Abstract
Chronic lymphocytic leukemia (CLL) is characterized by clonal proliferation and accumulation of malignant B lymphocytes in the blood, bone marrow, spleen, and lymph nodes. This process is associated with constitutively activated B cell receptor (BCR) signaling, and interference with BCR signaling provides therapeutic benefit. Specifically, the Bruton's Tyrosine Kinase (BTK) inhibitor ibrutinib prevents BTK tyrosine phosphorylation and thereby interferes with pathways downstream of BCR. It has shown high clinical response rates in patients with relapsed and refractory CLL, including patients with adverse cytogenetic profiles.
Despite the high response rates achieved by ibrutinib, the drug has important limitations. Ibrutinib treatment induces a redistribution of CLL cells from protected niches to the peripheral blood, and the cellular response to ibrutinib is slow and often incomplete. Further, there is no evidence that a cure can be achieved, and drug discontinuation (e.g., due to toxicity) is associated with rapid progression. Even among patients that tolerate long-term treatment with ibrutinib, a considerable percentage develops drug resistance (e.g., due to mutations in the BTK gene), BTK independent disease progression, or Richter's transformation, indicating drug synergies with ibrutinib may increase prognosis.
Recent studies have explored the combined use of ibrutinib with the proteasome inhibitor carfilzomib, the BCL2 inhibitor venetoclax, and the HDAC inhibitor abexinostat in preclinical models, which has shown promising initial results. However, these approaches were largely empirical, and little is known about the gene-regulatory effects of ibrutinib treatment in CLL cells.
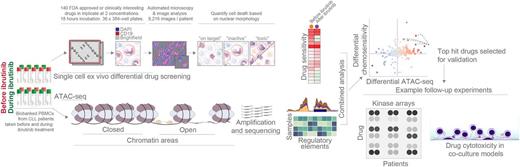
Here, we charted the ibrutinib-induced chromatin regulatory landscape of CLL, and in parallel mapped targetable pathways for synergistic combination therapies that could potentially improve disease control. Therefore, peripheral blood from 24 fully characterized CLL cases, including the clinical and hematological parameters, disease stage, cytogenetics (13q del, 11q del, 17p del, trisomy 12), p53 and IGHV mutation status were collected before and during therapy with ibrutinib. Chromatin accessibility was measured by ATAC-seq, a powerful assay for mapping the genome-wide regulatory landscape of cell. In addition, the ex vivo chemosensitivity to >140 drugs on paired CLL samples collected before and during ibrutinib treatment was measured using a novel method for ex vivo single-cell drug cytotoxicity profiling in primary samples (Snijder et al. submitted; NCT03096821).
We bioinformatically analyzed the changes in chromatin accessibility and differential drug responses before and during ibrutinib treatment, thereby establishing a comprehensive picture of the cellular responses to the drug. As a proof of principle for this approach, ex vivo results confirmed strong clinical activity of BCL2 inhibitors with and without ibrutinib. We identified increases in chromatin accessibility and chemosensitivity in proteasome, inflammatory NF-κB/TNF signaling, CoA biosynthesis, PI3K/Akt, caffeine metabolism pathways, along with changes affecting genes such as FOXO3 and IκBa. Integration of chromatin accessibility and differential chemosensitivity data revealed robust ibrutinib-induced signatures, which we exploited to prioritize approved drugs and drug candidates for synergistic treatment of CLL cells in co-culture models using primary bone marrow stromal cells. Cell viability assays revealed that ibrutinib treatment in vivo and in vitro sensitizes CLL cells to compounds such as the proteasome inhibitor bortezomib, the JAK inhibitor ruxolitinib, the PLK1 inhibitor volasertib, and the bisphosphate zoledronate.
In summary, our results show that the synergistic combination and bioinformatic integration of chromatin profiling with functional drug screening is a powerful tool to identify targetable pathways in CLL. This approach may be useful for designing personalized therapies as well as the rational planning of clinical studies. Our approach is directly transferable to other leukemic diseases in which malignant cells can be obtained for chromatin profiling and drug sensitivity analysis, thus providing a widely applicable tool for the rational development of combination therapies.
Vladimer: Allcyte Gmbh: Equity Ownership. Snijder: Allcyte: Equity Ownership. Krall: Allcyte Gmbh: Equity Ownership. Hoermann: Gilead: Honoraria, Research Funding; Amgen: Honoraria; Novartis: Honoraria; Ariad: Honoraria. Staber: Morphosys: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Gilad: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria. Superti-Furga: Allcyte GmbH: Equity Ownership. Jaeger: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal