respiration.37,43,44 This suggests that the exposure of HSCs to ROS negatively affects their function. Indeed, aging HSCs display DNA damage responses, although controversy exists about the nature of the underlying damage and whether the damage is induced during dormancy45 or during proliferation.46 Recently, the aging-associated decay of HSCs has been attributed to replication stress resulting from the decreasing expression of the minichromosome maintenance helicase.47,48 Here we use Rev1 mice to demonstrate that replication stress at helix-distorting oxidative DNA lesions at the nuclear genome is associated with the functional and genomic attrition of the hematopoietic system (Figures 1, 3, and 5; supplemental Figure 4). The hematopoietic phenotypes of Rev1 mice are synergistically aggravated when, additionally, ggNER is compromised (resulting from the disruption of Xpc; Figures 2-4 and supplemental Figures 2-5). These data reveal that ggNER and TLS jointly preserve the genomic and functional integrity of the hematopoietic system by, respectively, repairing endogenous helix-distorting DNA lesions and suppressing replication stress at these lesions (Figure 6). The observation that loss of HSCs occurred during ontogeny, and independent of tissue context (both in the prenatal liver and in postnatal bone), further confirms that these phenotypes are cell autonomous.
Model for the role of mutagenic TLS in maintenance of the hematopoietic system. (A) Genomic nucleotides, damaged by endogenous sources or by chemical decay, form a threat to DNA transactions such as transcription or replication, in case they remain unrepaired. (B) Processive replication is arrested by a nucleotide, damaged by a helix-distorting oxidative nucleotide lesion. (C) The damaged nucleotide is bypassed by TLS. This prevents replication stress, but at the expense of the frequent incorporation of an incorrect nucleotide opposite the lesion (in red). (D) Subsequent repair of the damaged nucleotide, or replication of the bottom DNA strand, fixates the mutation. This contributes to the acquisition of clonal mutations in the aging hematopoietic system. Mutations in hematopoietic cells acquired during aging have been associated with the development of myeloid neoplasms in humans. (E) Stalled replicons that are not released by TLS can collapse to dsDNA breaks. DNA damage signaling at ssDNA gaps opposing the lesions and at dsDNA breaks induces senescence or apoptosis, ultimately resulting in collapse of the hematopoietic system. (F) We hypothesize that failure to release arrested replicons may underlie the observed mitochondrial dysfunction, possibly via depletion of NAD+ that is required simultaneously at DNA breaks and for mitochondrial respiration. This may lead to increased ROS production and to the induction of additional oxidative DNA lesions. A positive feedback loop between replication stress at the nuclear genome and mitochondrial dysfunction is proposed to further accelerate the collapse of the hematopoietic system.
Model for the role of mutagenic TLS in maintenance of the hematopoietic system. (A) Genomic nucleotides, damaged by endogenous sources or by chemical decay, form a threat to DNA transactions such as transcription or replication, in case they remain unrepaired. (B) Processive replication is arrested by a nucleotide, damaged by a helix-distorting oxidative nucleotide lesion. (C) The damaged nucleotide is bypassed by TLS. This prevents replication stress, but at the expense of the frequent incorporation of an incorrect nucleotide opposite the lesion (in red). (D) Subsequent repair of the damaged nucleotide, or replication of the bottom DNA strand, fixates the mutation. This contributes to the acquisition of clonal mutations in the aging hematopoietic system. Mutations in hematopoietic cells acquired during aging have been associated with the development of myeloid neoplasms in humans. (E) Stalled replicons that are not released by TLS can collapse to dsDNA breaks. DNA damage signaling at ssDNA gaps opposing the lesions and at dsDNA breaks induces senescence or apoptosis, ultimately resulting in collapse of the hematopoietic system. (F) We hypothesize that failure to release arrested replicons may underlie the observed mitochondrial dysfunction, possibly via depletion of NAD+ that is required simultaneously at DNA breaks and for mitochondrial respiration. This may lead to increased ROS production and to the induction of additional oxidative DNA lesions. A positive feedback loop between replication stress at the nuclear genome and mitochondrial dysfunction is proposed to further accelerate the collapse of the hematopoietic system.
Mitochondrial dysfunction causes attrition of the hematopoietic system,49,50 and a genomic DNA damage-dependent communication between the nucleus and mitochondria, leading to mitochondrial attrition, has been associated with aging-related pathologies.38 ,51,52 Although Rev1 is not found in mitochondria,53 viable Rev1Xpc and, to some extent, also Rev1 bone marrow cells develop mitochondrial dysfunction suggesting that nuclear replication stress affects mitochondrial activity. In Rev1 liver and cultured fibroblasts, mitochondrial dysfunction is associated with elevated activity of poly(ADP) ribose polymerase 1 (PARP1), possibly at ssDNA breaks. This presumably results in depletion of the PARP1 substrate and essential mitochondrial cofactor, NAD+ (N.B.F., J. A. Durhuus, C. E. Regnell, M. Angleys, C. Desler, M. Hasan-Olive, A. Martin-Pardillos, A.T.-S., K. Thomsen, M. Laauritzen, V. A. Bohr, N.d.W., Bergensen, L.H., and L.J.R., submitted August 2017). A similar mechanism causes mitochondrial dysfunction in cells with a defect in the minor, transcription-coupled, NER subpathway.54 We hypothesize that the increase in genomic replication stress, caused by ROS production by dysfunctional mitochondria, accelerates the collapse of the Rev1Xpc hematopoietic system (Figure 6F). Nevertheless, direct evidence for this hypothesis is lacking, and we cannot formally exclude that the mitochondrial dysfunction reflects, rather than originates, the decay of the Rev1Xpc bone marrow.
The decay of the hematopoietic system of Rev1 and, to a greater extent, Rev1Xpc mice may represent dramatically accelerated hematopoietic aging. The hematopoietic phenotypes of Rev1 single-deficient mice, and the finding that Xpc or Rev1 single-deficient cells already display moderate sensitivity to UV light (supplemental Figure 2B), emphasize that ggNER and TLS are unable to either repair or bypass, respectively, all helix-distorting nucleotide lesions. We therefore hypothesize that the phenotypes of Rev1 and Rev1Xpc bone marrow represent exacerbated phenotypes that contribute to the physiological functional attrition of HSCs in the aging bone marrow.1,15 Also, small-chain endogenous aldehydes have been identified as a threat to the integrity of the hematopoietic system.55,56 Therefore, multiple types of unreplicated endogenous DNA damage in parallel may participate in the aging-associated functional decline of the hematopoietic system. Nucleotide substitutions and inefficient DNA repair are strongly correlated with aging-associated hematopoietic and other malignancies.6,16,57-61 Because Rev1-mediated TLS of damaged nucleotides, including lipid peroxidation–adducted nucleotides, is highly mutagenic (Figure 5B9 ), we hypothesize that the accumulation of such mutations is the price to pay for the protection of the hematopoietic system against endogenous helix-distorting oxidative nucleotide lesions by error-prone TLS. Finally, future investigations may also address the question of whether Rev1-mediated TLS is preserved, and perhaps provides a mechanism of survival, in leukemic cells and therefore represents a potential therapeutic target.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the skillful assistance of the personnel of the animal facilities and Mark Drost and Jacob G. Jansen for helpful comments on the manuscript. The authors thank Lemelinda Marques for assisting with mouse experiments.
A.M.-P., S.L., N.B.F., L.J.R., G.d.H., and N.d.W. were supported by an EU Marie Curie/ITN grant 316964 (“MARriAGE”). N.d.W. and A.T.-S. also received a grant from the Dutch Cancer Society (UL 2010-4851). G.d.H. and R.P.v.O. are supported by the Mouse Clinic for Cancer and Ageing, funded by a grant from The Netherlands Organization of Scientific Research. M.M. was supported by the National Institutes of Health, National Institute of Environmental Health Sciences (ES018833). B.v.L. was funded by the Swiss National Science foundation and the Onsager Fellowship. N.B.F. and L.J.R. additionally were supported by Nordea-fonden. M.H.G.P.R. was supported by grants from the Dutch Cancer Society, the Netherlands Organization of Scientific Research and the Netherlands Genomics Initiative.
Authorship
Contribution: M.M., L.J.R., G.d.H., M.H.G.P.R., and N.d.W. designed and supervised the experiments and interpreted the results; N.d.W. wrote the manuscript; A.M.-P., A.T.-S., S.C., S.L., R.P.v.O., A.D.-A., N.B.F., M.B., R.A., B.v.L., D.C.F.S., K.H., and C.D.-v.d.S. carried out experiments; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niels de Wind, Leiden University Medical Center, Einthovenweg 20, 2333ZC Leiden, The Netherlands; e-mail: n.de_wind@lumc.nl; and Marc H. G. P. Raaijmakers, Department of Hematology, Erasmus Medical Center Cancer Institute, Rotterdam, The Netherlands; e-mail: m.h.g.raaijmakers@erasmusmc.nl.
References
Author notes
A.M.-P. and A.T.-S. contributed equally to this study.
M.H.G.P.R. and N.d.W. are joint corresponding authors.




![Figure 5. Rev1-dependent TLS and ggNER converge on helix-distorting oxidative DNA lesions resulting from progressive mitochondrial dysfunction (see also supplemental Figure 6). We investigated the involvement of Rev1-dependent TLS at helix-distorting lipid peroxidation–derived nucleotide adduct by treating Rev1 HSCs with a radical scavenger, by using an in cellulo TLS assay, by investigating the sensitivity of Rev1 cells to a lipid peroxidation–derived aldehyde, to oxidative stress, and by measuring oxidative stress in bone marrow. We then characterized the quantity and functionality of Rev1Xpc mitochondria. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are mean ± SEM. (A) DNA breaks (γH2AX) in cultured HSCs (LSK-SLAM), WT (n = 3), and Rev1 (n = 3), treated or nontreated with the ROS scavenger NAC. The fraction of positive cells shown was normalized relative to WT. (B) The prototypic DNA-reactive lipid peroxidation–derived aldehyde 4-ONE and its adduction to a cytosine base (H-εdC). (C) Top: TLS assay at a site-specific H-εdC. MEFs were transfected with the substrate, followed by incubation to allow TLS, and by recovery of covalently closed progeny plasmids in Escherichia coli. The fraction of recovered substrate, compared with an undamaged internal control, is a measure of TLS activity of the MEFs. Bottom: Relative efficiency and mutation spectrum of TLS events at a site-specific H-εdC lesion. (D) Clonal survival of WT, Rev1, Xpc, and Rev1Xpc MEFs in response to the addition of the mitochondrial poison paraquat to the growth medium. (E) Clonal survival of Xpc and Rev1Xpc MEFs in response to the addition of 4-HNE to the growth medium. Oxidative stress in the bone marrow of Rev1Xpc mice as evidenced by: lipofuscin accumulation (brown inclusions) in bone marrow of moribund mice (F), 4-HNE-positive cells (G), activation of p38 signaling [phospho (γ)p38 staining] (H), and accumulation of free radical-induced oxidative DNA lesions (OHdG-positive cells) (I) in Rev1Xpc mice: 1m (n = 3-4), 3m (n = 3-5), MB (n = 5-6). The fraction of positive cells shown was normalized relative to 3-month-old WT bone marrow. (J) Relative mtDNA contents, as determined by real-time PCR, in bone marrow from Xpc (n = 5-6) and Rev1Xpc (n = 5-6) mice. All mtDNA levels were normalized to those in 3-month-old Xpc mice. (K) Western blot of mitochondrial complexes I to IV in bone marrow from Xpc and Rev1Xpc mice (4 mice per group). Lamin B1: internal standard. (L) Expression of the mitochondrial stress proteins UCP2 and PGC-1α in WT, Xpc, Rev1, and Rev1Xpc bone marrow. Lamin B: internal standard. wk, weeks; m, months; MB, moribund (see Figure 2C for survival data). (M) Mitochondrial membrane potentials in bone marrow of Xpc and Rev1Xpc mice. All potentials in Rev1Xpc bone marrow were normalized to those in Xpc bone marrow of the same age. 1 m (n = 4), 1.5 m (n = 4), 3 m (n = 4-6), MB (n = 3-4). (N) Basal oxygen consumption rates in viable cells from bone marrow from Xpc and Rev1Xpc mice. All oxygen consumption rates in Rev1Xpc bone marrow were normalized to those in Xpc bone marrow of the same age. 2wk (n = 5-6), 3 m (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/13/10.1182_blood-2017-01-764274/4/m_blood764274f5.jpeg?Expires=1765929606&Signature=m~Lda6tTq94U5WMqV4MOBfPr-Mc5F~SvKpoUkZcokR7d9ayxvvcVzI6Bm4wo~NJXpu1FuqEP1lBuD0rZYE~xVGGfS5808bBK-Uu70b1SKP497AVMM23CXDmej2RYNaZla5RN0T~7jfMkS0PYsIPRHG~4PKEcNjZva1WUYNqtIsYskP8yfdz-rgQZ6gnap6iJ-Ty82hEBMDay8FK-HmkBwdqN5uvk6~BDe~mbEmYka7dOKYqTMIR0~MHWj-ZaIJC~xlcEWXxketor8wyzlzFO~ZRO-Mk6fhsgd-BAtxaKPDLePwH~ze38L-ueRmfhrW7S3J9SS84vQrfzf7~D3EtPaw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal