Abstract
Introduction: LT of myeloproliferative neoplasms (MPN), including primary or post-polycythemia vera (PV)/essential thrombocythemia (ET) MF, is associated with dismal prognosis, largely owing to the low rates and short duration of responses to currently available therapies. Ruxolitinib is the only approved therapy for MF. No data are available on the characteristics and outcomes of MF LT in pts who received ruxolitinib during the course of MF.
Methods: We reviewed our database of consecutive pts with MF followed at our institution from 1993 to 2016 and sorted those who experienced LT. We then divided pts into those who received ruxolitinib during the MF course and those who never did, and compared these two groups in terms of clinical characteristics, biology of LT, response to therapy, and outcome.
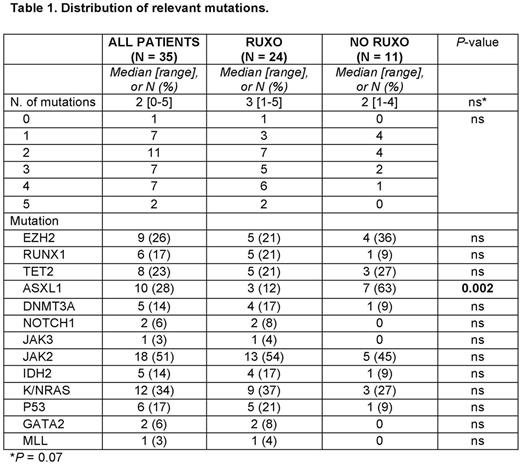
Results: 135 pts were analyzed: 39 had received ruxolitinib (RUXO), and 96 had not (NO-RUXO). Time to LT was similar between the two groups (45.7 and 45.6 months, P = 0.36), as were median age, gender distribution, proportion of post-PV/ET cases, distribution of driver mutations, or number of treatments for MF (besides ruxolitinib) (all P-values = ns). Similarly, there were no significant differences in peripheral blood and bone marrow parameters of the LT between the two groups, with the possible exception of a lower median Hgb in RUXO pts (8.5 g% vs 9.1 g%, P = 0.05). Complex karyotype was found in 40% and 36% of RUXO and NO-RUXO pts, respectively, and a monosomal karyotype in 20% and 23%, respectively (all P-values = ns). The distribution of relevant mutations, as detected by a previously described next-generation sequencing 28-gene panel (Patel et al. Blood 2015), presented some differences, mostly not statistically significant, likely due to small number of tested pts (Table 1). Notably, however, ASXL1 mutations appeared to be less frequent in RUXO than NO-RUXO pts (12% vs. 63%, respectively P = 0.002). For the purposes of this analysis, induction therapies were grouped into high-dose ara-C (HiDAC)-based, low-dose (Lo)DAC-based, hypomethylating agent (HMA)-based, and others. Similar proportions of patients in the RUXO and NO-RUXO groups received each of these treatment types. Finally, 20% and 25% of the RUXO and NO-RUXO pts, respectively, underwent SCT. The overall response rates (ORR) and complete RR (CRR) were similar between the two groups (40% and 23% for RUXO pts, respectively, and 42% and 38% for NO-RUXO pts, respectively). Similar CRR were achieved in pts who received JAK inhibitors as part of their LT induction regimen and those who did not (20% vs. 37%, P = 0.21). As of this writing, 17 pts are alive. The cause of death was leukemia in the overwhelming majority of pts. The median OS for the entire population, RUXO and NO-RUXO pts is 6.1, 7.9, and 6.1 months, respectively (P = ns). The median PFS is 2.8, 3.1, and 2.6 months, respectively (P = ns). In each group, pts who underwent SCT fared better than those who did not (OS 4.2 vs 18.2 months P < 0.001 and 3.7 vs 18.2 months, respectively, P = 0.004) The presence of ≥3 mutations was independently associated with shorter OS in MF patients treated with ruxolitinib (Patel et al. Blood 2015). We found such association to be of borderline significance in the overall MF LT population (11.7 vs. 1.9 months, P = 0.07) and not statistically significant in the RUXO population (OS 10.1 vs 1.7 months, P = 0.29), in either case likely due to small sample size.
Conclusions: RUXO and NO-RUXO pts appeared to have similar responses to induction therapy for MF LT. We did not observe a statistically significantly longer PFS or OS in RUXO pts compared to NO-RUXO pts. Among MF pts who experience LT, prior therapy with ruxolitinib might be associated with a distinct pattern of mutations in the leukemic clone, including lower incidence of ASXL1 mutation. Analysis of a larger sample will be necessary to solidify these findings.
Bose: Incyte Corporation: Honoraria. Kantarjian: Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; Delta-Fly Pharma: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding. Verstovsek: Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Pfizer: Research Funding; Gilead: Research Funding; Pfizer: Research Funding; Seattle Genetics: Research Funding; Bristol Myers Squibb: Research Funding; Lilly Oncology: Research Funding; Celgene: Research Funding; Astrazeneca: Research Funding; Celgene: Research Funding; NS Pharma: Research Funding; Roche: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Promedior: Research Funding; Blueprint Medicines Corp: Research Funding; Bristol Myers Squibb: Research Funding; Astrazeneca: Research Funding; Galena BioPharma: Research Funding; Genentech: Research Funding; Blueprint Medicines Corp: Research Funding; Galena BioPharma: Research Funding; CTI BioPharma Corp: Research Funding; Incyte: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Lilly Oncology: Research Funding; Roche: Research Funding; NS Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal