Key Points
CK2β is critically required for thrombopoiesis by regulating tubulin polymerization, MK fragmentation, and proplatelet formation.
CK2β facilitates inositol triphosphate–mediated increase of cytosolic Ca2+ and is essential for platelet activation in arterial thrombosis in vivo.
Abstract
Platelets, anucleated megakaryocyte (MK)-derived cells, play a major role in hemostasis and arterial thrombosis. Although protein kinase casein kinase 2 (CK2) is readily detected in MKs and platelets, the impact of CK2-dependent signaling on MK/platelet (patho-)physiology has remained elusive. The present study explored the impact of the CK2 regulatory β-subunit on platelet biogenesis and activation. MK/platelet-specific genetic deletion of CK2β (ck2β−/−) in mice resulted in a significant macrothrombocytopenia and an increased extramedullar megakaryopoiesis with an enhanced proportion of premature platelets. Although platelet life span was only mildly affected, ck2β−/− MK displayed an abnormal microtubule structure with a drastically increased fragmentation within bone marrow and a significantly reduced proplatelet formation in vivo. In ck2β−/− platelets, tubulin polymerization was disrupted, resulting in an impaired thrombopoiesis and an abrogated inositol 1,4,5-triphosphate receptor–dependent intracellular calcium (Ca2+) release. Presumably due to a blunted increase in the concentration of cytosolic Ca2+, activation-dependent increases of α and dense-granule secretion and integrin αIIbβ3 activation, and aggregation were abrogated in ck2β−/− platelets. Accordingly, thrombus formation and stabilization under high arterial shear rates were significantly diminished, and thrombotic vascular occlusion in vivo was significantly blunted in ck2β−/− mice, accompanied by a slight prolongation of bleeding time. Following transient middle cerebral artery occlusion, ck2β−/− mice displayed significantly reduced cerebral infarct volumes, developed significantly less neurological deficits, and showed significantly better outcomes after ischemic stroke than ck2βfl/fl mice. The present observations reveal CK2β as a novel powerful regulator of thrombopoiesis, Ca2+-dependent platelet activation, and arterial thrombosis in vivo.
Introduction
Platelets are small, anucleated cells derived from proplatelets, which are generated by polyploid megakaryocytes (MKs) within sinusoids of the bone marrow (BM).1 Microtubules, polarized tubular filaments of α- and β-tubulin heterodimers, are the major structural components involved in proplatelet formation.2,3 Polymerization of microtubules is necessary to support the enlarging proplatelet mass.4,5 β1-Tubulin expression is restricted in the MK/platelet lineage and is essential for late MK differentiation and platelet biogenesis.6 Genetic loss of β1-tubulin, the major β-tubulin isoform in MKs, results in macrothrombocytopenia due to pathological MK fragmentation, impaired proplatelet formation, and release of large, immature platelets with an attenuated response to activation.6,7
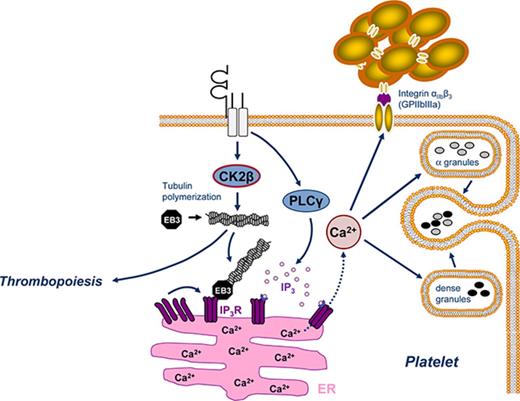
The microtubule plus end-binding protein 3 (EB3) transiently binds to growing polymerized microtubules by recognizing the GTP-bound state of β-tubulin.3,8,9 EB3 provides an essential hub for assembly of plus-end-tracking proteins that facilitate interactions of microtubules with the endoplasmic reticulum (ER).8,10 The ER is the major intracellular calcium (Ca2+) store in platelets and is continuously remodeled through its interactions with microtubules.11 Inositol 1,4,5-triphosphate receptors (IP3Rs) within ER membranes facilitate rapid Ca2+ release from internal stores on binding of Inositol 1,4,5-triphosphate (IP3) generated in activated platelets.12,13 Clustering of IP3Rs in the ER membranes is essential for IP3R activity and intracellular Ca2+ release.14,15 Microtubule cytoskeleton dynamics are crucial to IP3R clustering and IP3R-driven release of Ca2+ from internal stores.8,16,17 Microtubule-associated EB3 binds to IP3Rs, thus promoting IP3R clustering and Ca2+ release.8
Platelet adhesion, activation, and aggregation are essential for primary hemostasis, but are also critically involved in acute arterial thrombotic occlusion, leading to myocardial infarction or ischemic stroke.18 Moreover, platelets participate in the orchestration of vascular inflammation and atherogenesis.19,20 Subendothelial collagen, collagen-related peptide (CRP), and thrombin are the main triggers of platelet activation, characterized by granule release, integrin αIIbβ3 activation, aggregation, and consecutive thrombus formation.12,21 All those platelet responses depend on a rapid increase in the concentration of cytosolic Ca2+ ([Ca2+]i), which is accomplished by IP3-mediated Ca2+ release from intracellular stores triggering subsequent extracellular Ca2+ influx via the stimulation of Orai1-mediated store-operated Ca2+ entry.22
Casein kinase 2 (CK2) is a constitutively active serine-threonine protein kinase with a tetrameric structure, in which 2 regulatory β-subunits dimerize to link the 2 catalytic subunits α or α′.23 By bridging the 2 catalytic subunits, CK2β is required for the assembly of tetrameric CK2 complexes, thus enhancing CK2 holoenzyme stability to modulate catalytic activity and substrate specifity.24-26 The regulatory CK2 β-subunit plays an essential role in development because ubiquitous Ck2β-deficiency in mice causes lethality before embryonic day 7.5.27 CK2 controls various cellular processes, such as cell cycle progression, proliferation, apoptosis, and signal transduction, involving phosphatidylinositol 3-kinase (PI3K)/Akt and JAK/STAT pathways in a number of human cancers, including hematological malignancies.28 Recently, cell-specific deletion of CK2β in regulatory T cells identified CK2 as an important modulator of immune response.29 Growing evidence points to an important role of CK2 in the maintenance of cell morphology as well as in the regulation of tubulin cytoskeleton and microtubule dynamics.23,25,30 Although CK2 is strongly expressed in platelets,31 its role in thrombopoiesis as well as platelet activation and platelet-dependent arterial thrombosis still remains poorly defined.
The present study explored the role of CK2β in MK and platelet biology as well as its impact on platelet biogenesis and function. To circumvent prenatal death and to investigate the MK/platelet-specific effects of CK2β on mechanisms regulating thrombopoiesis, hemostasis, and arterial thrombosis in vivo, mice expressing ck2β alleles flanked with the loxP-Cre excision sequence (cskn2bfl/fl) were used to achieve MK/platelet-specific deletion of ck2β (ck2β−/−).
Material and methods
Mice
CK2β-floxed mice (csnk2bfl/fl) were generated by Thierry Buchou (INSERM, Grenoble, France). Origin, breeding, and genotyping of csnk2bfl/fl mice were described previously.27 For platelet-specific deletion of CK2β, cskn2bfl/fl mice were crossed with PF4-Cre mice (The Jackson Laboratory) to obtain csnk2bfl/flpf4-Cre+ (ck2β−/−) and cskn2bfl/flpf4-Cre– (ck2βfl/fl) littermates. Mice were studied at the age of 6 to 12 weeks. All animal experiments were performed according to the German animal protection law and were in accordance with the recently published Animal Research: Reporting In Vivo Experiments guidelines (National Centre for the Replacement, Refinement and Reduction of Animals in Research) and approved by the local authorities. Animal handling and care complied with published regulations by the German law for the protection of animals, and experiments were approved by the local authorities (Regierungspräsidium Tübingen).
Methods
Platelet preparation, in vitro differentiation, and cultivation of fetal liver– and BM-derived MKs, platelet and MK immunoblot analysis, determination of reticulated platelets, platelet life span assay, determination of thrombopoietin (TPO) levels and analysis of thrombopoiesis after TPO treatment, immunohistochemistry of murine BM and spleens, immunofluorescence staining on femora cryosections and cultured MKs, ultrastructural transmission electron microscopy (TEM) analysis of MKs, determination of MK ploidy, multiphoton intravital microscopy analysis of in vivo proplatelet formation, microtubule sedimentation, real-time polymerase chain reaction, spectrofluorometry for Ca2+ measurements, flow cytometry, platelet aggregometry and adenosine triphosphate (ATP) release, in vitro thrombus formation and stabilization, tail bleeding time, in vivo thrombus formation in mesenteric arterioles and in carotid arteries, ischemic stroke in mice (transient middle cerebral artery occlusion [tMCAO]), and the statistical analysis are described in detail in supplemental Material and methods, available on the Blood Web site.
Results
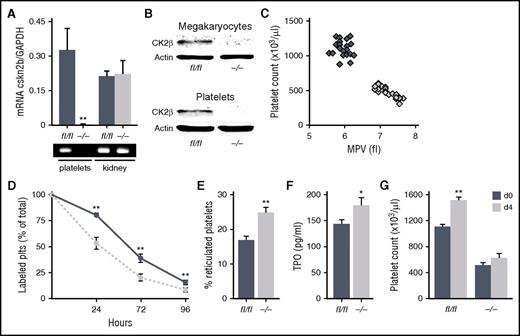
Genetic deletion of CK2β results in embryonic lethality in mice. Therefore, to specifically delete ck2β in the MK lineage, cskn2bfl/fl mice were crossed with transgenic mice expressing Cre-recombinase under the control of the MK- and platelet-specific platelet factor 4 (Pf4-Cre) to obtain csnk2bfl/flpf4-Cre+ (ck2β−/−) and cskn2bfl/flpf4-Cre− (ck2βfl/fl) littermates. Real-time polymerase chain reaction analysis revealed that CK2β was totally ablated in ck2β−/− platelets on the transcript level, whereas CK2β expression in other tissues was unaffected (Figure 1A; supplemental Figure 1). Immunoblotting confirmed that platelets and MKs derived from ck2β−/− mice completely lacked CK2β protein abundance (Figure 1B). Ck2β−/− mice were viable and fertile, but, notably, mice with the ck2β-deficient MK lineage developed distinct macrothrombocytopenia without obvious signs of spontaneous hemorrhage. Macrothrombocytopenia in ck2β−/− mice was reflected by a reduction of platelet counts of 55% and a significant increase in platelet volume (Figure 1C). To examine whether the macrothrombocytopenia in ck2β−/− mice resulted from increased platelet turnover, platelet life span was determined in ck2β−/− mice. Clearance of ck2β-null platelets was only moderately accelerated, which may have contributed to, but does not fully explain, the severely reduced platelet counts of ck2β−/− mice (Figure 1D). Further, according to the determination of major surface glycoproteins by flow cytometric analysis (Table 1), CK2β deficiency resulted in a mildly but significantly reduced expression of von Willebrand factor (vWF) receptor complex subunits, whereas the expression of other surface receptors was comparable in ck2β−/− and ck2βfl/fl platelets.
CK2β is strongly expressed in MKs and platelets, and CK2β deficiency results in macrothrombocytopenia, reduced platelet life span, and an increased amount of immature reticulated platelets. (A) Representative image (bottom) and arithmetic means ± standard errors of the means (n = 6; top) of CK2β transcript levels in platelets and kidneys from ck2βfl/fl and ck2β−/− mice indicating a cell-specific knockout of CK2β in the MK/platelet lineage. (B) Representative immunoblots of CK2β protein levels of MKs (top) and platelets (bottom) from ck2βfl/fl and ck2β−/− mice (n = 4) confirming a complete knockout of CK2β in the MK/platelet lineage. (C) Count and mean platelet volume (MPV) of ck2βfl/fl (blue diamonds) and ck2β−/− (gray diamonds) platelets (n = 24). Each diamond represents 1 individual mouse. (D) Endogenous survival of ck2βfl/fl (blue trace) and ck2β−/− (gray trace) platelets measured by the determination of the percentage of fluorescently labeled platelets in vivo at indicated time points after injection of DyLight 488 α-GPIX (n = 6). (E) Flow cytometric quantification of the percentage of new, reticulated platelets as a percentage of total platelets in ck2βfl/fl and ck2β−/− mice. Arithmetic means ± standard errors of the means (n = 6) are shown. (F) TPO levels of ck2βfl/fl (blue bar) and ck2β−/− (gray bar) mice. Arithmetic means ± standard errors of the means (n = 15) are shown. (G) Platelet counts of ck2βfl/fl and ck2β−/− mice before and 4 days after treatment with TPO (2 µg/animal per day). Arithmetic means ± standard errors of the means (n = 8) are shown. Unpaired Student t test in panels A and D-G. *P < .05; **P < .01.
CK2β is strongly expressed in MKs and platelets, and CK2β deficiency results in macrothrombocytopenia, reduced platelet life span, and an increased amount of immature reticulated platelets. (A) Representative image (bottom) and arithmetic means ± standard errors of the means (n = 6; top) of CK2β transcript levels in platelets and kidneys from ck2βfl/fl and ck2β−/− mice indicating a cell-specific knockout of CK2β in the MK/platelet lineage. (B) Representative immunoblots of CK2β protein levels of MKs (top) and platelets (bottom) from ck2βfl/fl and ck2β−/− mice (n = 4) confirming a complete knockout of CK2β in the MK/platelet lineage. (C) Count and mean platelet volume (MPV) of ck2βfl/fl (blue diamonds) and ck2β−/− (gray diamonds) platelets (n = 24). Each diamond represents 1 individual mouse. (D) Endogenous survival of ck2βfl/fl (blue trace) and ck2β−/− (gray trace) platelets measured by the determination of the percentage of fluorescently labeled platelets in vivo at indicated time points after injection of DyLight 488 α-GPIX (n = 6). (E) Flow cytometric quantification of the percentage of new, reticulated platelets as a percentage of total platelets in ck2βfl/fl and ck2β−/− mice. Arithmetic means ± standard errors of the means (n = 6) are shown. (F) TPO levels of ck2βfl/fl (blue bar) and ck2β−/− (gray bar) mice. Arithmetic means ± standard errors of the means (n = 15) are shown. (G) Platelet counts of ck2βfl/fl and ck2β−/− mice before and 4 days after treatment with TPO (2 µg/animal per day). Arithmetic means ± standard errors of the means (n = 8) are shown. Unpaired Student t test in panels A and D-G. *P < .05; **P < .01.
Glycoprotein surface expression of ck2βfl/fl and ck2β−/− platelets
| Platelet GP . | ck2βfl/fl . | ck2β−/− . | P . |
|---|---|---|---|
| GPIbα | 343 ± 10 | 311 ± 4 | <.01 |
| GPV | 145 ± 3 | 121 ± 2 | <.001 |
| GPVI | 66 ± 3 | 62 ± 2 | .08 |
| GPIX | 175 ± 5 | 145 ± 1 | <.001 |
| Integrin β1 | 147 ± 5 | 141 ± 7 | .50 |
| Integrin β3 | 184 ± 4 | 182 ± 3 | .83 |
| Platelet GP . | ck2βfl/fl . | ck2β−/− . | P . |
|---|---|---|---|
| GPIbα | 343 ± 10 | 311 ± 4 | <.01 |
| GPV | 145 ± 3 | 121 ± 2 | <.001 |
| GPVI | 66 ± 3 | 62 ± 2 | .08 |
| GPIX | 175 ± 5 | 145 ± 1 | <.001 |
| Integrin β1 | 147 ± 5 | 141 ± 7 | .50 |
| Integrin β3 | 184 ± 4 | 182 ± 3 | .83 |
Results are expressed as mean fluorescence intensity ± standard error of the mean for 10 mice per group.
GPIbα, glycoprotein Ibα.
The significant thrombocytopenia that was observed despite the only moderately reduced platelet life span prompted us to assess platelet production. To this end, we labeled blood samples ex vivo with thiazole orange. As revealed by flow cytometry, we found a significantly higher amount of larger young reticulated platelets in ck2β−/− mice (Figure 1E). Because TPO is the main trigger of (compensatory) megakaryopoiesis and its plasma concentrations are reciprocally regulated by total platelet mass, we measured plasma TPO levels in thrombocytopenic ck2β−/− mice. TPO concentrations were slightly but significantly increased in ck2β−/− mice (Figure 1F). TPO injection was followed by significant elevations in the platelet counts of ck2βfl/fl mice after 96 hours, whereas exogenous TPO did not sufficiently elevate the platelet counts of ck2β−/− mice and failed to prevent thrombocytopenia in these mice (Figure 1G). Of note, protein expression levels and surface coverage of the TPO receptor myeloproliferative leukemia virus oncogene were similar in platelets and MKs (supplemental Figure 2). Platelet sialylation and hepatic TPO transcript levels were comparable in ck2β−/− and ck2βfl/fl mice as well (supplemental Figure 3), indicating that Ashwell-Morell receptor–dependent platelet clearance is not affected by CK2β.
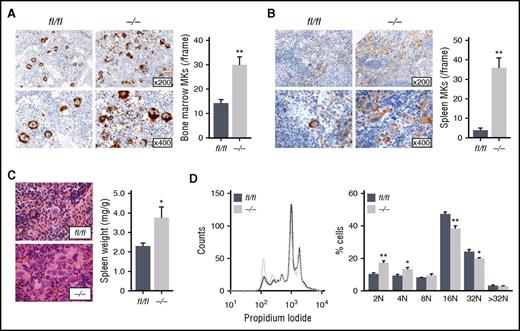
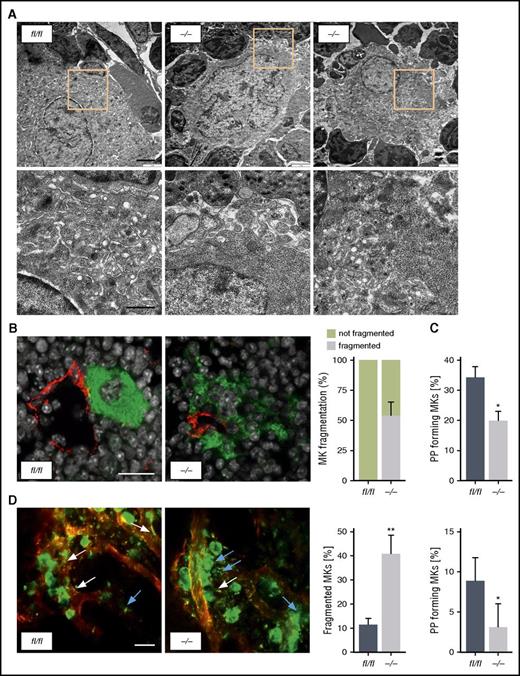
As revealed by immunohistological analysis, the BM of ck2β−/− mice showed remarkable MK hyperplasia, reflected by a significantly increased number of MKs within the BM (Figure 2A). Moreover, a significant number of ck2β−/− BM MKs displayed signs of fragmentation with abrogated cellular demarcation. Further examination of MKs flushed out of BM revealed a significantly reduced ploidy in ck2β-null MKs with a significant reduction of 16N- and 32N-containing MKs (Figure 2D), indicating that CK2β-deficiency results in the accumulation of immature MKs. Although only a mild splenomegaly was observed in ck2β−/− mice (Figure 2C), after spleen immunostaining, we found drastically increased extramedullary thrombopoiesis in ck2β−/− mice, with distortion of splenic architecture strongly enriched with GPIb-positive MKs (Figure 2B). Nevertheless, ck2β−/− mice displayed neither a modification of the hematopoietic stem and progenitor cell compartment in the BM and spleen nor an excess of hematopoietic progenitors in the peripheral blood (supplemental Figure 4). Hematoxylin and eosin (H&E) staining of formalin-fixed, paraffin-embedded spleens confirmed a markedly increased number of MKs in the spleens from ck2β−/− mice (Figure 2B), whereas BM and spleen fibrosis was not accelerated in CK2β-deficient mice (supplemental Figure 5). GPIb and H&E staining of the lungs ruled out any difference in the presence of MKs and platelets in the lung circulation or parenchyma in ck2β−/− mice as compared with wild-type mice (supplemental Figure 6). Because ck2β−/− MKs were hardly identifiable due to their altered morphology and demarcation from surrounding cells, we analyzed the ultrastructure of BM MKs by TEM, revealing impaired DMS formation and prominent granule-free zones in ck2β−/− MKs (Figure 3A). To evaluate the functional consequence of these findings, we next visualized MKs in BM cryosections of intact femora. Immunostaining clearly indicated that ck2β-null MKs had less contact with BM sinusoids and displayed markedly increased fragmentation (Figure 3B). To further study the role of CK2β in MK-dependent platelet biogenesis, we performed in vitro proplatelet formation assays. Accordingly, significantly fewer numbers of ck2β−/− MKs formed proplatelets (Figure 3C). For examination of these findings in vivo, we analyzed proplatelet formation under steady-state conditions, visualizing the BM in the mouse skull by using 2-photon intravital microscopy (2P-IVM). 2P-IVM confirmed that ck2β−/− MKs undergo premature ectopic fragmentation and are unable to efficiently release proplatelets into the vascular sinusoids in vivo because 40.6% ± 7.6% of the ck2β-deficient MKs had an altered morphology, appearing fragmented as if the MKs were somewhat unstable, whereas only 11.5% ± 2.6% of the ck2βfl/fl MKs were fragmented (Figure 3D; supplemental Videos 1 and 2). As further revealed by 2P-IVM, premature platelet release by ck2β−/− MKs is probably caused by defective in vivo proplatelet formation. Only 3.2% ± 2.9% of ck2β-null MKs produce proplatelets in vivo compared with 8.5% ± 2.7% of ck2βfl/fl MKs, indicating that CK2β is required for proplatelet formation and is a critical regulator of thrombopoiesis (Figure 3D). To eliminate MK fragmentation of ck2β−/− MKs as consequence of increased apoptosis in these cells, we determined caspase 3/7 activity. As illustrated in supplemental Figure 7, caspase 3/7 activity in ck2β−/− MKs was similar to that in ck2βfl/fl MKs.
Ck2β−/−mice display BM MK hyperplasia and an upregulation of extramedullary thrombopoiesis. (A) Representative images of GPIb immunostaining of BM sections from 2-month-old ck2βfl/fl and ck2β−/− mice (left). Quantification of ck2βfl/fl and ck2β−/− BM MKs per visual field. Arithmetic means ± standard errors of the means (n = 6; right) are shown. (B) Representative images of GPIb immunostaining of spleen sections from 6-month-old ck2βfl/fl and ck2β−/− mice (left). Quantification of ck2βfl/fl and ck2β−/− spleen MKs per visual field. Arithmetic means ± standard errors of the means (n = 6; right) are shown. (C) Representative H&E staining of spleen sections from 6-month-old ck2βfl/fl and ck2β−/− mice (left). Arithmetic means ± standard errors of the means (n = 8; right) of relative spleen weight in ck2βfl/fl and ck2β−/− mice. (D) Ploidy histogram of ck2βfl/fl and ck2β−/− MKs (left) and arithmetic means ± standard errors of the means (n = 6; right) are shown. Unpaired Student t test. *P < .05; **P < .01.
Ck2β−/−mice display BM MK hyperplasia and an upregulation of extramedullary thrombopoiesis. (A) Representative images of GPIb immunostaining of BM sections from 2-month-old ck2βfl/fl and ck2β−/− mice (left). Quantification of ck2βfl/fl and ck2β−/− BM MKs per visual field. Arithmetic means ± standard errors of the means (n = 6; right) are shown. (B) Representative images of GPIb immunostaining of spleen sections from 6-month-old ck2βfl/fl and ck2β−/− mice (left). Quantification of ck2βfl/fl and ck2β−/− spleen MKs per visual field. Arithmetic means ± standard errors of the means (n = 6; right) are shown. (C) Representative H&E staining of spleen sections from 6-month-old ck2βfl/fl and ck2β−/− mice (left). Arithmetic means ± standard errors of the means (n = 8; right) of relative spleen weight in ck2βfl/fl and ck2β−/− mice. (D) Ploidy histogram of ck2βfl/fl and ck2β−/− MKs (left) and arithmetic means ± standard errors of the means (n = 6; right) are shown. Unpaired Student t test. *P < .05; **P < .01.
Ck2β−/−MKs exhibit abnormal ultrastructure, resulting in premature MK fragmentation in the BM and impaired proplatelet formation. (A) Representative TEM images of ck2βfl/fl (left) and ck2β−/− (middle and right) MKs in the BM showing increased fragmentation, decreased presence of demarcation membranes, and large granule-free zones on ck2β deficiency. Upper, overview (bar represents 3 μm). Lower, detail (bar represents 1 μm). (B) Representative confocal microscopy images of immunostained BM sections (left) and arithmetic means ± standard errors of the means (n = 6; right) of MK fragmentation in the BM of ck2βfl/fl and ck2β−/− mice (n = 6). Green, MKs (GPIb); red, sinusoids (CD105); gray, nuclei (4′,6-diamidino-2-phenylindole). Bar represents 20 μm. (C) Quantification of the percentage of proplatelet-forming fetal liver cell–derived MKs from ck2βfl/fl and ck2β−/− mice in vitro on day 4 of culture. Ck2βfl/fl and ck2β−/− MKs extending proplatelets were counted 18 hours after bovine serum albumin gradient and expressed as percentage of total MKs. Arithmetic means ± standard errors of the means (n = 10) are shown. (D) 2P-IVM revealing MK instability and reduced proplatelet formation in ck2β−/− MKs in vivo. Platelets and MKs were stained with anti-GPIX antibodies (green), and the vessel lumen was labeled using fluorescein isothiocyanate-bovine serum albumin and anti-CD105 antibodies (red). Proplatelet-forming MKs (white arrows indicated proplatelets) were counted, and the ratio per mouse was assessed (n = 5). MK morphology was categorized by a blinded experimenter in normal and fragmented (blue arrows). Representative images from the BM of ck2βfl/fl and ck2β−/− MKs (left) and arithmetic means ± standard errors of the means (n = 5; right) of proplatelet-forming MKs and of MKs with altered morphology are shown. Bar represents 50 µm. Unpaired Student t test in panels C and D. *P < .05; **P < .01.
Ck2β−/−MKs exhibit abnormal ultrastructure, resulting in premature MK fragmentation in the BM and impaired proplatelet formation. (A) Representative TEM images of ck2βfl/fl (left) and ck2β−/− (middle and right) MKs in the BM showing increased fragmentation, decreased presence of demarcation membranes, and large granule-free zones on ck2β deficiency. Upper, overview (bar represents 3 μm). Lower, detail (bar represents 1 μm). (B) Representative confocal microscopy images of immunostained BM sections (left) and arithmetic means ± standard errors of the means (n = 6; right) of MK fragmentation in the BM of ck2βfl/fl and ck2β−/− mice (n = 6). Green, MKs (GPIb); red, sinusoids (CD105); gray, nuclei (4′,6-diamidino-2-phenylindole). Bar represents 20 μm. (C) Quantification of the percentage of proplatelet-forming fetal liver cell–derived MKs from ck2βfl/fl and ck2β−/− mice in vitro on day 4 of culture. Ck2βfl/fl and ck2β−/− MKs extending proplatelets were counted 18 hours after bovine serum albumin gradient and expressed as percentage of total MKs. Arithmetic means ± standard errors of the means (n = 10) are shown. (D) 2P-IVM revealing MK instability and reduced proplatelet formation in ck2β−/− MKs in vivo. Platelets and MKs were stained with anti-GPIX antibodies (green), and the vessel lumen was labeled using fluorescein isothiocyanate-bovine serum albumin and anti-CD105 antibodies (red). Proplatelet-forming MKs (white arrows indicated proplatelets) were counted, and the ratio per mouse was assessed (n = 5). MK morphology was categorized by a blinded experimenter in normal and fragmented (blue arrows). Representative images from the BM of ck2βfl/fl and ck2β−/− MKs (left) and arithmetic means ± standard errors of the means (n = 5; right) of proplatelet-forming MKs and of MKs with altered morphology are shown. Bar represents 50 µm. Unpaired Student t test in panels C and D. *P < .05; **P < .01.
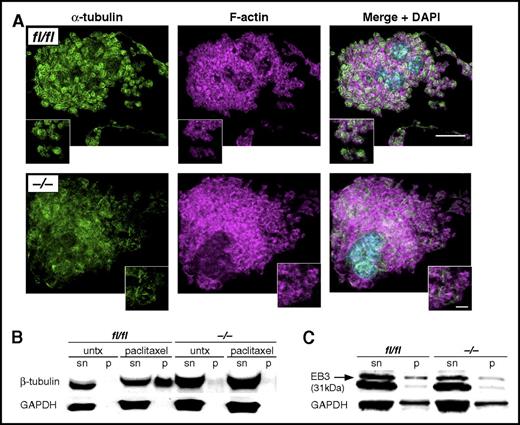
Microtubules and tubulin polymerization are essential for platelet structure and function. Because CK2β has been described as a critical regulator of tubulin and microtubule architecture, we next examined tubulin structure and polymerization. Of note, α-tubulin filaments appeared fragmented in many cultured ck2β−/− MKs, indicating that filament stability or polymerization were affected in the absence of CK2β (Figure 4A). Along those lines, treatment with the microtubule-stabilizing toxin, paclitaxel, resulted in an increase of polymerized β-tubulin in ck2βfl/fl, whereas ck2β−/− platelets failed to stabilize polymerized tubulin (Figure 4B), pointing to a critical role of CK2β in platelet microtubule stability. Presumably as a result of impaired tubulin polymerization, ck2β−/− platelets also showed significantly less EB3 binding (Figure 4C).
CK2β is essential for microtubule architecture and tubulin polymerization as well as EB3 binding to polymerized tubulin. (A) Representative immunofluorescence confocal microscopy images of cultured fetal liver–derived ck2βfl/fl and ck2β−/− MKs. α-Tubulin staining appears fragmented in Ck2β−/− MKs undergoing proplatelet formation. Bar represents 20 μm (overview) and 5 μm (detail). (B) Representative immunoblots of 3 independent experiments of free and polymerized tubulin in ck2βfl/fl and ck2β−/− platelets (n = 3) in the absence and presence of paclitaxel (10 μM) in supernatant (sn) and pellet (p). (C) Representative immunoblots of EB3 bound to polymerized tubulin in ck2βfl/fl and ck2β−/− platelets (n = 3) after treatment with paclitaxel (10 μM) in supernatant (sn) and pellet (p).
CK2β is essential for microtubule architecture and tubulin polymerization as well as EB3 binding to polymerized tubulin. (A) Representative immunofluorescence confocal microscopy images of cultured fetal liver–derived ck2βfl/fl and ck2β−/− MKs. α-Tubulin staining appears fragmented in Ck2β−/− MKs undergoing proplatelet formation. Bar represents 20 μm (overview) and 5 μm (detail). (B) Representative immunoblots of 3 independent experiments of free and polymerized tubulin in ck2βfl/fl and ck2β−/− platelets (n = 3) in the absence and presence of paclitaxel (10 μM) in supernatant (sn) and pellet (p). (C) Representative immunoblots of EB3 bound to polymerized tubulin in ck2βfl/fl and ck2β−/− platelets (n = 3) after treatment with paclitaxel (10 μM) in supernatant (sn) and pellet (p).
Because we know from other cell types that EB3 may act as an important linker between polymerized microtubules and the regulation of internal Ca2+ stores,8 we next aimed to elucidate the impact of CK2β-driven tubulin polymerization on intracellular Ca2+ release and subsequent Ca2+-dependent platelet activation. To discriminate between intracellular Ca2+ store release and Ca2+ influx from extracellular space, we used spectrofluorometry to determine the activation-dependent changes of [Ca2+]i in the absence or presence of extracellular Ca2+ (Figure 5A-B). Before stimulation, the cytosolic Ca2+ concentration was similar. By comparison, in the presence and in the absence of extracellular Ca2+, ck2β−/− platelets displayed a significantly blunted [Ca2+]i increase in response to stimulation with CRP or thrombin, pointing to an impaired intracellular regulation of cytosolic Ca2+ release in ck2β-null platelets. IP3 production occurs due to the activation of phospholipase C (PLC) induced by thrombin downstream of G protein–coupled signaling or after stimulation of the tyrosine kinase–linked collagen receptor glycoprotein VI (GPVI) by collagen or CRP.32 To test if the decreased intracellular Ca2+ release in ck2β−/− platelets could have been due to defective PLC-triggered IP3 production, we investigated whether IP3 production or activation of PLC is regulated by CK2β. Neither activation-dependent myo-inositol 1 phosphate production, reflecting the levels of the unstable IP3, nor PLC phosphorylation were significantly affected in ck2β−/− platelets (supplemental Figure 8). However, as revealed by light pulse-triggered activation of caged IP3, the IP3-dependent release of Ca2+ from intracellular stores was significantly blunted in ck2β-deficient platelets (Figure 5C), pointing to CK2β-sensitive regulation of IP3R activation on platelet stimulation. The total expression of platelet IP3Rs and phosphorylation of IP3R1, mainly located in the ER of platelets, were comparable (supplemental Figure 9). However, the abundance of IP3R1 bound to the fraction of polymerized tubulin was significantly diminished in ck2β−/− platelets (supplemental Figure 9).
CK2β is a critical regulator of platelet IP3-triggered Ca2+release from internal stores with subsequent extracellular Ca2+influx, resulting in impaired granule secretion, integrin αIIbβ3activation, and aggregation in ck2β-null platelets. (A) Representative tracings of Fura-2-fluorescence reflecting the cytosolic Ca2+ concentration [Ca2+]i (right) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; left) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with CRP (5 µg/ml) and thrombin (20 mU/ml) in the absence (0.5 mM EGTA) of extracellular Ca2+. (B) Representative tracings of Fura-2-fluorescence reflecting the cytosolic Ca2+ concentration [Ca2+]i (right) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; left) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with CRP (5 µg/ml) and thrombin (20 mU/ml) in the presence (1 mM Ca2+) of extracellular Ca2+. (C) Representative tracings of Fura-2-fluorescence reflecting cytosolic Ca2+ concentration [Ca2+]i (lower) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; upper) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with IP3 (2.5 µM). The cytosolic Ca2+ concentration before and after flash photolysis (365 nm) in the absence of IP3 was measured as a control (tracing not shown). (D) Flow cytometric analysis of P-selectin exposure reflecting α-granule release in ck2βfl/fl (blue bars) and ck2β−/− (gray bars) platelets in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Arithmetic means ± standard errors of the means (n = 6) are shown. (E) Luminescence analysis of ATP release reflecting the secretion of dense granules in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Representative ATP release tracings of ck2βfl/fl (blue lines) and ck2β−/− (gray lines) mice are shown (n = 6). (F) Flow cytometric analysis of αIIbβ3 integrin activation in ck2βfl/fl (blue bars) and ck2β−/− (gray bars) platelets in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Arithmetic means ± standard errors of the means (n = 6) are shown. (G) Light transmission aggregometry after stimulation with increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Representative aggregation tracings of ck2βfl/fl (blue lines) and ck2β−/− (gray lines) platelets are shown (n = 4). Unpaired Student t test in panels A-D. *P < .05; **P < .01.
CK2β is a critical regulator of platelet IP3-triggered Ca2+release from internal stores with subsequent extracellular Ca2+influx, resulting in impaired granule secretion, integrin αIIbβ3activation, and aggregation in ck2β-null platelets. (A) Representative tracings of Fura-2-fluorescence reflecting the cytosolic Ca2+ concentration [Ca2+]i (right) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; left) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with CRP (5 µg/ml) and thrombin (20 mU/ml) in the absence (0.5 mM EGTA) of extracellular Ca2+. (B) Representative tracings of Fura-2-fluorescence reflecting the cytosolic Ca2+ concentration [Ca2+]i (right) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; left) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with CRP (5 µg/ml) and thrombin (20 mU/ml) in the presence (1 mM Ca2+) of extracellular Ca2+. (C) Representative tracings of Fura-2-fluorescence reflecting cytosolic Ca2+ concentration [Ca2+]i (lower) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; upper) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with IP3 (2.5 µM). The cytosolic Ca2+ concentration before and after flash photolysis (365 nm) in the absence of IP3 was measured as a control (tracing not shown). (D) Flow cytometric analysis of P-selectin exposure reflecting α-granule release in ck2βfl/fl (blue bars) and ck2β−/− (gray bars) platelets in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Arithmetic means ± standard errors of the means (n = 6) are shown. (E) Luminescence analysis of ATP release reflecting the secretion of dense granules in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Representative ATP release tracings of ck2βfl/fl (blue lines) and ck2β−/− (gray lines) mice are shown (n = 6). (F) Flow cytometric analysis of αIIbβ3 integrin activation in ck2βfl/fl (blue bars) and ck2β−/− (gray bars) platelets in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Arithmetic means ± standard errors of the means (n = 6) are shown. (G) Light transmission aggregometry after stimulation with increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Representative aggregation tracings of ck2βfl/fl (blue lines) and ck2β−/− (gray lines) platelets are shown (n = 4). Unpaired Student t test in panels A-D. *P < .05; **P < .01.
To assess the functional significance of impaired IP3R activation and the corresponding defective [Ca2+]i increase in ck2β−/− platelets, we determined platelet degranulation, integrin αIIbβ3 activation, and aggregation after platelet stimulation via ITAM-coupled (CRP) or G-protein–coupled (thrombin) receptors. As quantified by flow cytometry and luminescence analysis, the activation-dependent secretion of platelet α and dense-granules after stimulation with low and intermediate concentrations of CRP or thrombin were significantly decreased in ck2β−/− platelets (Figure 5D-E). Moreover, integrin αIIbβ3 activation, which is required for fibrinogen binding, was significantly diminished in ck2β−/− platelets after stimulation with CRP or thrombin (Figure 5F). Light transmission aggregometry was used to explore whether impaired degranulation and integrin αIIbβ3 activation would translate into reduced activation-dependent platelet aggregation. After stimulation with increasing concentrations of CRP or thrombin, aggregation of ck2β-null platelets was significantly blunted (Figure 5G). Defective degranulation, integrin αIIbβ3 activation, and aggregation in ck2β−/− platelets were overcome by high agonist concentrations. Interestingly, as illustrated in supplemental Figures 10-14, comparable with our findings in murine ck2β−/− platelets, the treatment of mature murine and human platelets with the CK2 inhibitors (E)-3-(2,3,4,5-tetrabromophenyl)acrylic acid (TBCA) and CX-4945 significantly affected platelet Ca2+ influx, secretion, and aggregation in response to CRP.
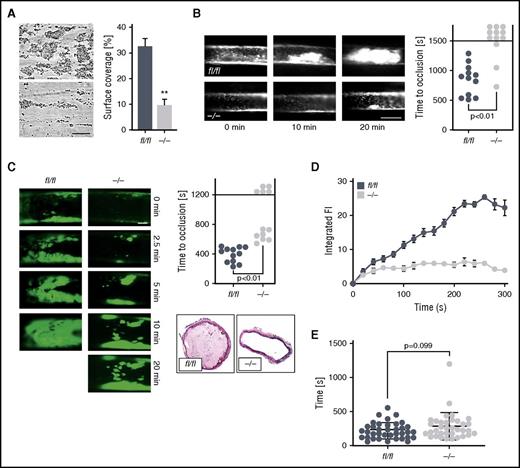
A further series of experiments elucidated the consequences of impaired activation of ck2β-deficient platelets for platelet adhesion to collagen-coated surfaces under flow at high arterial shear rates (1700 s−1). Although ck2βfl/fl platelets rapidly adhered to collagen and formed stable thrombi, thrombus formation by ck2β−/− platelets was significantly decreased because the thrombus surface coverage was reduced by ∼71% (Figure 6A). Because Ca2+-dependent platelet activation may contribute to pathological thrombus formation in vivo, we next assessed the time to occlusion of mesenteric arterioles and carotid arteries due to thrombus formation after ferric chloride (FeCl3)-induced injury in mice with a platelet-specific deletion of CK2β. Arterial thrombotic occlusion was significantly delayed and decreased in ck2β−/− mice after FeCl3-triggered vascular injury of mesenteric arterioles (Figure 6B). Seventy-five percent of the ck2β−/− mice failed to form an occlusive thrombus within 25 minutes, whereas in ck2βfl/fl mice, the mean time to thrombotic vascular occlusion was ∼14 minutes. Ck2β−/− mice were similarly protected from acute arterial thrombosis after FeCl3-induced injury of carotid arteries. Whereas all ck2βfl/fl mice displayed a rapid and complete vascular occlusion, the time to occlusion was significantly prolonged in ck2β−/− mice. No discontinuation of blood flow was found in 42% of the ck2β−/− mice due to the absence of thrombotic occlusion at the end of the 20-minute observation period (Figure 6C). Real-time imaging of thrombus formation under flow that was achieved by capturing images of fluorescently labeled platelets indicated a consistent and gradual increase in fluorescence intensity from the deposited ck2βfl/fl platelets (Figure 6D). With platelets from the ck2β−/− mice, the slope of fluorescence increase was diminished, and the time traces pointed to a slow and insufficient thrombus buildup. These findings from both in vitro and in vivo models of arterial thrombosis indicate that CK2β-mediated activation of IP3R is required for efficient platelet activation underlying thrombotic vascular occlusion. To test whether the defect in ck2β−/− platelets was associated with impaired hemostasis, we measured tail bleeding time. As illustrated in Figure 6E, ck2β−/− mice showed a minor bleeding tendency without a significantly prolonged bleeding time as compared with ck2βfl/fl mice (Figure 6E).
Ck2β deficiency results in significantly reduced platelet adhesion and thrombus stability under high arterial shear rates as well as abrogated thrombotic vascular occlusion in vivo, whereas primary hemostasis is not significantly affected. (A) Flow chamber analysis of platelet adhesion to collagen and thrombus formation in vitro under high arterial shear rates. Whole blood from ck2βfl/fl and ck2β−/− mice was perfused over a collagen-coated surface for 5 minutes at a shear rate of 1700 s−1. Arithmetic means ± standard errors of the means (n = 6; right) and representative phase contrast images (left) of surface coverage are shown. Bar represents 50 µm. (B) Time to arterial occlusion after FeCl3-induced injury of mesenteric arterioles (right, n = 12) and representative images of occlusive in vivo thrombus formation after 0, 10, and 20 minutes (left) in ck2βfl/fl and ck2β−/− mice. Each dot represents 1 individual mouse. Bar represents 50 µm. (C) Representative images of in vivo thrombus formation after 0, 2.5, 5, 10, and 20 minutes (left) and time to arterial occlusion after FeCl3-induced injury of carotid arteries (upper right, n = 12) and representative immunohistological H&E staining (lower right) of occlusive in vivo thrombus formation after 7 minutes in ck2βfl/fl and ck2β−/− mice. Each dot represents 1 individual mouse. Bar represents 100 µm. (D) Arithmetic means ± standard errors of the means (n = 6) of integrated fluorescence intensity of adherent platelets after perfusion of whole blood from ck2βfl/fl (blue dots) and ck2β−/− (gray dots) mice over a collagen-coated surface representing thrombus growth in vitro over 5 minutes. (E) Tail bleeding time measured after amputating the tail tip of ck2βfl/fl and ck2β−/− mice. Each dot represents 1 individual mouse (n = 36). Unpaired Student t test in panels A-E. **P < .01.
Ck2β deficiency results in significantly reduced platelet adhesion and thrombus stability under high arterial shear rates as well as abrogated thrombotic vascular occlusion in vivo, whereas primary hemostasis is not significantly affected. (A) Flow chamber analysis of platelet adhesion to collagen and thrombus formation in vitro under high arterial shear rates. Whole blood from ck2βfl/fl and ck2β−/− mice was perfused over a collagen-coated surface for 5 minutes at a shear rate of 1700 s−1. Arithmetic means ± standard errors of the means (n = 6; right) and representative phase contrast images (left) of surface coverage are shown. Bar represents 50 µm. (B) Time to arterial occlusion after FeCl3-induced injury of mesenteric arterioles (right, n = 12) and representative images of occlusive in vivo thrombus formation after 0, 10, and 20 minutes (left) in ck2βfl/fl and ck2β−/− mice. Each dot represents 1 individual mouse. Bar represents 50 µm. (C) Representative images of in vivo thrombus formation after 0, 2.5, 5, 10, and 20 minutes (left) and time to arterial occlusion after FeCl3-induced injury of carotid arteries (upper right, n = 12) and representative immunohistological H&E staining (lower right) of occlusive in vivo thrombus formation after 7 minutes in ck2βfl/fl and ck2β−/− mice. Each dot represents 1 individual mouse. Bar represents 100 µm. (D) Arithmetic means ± standard errors of the means (n = 6) of integrated fluorescence intensity of adherent platelets after perfusion of whole blood from ck2βfl/fl (blue dots) and ck2β−/− (gray dots) mice over a collagen-coated surface representing thrombus growth in vitro over 5 minutes. (E) Tail bleeding time measured after amputating the tail tip of ck2βfl/fl and ck2β−/− mice. Each dot represents 1 individual mouse (n = 36). Unpaired Student t test in panels A-E. **P < .01.
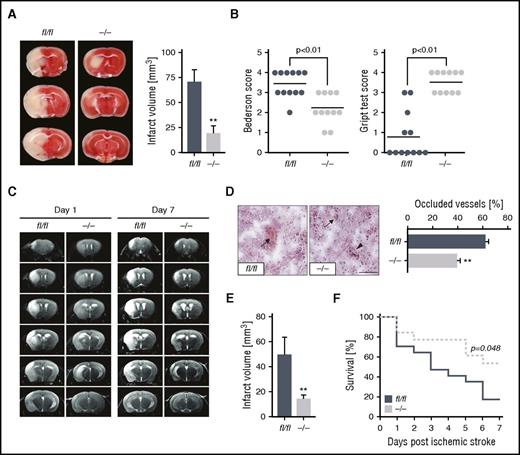
As a logical extension of our findings that CK2β can modulate arterial thrombosis, we investigated the impact of platelet CK2β on responses and outcome after ischemic stroke. Mice with a platelet-specific knockout of ck2β were studied in the tMCAO model. As determined by 2,3,5-triphenyltetrazolium chloride staining (Figure 7A), brain infarct volumes were significantly reduced in ck2β−/− mice 24 hours after tMCAO. Moreover, the platelet-specific CK2β deficiency resulted in a significantly decreased Bederson score reflecting the loss of global neurological function and a significantly improved grip test, assessing motor function and coordination. Collectively, these indicators demonstrate that the marked reduction of infarct volumes in ck2β−/− mice was functionally relevant (Figure 7B). Histological analysis of brain sections revealed significantly less thrombotic-occluded vessels in ck2β−/− mice (Figure 7D). To confirm the protective effect of a genetic CK2β knockout on ischemic brain infarct development, magnetic resonance imaging was performed on living mice. As illustrated by serial T2-weighted magnetic resonance imaging, hyperintense ischemic brain infarcts were markedly reduced in ck2β−/− mice after tMCAO, a protective effect that was sustained over 7 days (Figure 7C-E). Ck2β−/− mice further displayed significantly improved outcomes because CK2β deficiency resulted in increased survival 7 days after tMCAO. As illustrated by the Kaplan-Maier curve, 53.8% of ck2β−/− mice survived the 7-day observation period after tMCAO, whereas 82.5% of the ck2βfl/fl mice died within 7 days after ischemic stroke. Notably, we found no intracranial hemorrhages in ck2β−/− mice after tMCAO (Figure 7F).
Ck2β−/− mice are protected from ischemic stroke in vivo and display less thrombotic cerebral vascular occlusions with a significantly better neurological outcome and survival after stroke. (A) Representative images of 3 corresponding coronal sections of 2,3,5-triphenyltetrazolium chloride–stained brains from ck2βfl/fl and ck2β−/− mice 24 hours after tMCAO (left). Arithmetic means ± standard errors of the mean (n = 11-12) of brain infarct volumes in ck2βfl/fl (blue bar) and ck2β−/− mice (gray bar) 24 hours after tMCAO (right). (B) Bederson score reflecting global neurological defects (0 indicates best, 5 indicates worst; left) and grip test indicating motor functional and coordination deficits (0 indicates worst, 5 indicates best; right) assessed 24 hours after tMCAO. Each dot represents 1 individual mouse. (C) Representative coronal T2-weighted magnetic resonance imaging images of hyperintense ischemic brain infarct lesions of ck2βfl/fl and ck2β−/− mice at day 1 and day 7 (infarct maturation) after tMCAO (n = 3-7/group). Hypointense areas (reflecting intracerebral hemorrhage) were not observed. (D) Determination of thrombosis index by quantification of thrombotic occluded vs nonoccluded vessels within the ischemic hemisphere 24 hours after tMCAO. Arithmetic means ± standard errors of the means (n = 5, right) and representative images (left) of H&E-stained cryosections are shown. Bar represents 100 μm. (E) Arithmetic means ± standard errors of the means (n = 6) of infarct volume in ck2βfl/fl and ck2β−/− mice 7 days after tMCAO (infarct maturation). (F) Analysis of survival of ck2βfl/fl (blue line) and ck2β−/− (gray line) mice 7 days after ischemic stroke (n = 13-17). Student t test in panels A-D and log-rank (Mantel-Cox) analysis in panel F. **P < .01.
Ck2β−/− mice are protected from ischemic stroke in vivo and display less thrombotic cerebral vascular occlusions with a significantly better neurological outcome and survival after stroke. (A) Representative images of 3 corresponding coronal sections of 2,3,5-triphenyltetrazolium chloride–stained brains from ck2βfl/fl and ck2β−/− mice 24 hours after tMCAO (left). Arithmetic means ± standard errors of the mean (n = 11-12) of brain infarct volumes in ck2βfl/fl (blue bar) and ck2β−/− mice (gray bar) 24 hours after tMCAO (right). (B) Bederson score reflecting global neurological defects (0 indicates best, 5 indicates worst; left) and grip test indicating motor functional and coordination deficits (0 indicates worst, 5 indicates best; right) assessed 24 hours after tMCAO. Each dot represents 1 individual mouse. (C) Representative coronal T2-weighted magnetic resonance imaging images of hyperintense ischemic brain infarct lesions of ck2βfl/fl and ck2β−/− mice at day 1 and day 7 (infarct maturation) after tMCAO (n = 3-7/group). Hypointense areas (reflecting intracerebral hemorrhage) were not observed. (D) Determination of thrombosis index by quantification of thrombotic occluded vs nonoccluded vessels within the ischemic hemisphere 24 hours after tMCAO. Arithmetic means ± standard errors of the means (n = 5, right) and representative images (left) of H&E-stained cryosections are shown. Bar represents 100 μm. (E) Arithmetic means ± standard errors of the means (n = 6) of infarct volume in ck2βfl/fl and ck2β−/− mice 7 days after tMCAO (infarct maturation). (F) Analysis of survival of ck2βfl/fl (blue line) and ck2β−/− (gray line) mice 7 days after ischemic stroke (n = 13-17). Student t test in panels A-D and log-rank (Mantel-Cox) analysis in panel F. **P < .01.
Discussion
The results of the present study disclose a key role for the protein kinase CK2 in the tightly regulated signaling processes in MKs and platelets. CK2β is required for thrombopoiesis and Ca2+-triggered platelet activation and, by the same token, contributes to acute arterial thrombotic occlusion and consecutive ischemic diseases, such as acute myocardial infarction and ischemic stroke, the leading causes of death and permanent disability in industrialized countries.18
Ck2β−/− mice suffer from severe macrothrombocytopenia resulting from defective thrombopoiesis due to premature MK fragmentation with prematurely released, enlarged platelets and crippled proplatelet formation, potentially driven by significantly altered tubulin polymerization and impaired microtubule assembly. Similar macrothrombocytopenia was observed in mice lacking other regulators of platelet tubulin organization and microtubule dynamics, such as Filamin A (FlnA)33 or the GTPases, Rac1/Cdc42.34 Similar to Rac1/Cdc42-null platelets or platelets with a genetic ablation of FlnA, ck2β−/− platelets harbor less vWF receptor complex abundance than ck2βfl/fl platelets (Table 1). FlnA cross-links actin filaments, connecting vWF receptor GPIb-IX-V to the platelet cytoskeleton, and interacts with Syk, regulating platelet ITAM-mediated receptor signaling and function.33,35 Similar to ck2β−/− platelets, FlnA-null platelets are relatively insensitive to activation.35 However, neither FlnA expression (supplemental Figure 15) nor Syk phosphorylation (supplemental Figure 8C) were significantly affected in CK2β-deficient platelets. Double knockout of 2 additional major regulators of tubulin organization, Rac1 and Cdc42, resulted in altered MK morphology and uncontrolled MK fragmentation,34 similar to ck2β−/−. Nevertheless, the activity of Rac1 and Cdc42 was unaffected in ck2β−/− platelets (supplemental Figure 16), excluding Rac1/Cdc42 as potential downstream targets of CK2 in MKs or platelets.
In vivo visualization of thrombopoiesis in MK/platelet-specific knockout of CK2β revealed significant alterations in the MK cytoskeleton, resulting in premature ectopic M-fragmentation and severely affected proplatelet formation. During MK maturation, polymerization of microtubules assembled from α- and β-tubulin heterodimers is essential for maintaining reorganization of the MK cytoskeleton underlying proplatelet formation and subsequent platelet production.4,5 Recently, tubulin has been reported as a direct target of CK2.25,36 Accordingly, CK2 directly binds tubulin heterodimers, thus strongly stabilizing microtubule polymerization.23 Even the microtubule-stabilizing agent paclitaxel failed to stabilize polymerized tubulin and to prevent depolymerization of β1-tubulin in ck2β−/− MKs. β1-tubulin, restrictively expressed in the MK lineage,37 is the major tubulin isotype in platelets and is indispensable for microtubule assembly and thus platelet production in MKs.6 Loss of β1-tubulin in mice and loss-of-function β1-tubulin polymorphisms in humans both result in severely impaired proplatelet formation with significant macrothrombocytopenia due to deformed microtubules, premature MK fragmentation, and release of large platelets,6,7,37,38 a phenotype virtually identical to the deletion of CK2β in the MK/platelet lineage reported in this article. Due to the abrogated tubulin polymerization, binding of EB3 to polymerizing microtubules was impaired in ck2β−/− platelets and MKs. Accordingly, proplatelet maturation and platelet release in MKs may be affected by CK2β deficiency. However, despite the analogy between loss of β1-tubulin and CK2β in the MK/platelet lineage for platelet biogenesis, the phenotype regarding platelet activation is different. As reported recently,37 even if aggregation of β1-tubulin−/− platelets was blunted after stimulation with a low dose of thrombin, thrombus formation in mice lacking platelet β1-tubulin was not as strongly impaired as in ck2β−/− mice, suggesting that CK2 deficiency affects additional mechanisms in platelets that are essential for activation and thrombus formation beyond modulating platelet β1-tubulin.
Ck2β-deficient mice compensate for defective proplatelet formation and insufficient thrombopoiesis by increasing BM megakaryopoiesis and extramedullary thrombopoiesis. Similar to what was observed in other mice with impaired platelet production as a result of abolished microtubule assembly,39 splenomegaly was only mild in ck2β−/− mice despite the profound spleen MK hyperplasia. The reticuloendothelial system in the liver and spleen clears platelets from the circulation due to altered cellular morphology34 or platelet apoptosis.40,41 In theory, CK2 could suppress apoptosis by the stimulation of PI3K/Akt signaling,42 direct interaction with the Bcl-2-associated death promoter (BAD),43 or decreased susceptibility of caspase substrates, such as the BH3 interacting-domain death agonist (BID), to caspase cleavage.36,44 However, phosphorylation of Akt and its downstream target, BAD, both critical elements in prosurvival signaling of platelets,45 were not significantly affected in ck2β−/− platelets (supplemental Figure 17).
Besides the critical role of CK2 in thrombopoiesis, the present study revealed that CK2 is essential for the activation-dependent [Ca2+]i increase via the regulation of IP3-dependent Ca2+ release from internal stores. IP3 is generated by several PLC isoforms and mediates intracellular Ca2+ release by activating IP3Rs located on the ER.13,46 Although platelet activation by collagen and CRP culminates in PLCγ2-activation downstream of GPVI, stimulation with thrombin activates PLCβ via G-protein–coupled signaling.47 IP3R function is presumably regulated in part by the modulation of IP3R expression, clustering, and phosphorylation.8,13 Cyclic nucleotide cyclic adenosine monophosphate (cAMP) mediates its inhibitory effects on platelet activation potentially by phosphorylating IP3Rs with subsequent inhibition of intracellular Ca2+ release.13,46 However, even though CK2 has recently been identified as a negative regulator of cAMP generation,48 neither cAMP levels (data not shown) nor phosphorylation of IP3R1, mainly expressed in the ER of platelets, were significantly affected in ck2β−/− platelets (supplemental Figure 9). Total IP3R protein abundance was similar in ck2β−/− platelets and ck2βfl/fl platelets (supplemental Figure 9). The major regulators of IP3-induced Ca2+-signaling also includes microtubule-associated EB3, which directly interacts with the S/TxIP motif within IP3Rs, thus facilitating IP3R clustering and activation.8 Because ck2β−/− platelets display blunted tubulin polymerization as well as abrogated binding of EB3 and IP3R1 to the fraction of polymerized tubulin, a disordered IP3R clustering may contribute to impaired Ca2+ release from intracellular stores in ck2β−/− platelets. These findings are in line with previous studies reporting that the disruption of the cortical microtubule system results in abrogated intracellular Ca2+ release.49
In platelets, IP3-mediated Ca2+ release from the ER triggers Ca2+ entry from the extracellular compartment, potentiating the [Ca2+]i increase and thus facilitating subsequent platelet activation and aggregation with consecutive thrombus formation. Intracellular Ca2+ store depletion is sensed by STIM1, which activates store-operated Ca2+ entry via the pore-forming Ca2+ release–activated channel Orai1.47 Similar to ck2β−/− mice, animals expressing mutant Stim1 displayed macrothrombocytopenia, MK hyperplasia, and decreased platelet life span, pointing to an important role of intracellular Ca2+ regulation in MKs/platelets for thrombopoiesis.32 In STIM1-deficient platelets, the Ca2+ response on platelet activation with CRP and thrombin is blunted,47 mimicking the phenotype of ck2β−/− platelets. However, STIM1 expression was similar in ck2β-null platelets and wild-type platelets (supplemental Figure 18).
Although CK2 is readily detected in platelets, its role in platelet function remained ill defined. CK2 is constitutively active in platelets, suggesting that its effects are tightly modulated by phosphatases.31 According to pharmacological studies using CK2 inhibitors, CK2 may play a role in platelet activation.50-52 However, the precise role of CK2 in platelet function and arterial thrombosis has remained elusive due to the lack of selectivity of the reagents available to target CK2 and its subunits. Pharmacological approaches suggested that CK2 modulates PI3K-Akt–dependent signaling in platelets.51,52 In our genetic knockout approach, we did not find evidence for CK2-dependent activation of the PI3K downstream effectors Akt and GSK3β in platelets (supplemental Figure 17). We do not have a precise explanation for these apparent discrepancies other than the limited selectivity of the pharmacological CK2 inhibitors used.53-55 For example the CK inhibitor 5,6-dichloro-1-(β-d-ribo-furanosyl) benzimidazole, used in some of previous studies on platelets, inhibits both CK2 and CK1 isoforms as well as other cellular targets.50 In the present study, we were able to define CK2β as a critical regulator of Ca2+-triggered platelet activation using MK/platelet-specific deletion of the CK2 regulatory β-subunit. Similar to our observations in ck2β-null platelets, treatment of murine and human platelets with TBCA and CX-4945 significantly affected platelet Ca2+ influx, secretion, and aggregation in response to CRP (supplemental Figure 10-14) without affecting Akt phosphorylation (supplemental Figure 19), indicating that CK2 is crucial for GPVI-dependent signaling without involving Akt-dependent intracellular pathways. Because TBCA and CX-4945 sufficiently blocked platelet Akt phosphorylation after activation with the Par-4 agonist AYPGKF or 2-methylthioadenosine diphosphate (2-MeSADP), we speculate that CK2 may play a role in modifying Akt signaling downstream of PAR-4 or the purinergic receptor P2Y12. However, further studies will be necessary to clarify the role of CK2 in signaling downstream of purinergic receptors, in particular P2Y12. Inhibition of CK2 in thrombin-driven platelet activation did not affect Akt phosphorylation (supplemental Figure 19), pointing to Akt-independent CK2 signaling in response to platelet stimulation with thrombin. Nevertheless, consistent with the findings by Ampofo et al,56 using the CK2 inhibitor CX-4945, we show for the first time that CK2β is an essential player in thrombopoiesis and arterial thrombosis in vivo.
According to our observations, activation-dependent Ca2+ release is mainly under the control of the constitutively active CK2. As a consequence of the severely impaired Ca2+ response, platelet adhesion, secretion, and integrin αIIbβ3 activation are significantly abolished in ck2β−/− platelets. The marked knockdown of integrin αIIbβ3 activation in response to thrombin or CRP in platelets lacking CK2β paralleled a deficit in the ability of these platelets to undergo aggregation at submaximal agonist concentrations. Increasing the agonist concentrations dissipated the differences between ck2β−/− and ck2βfl/fl platelets, indicating that other signaling processes are able to bypass intracellular platelet pathways that are controlled by CK2. The defective activation of ck2β−/− platelets in vitro translated into a significantly blunted arterial occlusive thrombus formation in vivo, revealing CK2β to be a potential drug target for antithrombotic therapy, because tail bleeding time, partially reflecting primary hemostasis, was not significantly affected. Platelet-specific deletion of CK2β in mice resulted in a significant reduction of intravascular thrombosis and a profound protection against ischemic neuronal damage, with significantly improved neurological outcomes and survival 7 days after ischemic stroke. However, it is important to note that the data obtained in the murine tMCAO model cannot be extrapolated to humans without reservations due to possible differences in the underlying pathomechanisms.47 Of note, not only does CK2 regulate platelet-triggered thrombus formation, but also a considerable number of proteins involved in the coagulation cascade have been identified as substrates of CK2.57 CK2 phosphorylates several coagulation factors, tissue factor pathway inhibitor, and fibrinogen, suggesting that CK2 may also directly interfere with plasmatic coagulation.57,58
In conclusion, using MK/platelet-specific deletion of the CK2 regulatory β-subunit, we provide strong evidence that CK2 is essential for platelet biogenesis and activation. CK2 is critically required for the regulation of MK maturation and fragmentation, both underlying sufficient proplatelet formation, due to the fact that tubulin polymerization is controlled by CK2. Further, CK2 was identified as a major player in the activation-dependent [Ca2+]i increase by facilitating the release from stores as well as Ca2+-dependent platelet activation and arterial thrombus formation in vivo. These findings may have important implications for the development of novel antithrombotic therapies because the absence of CK2β provides profound protection from occlusive arterial thrombosis and ischemic stroke in vivo with substantially improved outcomes. Further, this study may deepen the understanding of the (patho-)physiological mechanisms underlying proplatelet formation, thrombopoiesis, and the development of macrothrombocytopenia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the microscopy platform of the Bioimaging Centre (Rudolf Virchow Centre) for providing technical infrastructure and support. The authors also thank Daniela Eißler for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft grants BO 3786/1-1 (O.B.), KFO274 (M.G., O.B., and F.L.), SFB688 (D. Stegner and B.N.), the Fortüne Research Programme (grants 2133-0 and 1895-0), the German Cardiac Foundation (grant F16/15), and the Dr Karl Kuhn Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: P.M., B.W.-A., S.G., F.L., M.G., and O.B. designed the research; P.M., B.W.-A., S.G., F.L., E.G., D. Stegner, K.A., D. Semeniak, M.C., I.G.M., M.M., and L.Q.-M. conducted the experiments; P.M., B.W.-A., S.G., F.L., E.G., D. Stegner, D. Semeniak, M.C., L.Q.-M., H.R.S., C.K., I.P., H.S., and O.B. analyzed the data; D.W.L., T.B., F.L., B.N., and H.S. provided reagents; and P.M., B.W.-A, D.W.L., F.L., B.N., I.P., H.S., M.G., and O.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oliver Borst, Department of Cardiology and Cardiovascular Medicine, University of Tübingen, Otfried Müller-Str10, 72076 Tübingen, Germany; e-mail: oliver.borst@med.uni-tuebingen.de.
References
Author notes
P.M. and B.W.-A. contributed equally and are joint first authors.

![Figure 5. CK2β is a critical regulator of platelet IP3 -triggered Ca2+ release from internal stores with subsequent extracellular Ca2+ influx, resulting in impaired granule secretion, integrin αIIb β3 activation, and aggregation in ck2β-null platelets. (A) Representative tracings of Fura-2-fluorescence reflecting the cytosolic Ca2+ concentration [Ca2+]i (right) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; left) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with CRP (5 µg/ml) and thrombin (20 mU/ml) in the absence (0.5 mM EGTA) of extracellular Ca2+. (B) Representative tracings of Fura-2-fluorescence reflecting the cytosolic Ca2+ concentration [Ca2+]i (right) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; left) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with CRP (5 µg/ml) and thrombin (20 mU/ml) in the presence (1 mM Ca2+) of extracellular Ca2+. (C) Representative tracings of Fura-2-fluorescence reflecting cytosolic Ca2+ concentration [Ca2+]i (lower) and arithmetic means of maximal ∆[Ca2+]i ± standard deviations (n = 6; upper) of ck2βfl/fl (blue line) and ck2β−/− (gray line) platelets before and after stimulation with IP3 (2.5 µM). The cytosolic Ca2+ concentration before and after flash photolysis (365 nm) in the absence of IP3 was measured as a control (tracing not shown). (D) Flow cytometric analysis of P-selectin exposure reflecting α-granule release in ck2βfl/fl (blue bars) and ck2β−/− (gray bars) platelets in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Arithmetic means ± standard errors of the means (n = 6) are shown. (E) Luminescence analysis of ATP release reflecting the secretion of dense granules in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Representative ATP release tracings of ck2βfl/fl (blue lines) and ck2β−/− (gray lines) mice are shown (n = 6). (F) Flow cytometric analysis of αIIbβ3 integrin activation in ck2βfl/fl (blue bars) and ck2β−/− (gray bars) platelets in response to increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Arithmetic means ± standard errors of the means (n = 6) are shown. (G) Light transmission aggregometry after stimulation with increasing concentrations of CRP (in micrograms per milliliter) or thrombin (in microunits per milliliter). Representative aggregation tracings of ck2βfl/fl (blue lines) and ck2β−/− (gray lines) platelets are shown (n = 4). Unpaired Student t test in panels A-D. *P < .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/25/10.1182_blood-2017-05-784413/4/m_blood784413f5.jpeg?Expires=1765920714&Signature=jBgcHZZLLPVLfJ-8KuT-KFsuunRjTN2xqYCLj69HZea~ri6dcbShNtCMMCSE9XQgXl5vL3bJX2lhdAIQWMqwyqzw9cqXQkRz9LyYFlegfu8SmoazDwmPbWN3vo6e9VW6Q4rqepXzXWeUL9bNuh~amSK769LTH3qodREfqkZHKP71sI-ggwUXu~h~rK~KYOUS3LTSGnh~10IV2m3WdC4mtD6J8CbBKwL3CSGUitrw1OXVA2Eg~x-nlbQYQI-8Gdi5nARFMett-qOYusIR~sLcvBxxLxsfGkSjmDm3qjP~gQ7AiGW8KiMbLQXr7wmTtM8T4DMNeIat2fE-UiUG0vSWSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal