Key Points
BRAFV600E mutations are detectable in hematopoietic stem and progenitors in adults with histiocytosis.
Transplantation of CD34+ cells from histiocytosis patients can give rise to genetically and phenotypically accurate xenografts.
Abstract
Langerhans cell histiocytosis (LCH) and the non-LCH neoplasm Erdheim-Chester disease (ECD) are heterogeneous neoplastic disorders marked by infiltration of pathologic macrophage-, dendritic cell–, or monocyte-derived cells in tissues driven by recurrent mutations activating MAPK signaling. Although recent data indicate that at least a proportion of LCH and ECD patients have detectable activating kinase mutations in circulating hematopoietic cells and bone marrow–based hematopoietic progenitors, functional evidence of the cell of origin of histiocytosis from actual patient materials has long been elusive. Here, we provide evidence for mutations in MAPK signaling intermediates in CD34+ cells from patients with ECD and LCH/ECD, including detection of shared origin of LCH and acute myelomonocytic leukemia driven by TET2-mutant CD34+ cell progenitors in one patient. We also demonstrate functional self-renewal capacity for CD34+ cells to drive the development of histiocytosis in xenotransplantation assays in vivo. These data indicate that the cell of origin of at least a proportion of patients with systemic histiocytoses resides in hematopoietic progenitor cells prior to committed monocyte/macrophage or dendritic cell differentiation and provide the first example of a patient-derived xenotransplantation model for a human histiocytic neoplasm.
Introduction
Histiocytic neoplasms are heterogeneous disorders marked by tissue infiltration and accumulation of pathologic macrophage-, dendritic cell (DC)–, or monocyte-derived cells.1 Over the last 5 years, recurrent mutations activating MAPK signaling have been identified in adults and children affected by Langerhans cell histiocytosis (LCH)2-7 or Erdheim-Chester disease (ECD).7,8 While the discovery of these mutations has impacted treatment,7,9-13 these discoveries may also be useful in identifying the cellular origins of LCH and ECD, which have been debated for decades.14,15
LCH was historically thought to arise from epidermal Langerhans cells14,16 due to phenotypic similarities between LCH and normal Langherans cells. Recent data however suggest that LCH may be derived from myeloid precursor cells bearing somatic mutations in MAPK pathway members,17 including BRAFV600E mutations in ∼50% of patients, as well as mutations in NRAS,18 KRAS,18 ARAF,3,5,7 and MAP2K1.4,5,7,19 Gene expression profiling of CD207+ DCs from LCH patients revealed that LCH cells are more akin to bone marrow (BM)–derived, immature myeloid DCs than epidermal Langerhans cells.20 Moreover, BRAFV600E mutations have been identified in CD11c+ myeloid DC precursors in LCH, CD14+ monocytes in LCH and ECD, and BM CD34+ hematopoietic stem/progenitor cells (HSPCs) in a proportion of LCH patients. Finally, expression of the BRAFV600E mutation in committed DC precursors is sufficient to drive LCH-like disease in mice.17 Despite these data, however, functional evidence linking the cell of origin for histiocytosis to HSPCs in actual patient materials does not currently exist. Therefore, we attempted to understand the cell of origin of histiocytic neoplasms using genetic and functional analyses of HSPCs from histiocytosis patients. We identify that CD34+ cells in histiocytosis patients have functional self-renewal potential and describe the first successful patient-derived xenograft of a histiocytic neoplasm.
Methods
BM and/or peripheral blood (PB) cells were obtained from 41 patients with ECD or mixed histiocytosis (MH). A total of 37 patients had a BRAFV600E mutation, and 1 patient had KRASG12S mutation within histiocytosis-infiltrated tissues (supplemental Table 1, available on the Blood Web site); 10 patients had a concurrent myeloid neoplasm (chronic myelomonocytic leukemia or myeloproliferative neoplasm) in addition to histiocytosis (a recently identified clinical overlap21 ).
BM and PB cell subsets were purified by fluorescence-activated cell sorting or immunomagnetic selection. Purity was consistently >90% (supplemental Methods). CD34+ BM cells were assayed in methylcellulose (Methocult GF+ H4435, STEMCELL Technologies) and/or grown in liquid culture conditions allowing differentiation into myeloid dendritic cells (mDCs) or plasmacytoid dendritic cells.22 In some experiments, single CD34+ cells were expanded in liquid culture for 10 days.23
Detection of BRAFV600E and BRAFWT alleles within DNA extracted from BM, PB, or colonies was performed by real-time polymerase chain reaction (PCR) and/or picodroplet digital PCR as described previously.18 Mutations in colony-forming unit-macrophage (CFU-M), colony-forming unit-granulocyte (CFU-G), and burst-forming unit-erythroid (BFU-E) were analyzed by pools of 10 colonies.
Xenografts were performed by direct intrafemoral injection24 of a variable number of CD34+ cells from BM mononuclear cells from histiocytosis patients into sublethally irradiated (200 cGy) nonobese diabetic severe combined immunodeficiency IL2Rgamma-null mice with transgenic expression of human interleukin-3, stem cell factor, and granulocyte-macrophage colony-stimulating factor (NSGS)25 mice. Human engraftment was monitored by monthly flow cytometric analysis of PB until mice became moribund. Thereafter, animals were sacrificed, and tissues were analyzed using flow cytometry, histology, immunohistochemistry, and sequencing to detect driver mutations identified in the histiocytosis/co-occurring myeloid neoplasm. Studies were approved by the ethics committees of University Hospital La Pitié-Salpêtrière and Memorial Sloan Kettering Cancer Center and by the Institutional Review Board of the French Regulatory Agency and conducted in accordance to the Declaration of Helsinki. All patients signed informed consent.
Results and discussion
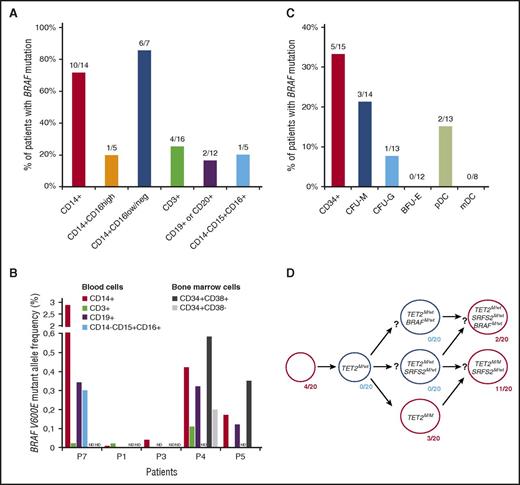
We first performed genotyping for the BRAFV600E mutation in blood cells from ECD or MH known to harbor the BRAFV600E mutations within infiltrated tissues. BRAFV600E mutations were detected in 71% (10/14) of blood CD14+ monocytes (Figure 1A). The BRAFV600E mutation was more frequent within CD14+ CD16low classical26 than CD14+ CD16high nonclassical monocytes. These results confirm prior detection of MAPK pathway mutations in CD14+ cells of children with systemic LCH17 and in 1 patient with ECD.18 The BRAFV600E mutation was also detected in T (CD3+) and B lymphocytes (CD19+ or CD20+) and/or polymorphonuclear (CD14− CD15+ CD16+) cells in some patients (Figure 1A). The BRAFV600E mutant allele frequency (BRAF-MAF) was low in all cell populations and ranged from 0.01 to 2.9 within monocytes (Figure 1B). The detection of the BRAFV600E mutation in multiple mature blood cells suggests that the oncogenic event involved a multipotent BM progenitor.
The BRAFV600Emutation is detectable in mature and immature hematopoietic cells from patients with histiocytosis. (A) Percentage of patients for whom BRAFV600E mutation was detected in mature PB cells. (B) BRAFV600E mutant allele frequency in fluorescence-activated cell sorting–purified cells from the BM or PB of 5 histiocytosis patients. ND indicates populations not studied. Patient numbering per supplemental Table 1. (C) Percentage of patients for whom the BRAFV600E mutation was detected in CD34+ BM cells or in CD34-derived CFU-M, CFU-G, BFU-E, and plasmacytoid DCs (pDC) or mDCs. (D) BRAFV600E, TET2L1819X, and SRSF2L95P status of 20 colonies derived from single CD34+ BM cells of a patient with BRAFV600E-mutant MH (#P10). The results suggest that histiocytosis and AMML cells of this patient derived from a common TET2-mutated HSPC. In panels A and C, each denominator indicates the total number of patients for whom results were obtained.
The BRAFV600Emutation is detectable in mature and immature hematopoietic cells from patients with histiocytosis. (A) Percentage of patients for whom BRAFV600E mutation was detected in mature PB cells. (B) BRAFV600E mutant allele frequency in fluorescence-activated cell sorting–purified cells from the BM or PB of 5 histiocytosis patients. ND indicates populations not studied. Patient numbering per supplemental Table 1. (C) Percentage of patients for whom the BRAFV600E mutation was detected in CD34+ BM cells or in CD34-derived CFU-M, CFU-G, BFU-E, and plasmacytoid DCs (pDC) or mDCs. (D) BRAFV600E, TET2L1819X, and SRSF2L95P status of 20 colonies derived from single CD34+ BM cells of a patient with BRAFV600E-mutant MH (#P10). The results suggest that histiocytosis and AMML cells of this patient derived from a common TET2-mutated HSPC. In panels A and C, each denominator indicates the total number of patients for whom results were obtained.
We next investigated BM progenitor populations for BRAFV600E in patients with ECD (n = 22) or MH (n = 8). BRAFV600E was detected within purified CD34+ BM cells from 5 out of 15 patients (33%) known to have BRAFV600E in lesional histiocytes (Figure 1C). The BRAF-MAF was always <1% and lower in the CD34+ CD38− multipotent progenitor/long-term hematopoietic stem cell compartment than in the CD34+ CD38+ myeloid progenitor compartment (Figure 1B). Given these low BRAF-MAFs in HSPCs, we further sought to evaluate if mutant BRAF could be detected in progeny derived from BRAF mutant HSPCs. Through evaluation of colonies obtained upon seeding CD34+ BM cells in methylcellulose clonogenic cultures, BRAFV600E was detected in CFU-M (3/14) and CFU-G (1/13), but not in BFU-E colonies. BRAFV600E mutations were also detected within in vitro–derived plasmacytoid dendritic cells (2/13), but not in mDCs. We also analyzed colonies derived from single CD34+ BM cells of 6 patients with BRAFV600E ECD. Only 3 out of 459 colonies (0.65%) were mutated for BRAF (supplemental Figure 1), which confirms the low BRAF-MAF detected by picodroplet digital PCR. The patient with MH who had 2 BRAF-mutated colonies (#P10) was further analyzed for TET2 and SRSF2 mutations, which were known to exist in a concurrent acute myelomonocytic leukemia (AMML) in this patient. Interestingly, the presence of colonies with BRAF/TET2 comutation, as well as with TET2 mutation alone, revealed that the mutation in TET2 occurred before the BRAF mutation and suggests a common clonal origin for both the histiocytosis and the AMML (Figure 1D). This was further reinforced by the detection of the same BRAF, TET2, and SRSF2 mutations within LCH cells in a skin biopsy from this patient (supplemental Figure 2).
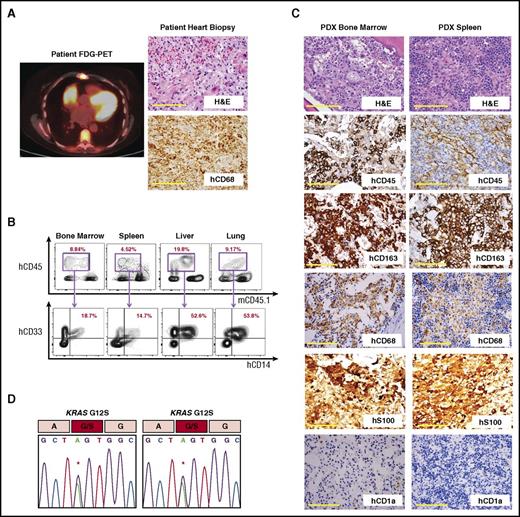
Next, to test the self-renewal and differentiation capacity of HSPCs from histiocytoses patients, CD34+ cells were purified from 8 patients, including 6 with ECD or MH plus a concomitant myeloid neoplasm and 2 with ECD alone (supplemental Table 1). A total of 0.1 to 0.8 × 106 CD34+ cells from each patient were transplanted into sublethally irradiated NSGS24 mice. Successful engraftment of human myeloid cells was observed in blood of recipient mice xenografted from 50% of patients (4 out of 8) after a median of 90 days (range: 60-120 days; supplemental Figure 3A). Of these 4 samples, CD34+ cells from 1 ECD patient sample induced histiocytosis-like lesions, as shown by infiltration of human CD45+ (hCD45) cells coexpressing the human myeloid and monocyte lineage markers hCD33 and hCD14 in the BM, spleen, liver, and lung (Figure 2A-B; supplemental Figure 3B). Immunohistochemistry revealed that tissues were infiltrated by an hCD45+ hCD163+ hCD68+ hS100+ hCD1a− population of foamy histiocytes, characteristic of ECD (Figure 2C). Furthermore, genomic analysis of hCD45+ cell DNA from the engrafted animal’s BM and spleen revealed the same KRASG12S mutation present in the patient’s ECD lesion (Figure 2D). All other mice that successfully engrafted with human cells revealed either no evidence of histiocytosis histologically and/or no evidence of the histiocytosis-associated mutation.
Analysis of a patient-derived xenograft from a KRASG12S-mutant patient with ECD. (A) Fludeoxyglucose positron emission tomography (FDG-PET) imaging and histologic and immunohistochemical analysis of the KRASG12S-mutant ECD patient’s heart biopsy specimen (scale bars represent 200 µm). (B) Flow cytometric analysis of BM, spleen, liver, and lung of recipient NSGS mouse 90 days postxenotransplantation with CD34+ cells from the same KRASG12S-mutant ECD patient. (C) Histologic and immunohistochemical analysis of engrafted tissue from the recipient mouse (scale bars represent 200 µm). (D) Sanger sequencing of genomic DNA from hCD45+ cells purified from recipient mouse BM (left) and spleen (right) revealing a KRASG12S mutation in engrafted human hematopoietic cells. H&E, hematoxylin and eosin.
Analysis of a patient-derived xenograft from a KRASG12S-mutant patient with ECD. (A) Fludeoxyglucose positron emission tomography (FDG-PET) imaging and histologic and immunohistochemical analysis of the KRASG12S-mutant ECD patient’s heart biopsy specimen (scale bars represent 200 µm). (B) Flow cytometric analysis of BM, spleen, liver, and lung of recipient NSGS mouse 90 days postxenotransplantation with CD34+ cells from the same KRASG12S-mutant ECD patient. (C) Histologic and immunohistochemical analysis of engrafted tissue from the recipient mouse (scale bars represent 200 µm). (D) Sanger sequencing of genomic DNA from hCD45+ cells purified from recipient mouse BM (left) and spleen (right) revealing a KRASG12S mutation in engrafted human hematopoietic cells. H&E, hematoxylin and eosin.
This study provides the first functional evidence that CD34+ cells from patients with histiocytosis can functionally initiate disease. Xenotransplantation successfully recapitulated the unique histologic, immunophenotypic, and genetic characteristics of human ECD. Moreover, these data confirm prior results suggesting that somatic mutations affecting MAPK signaling detectable in histiocytosis lesions are also detectable in the CD34+ compartment and in mature blood cells of a proportion of histiocytosis patients. Further analysis of HSPCs from histiocytosis patients for additional somatic mutations in histiocytosis lesional cells may therefore be very helpful in clarifying the cells of origin of systemic histiocytoses. Moreover, these data, together the wide clinical heterogeneity of histiocytoses, also suggest the possibility of alternate cells of origin for these conditions beyond HSPCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Isabelle Plo for help in quantification of the JAK2 mutation, Laetitia Claër for contribution in cell sorting, and Catherine Le Gall, Dominique Péchaud, Yolaine Pothin and Mariama Bakari and Rim Ben Jannet for contribution to BRAF mutation detection.
B.H.D. is supported by the American Society of Hematology Senior Research Training Award for Fellows and the New York State Council on Graduate Medical Education Empire Clinical Research Investigator Program Fellowship. A.Y. is supported by the Aplastic Anemia and MDS Research Foundation and the Lauri Strauss Leukemia Foundation. E.L.D. and O.A.-W. are supported by grants from the Erdheim Chester Disease Global Alliance and the Histiocytosis Association. O.A.-W. is supported by grants from the Edward P. Evans Foundation, the Department of Defense Bone Marrow Failure Research Program (BM150092 and W81XWH-12-1-0041), National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL128239), a National Institutes of Health K08 Clinical Investigator Award (1K08CA160647-01), the Josie Robertson Investigator Program, a Damon Runyon Clinical Investigator Award, an award from the Starr Foundation (I8-A8-075), the Leukemia and Lymphoma Society, and the Pershing Square Sohn Cancer Research Alliance. J.-F.E. is supported by grants from Association pour la Recherche et l'Enseignement en Pathologie. V.T. is supported by Ligue Nationale Contre le Cancer (Equipe labelisée EL2016.LNCC/VaT). O.A.B. is supported by Ligue Nationale Contre le Cancer (Equipe labelisée).
Authorship
Contribution: B.H.D., D.R.-W., C.B., A.Y., M.M., Z.H.-R., N.T., N.O., A.D., C.Y.P., G.G., V.T., O.A.B., O.A.-W., F.M.L., and J.-F.E. performed the experiments; F.C.-A., M.P., R.R., F.U., L.L.F., E.L.D., T.P., F.C., Z.A., and J.H. collected patient material and clinical data; and B.H.D., O.A.-W., J.H., and J.-F.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-François Emile, Service de Pathologie and EA4340, Hôpital Ambroise Paré, 9 Avenue Ch de Gaulle, 92104 Boulogne, France; e-mail: jean-francois.emile@uvsq.fr; and Julien Haroche, Département de Médecine Interne, Hôpital de la Pitié-Salpêtrière, 47-83 Boulevard de l’Hôpital, 75013 Paris, France; e-mail: julien.haroche@aphp.fr.
References
Author notes
B.H.D., D.R.-W., and C.B. contributed equally to this study.
O.A.-W., F.M.L., J.H., and J.-F.E. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal