Key Points
CD34+ progenitor-derived NK cells and HMAs potently cooperate against AML cells.
DAC-mediated modulation of CD34-derived NK cell phenotype, function, and trafficking results in enhanced anti-leukemic effect in vivo.
Abstract
Combining natural killer (NK) cell adoptive transfer with hypomethylating agents (HMAs) is an attractive therapeutic approach for patients with acute myeloid leukemia (AML). However, data regarding the impact of HMAs on NK cell functionality are mostly derived from in vitro studies with high nonclinical relevant drug concentrations. In the present study, we report a comparative study of azacitidine (AZA) and decitabine (DAC) in combination with allogeneic NK cells generated from CD34+ hematopoietic stem and progenitor cells (HSPC-NK cells) in in vitro and in vivo AML models. In vitro, low-dose HMAs did not impair viability of HSPC-NK cells. Furthermore, low-dose DAC preserved HSPC-NK killing, proliferation, and interferon gamma production capacity, whereas AZA diminished their proliferation and reactivity. Importantly, we showed HMAs and HSPC-NK cells could potently work together to target AML cell lines and patient AML blasts. In vivo, both agents exerted a significant delay in AML progression in NOD/SCID/IL2Rgnull mice, but the persistence of adoptively transferred HSPC-NK cells was not affected. Infused NK cells showed sustained expression of most activating receptors, upregulated NKp44 expression, and remarkable killer cell immunoglobulin-like receptor acquisition. Most importantly, only DAC potentiated HSPC-NK cell anti-leukemic activity in vivo. Besides upregulation of NKG2D- and DNAM-1–activating ligands on AML cells, DAC enhanced messenger RNA expression of inflammatory cytokines, perforin, and TRAIL by HSPC-NK cells. In addition, treatment resulted in increased numbers of HSPC-NK cells in the bone marrow compartment, suggesting that DAC could positively modulate NK cell activity, trafficking, and tumor targeting. These data provide a rationale to explore combination therapy of adoptive HSPC-NK cells and DAC in patients with AML.
Introduction
Harnessing natural killer (NK) cells against cancer is an emerging therapeutic approach, which is increasingly being explored for both hematological malignancies and solid tumors. Next to activation of the patient's own NK cells by means of cytokine administration or redirection through treatment with tumor-targeting antibodies, current research also invests in ex vivo NK cell generation and expansion methods for adoptive cell therapy. Previously, we reported on good manufacturing practice–compliant, cytokine-based culture systems for the generation of NK cell products from CD34+ hematopoietic stem and progenitor cells (HSPC-NK cells).1-3 Using this platform, we conducted a first-in-human phase 1 study in older patients with acute myeloid leukemia (AML) who were ineligible for allogeneic stem cell transplantation.4 In this study, escalating dosages of ex vivo–generated HSPC-NK cells were infused after lymphodepleting chemotherapy. It is important to note that we demonstrated infusion of up to 30 million allogeneic HSPC-NK cells per kg body weight was feasible, well tolerated, and safe, without inducing graft-versus-host disease or nonhematological toxicities. Although this study included older patients with AML who were in morphological complete remission, 2 patients showed persistent minimal residual disease (MRD) before treatment. It is interesting to note that MRD decreased below the detection level by day 90 after infusion of HSPC-NK cells, emphasizing a possible therapeutic activity of adoptively transferred HSPC-NK cells. In a likewise manner, promising anti-tumor responses have been reported worldwide from patients with cancer undergoing NK cell adoptive immunotherapy.5-10 However, responses are mostly transient and concern a minority of the patients. Therefore, efforts have to be made to further maximize tumor-targeting efficacy and NK cell responsiveness in vivo, likely through combinatorial treatment strategies.11 In the context of AML, an attractive approach resides in the combination of HSPC-NK cells with generally well-tolerated hypomethylating agents (HMAs).12
Azacitidine/5-azacytidine (Vidaza, AZA) and decitabine/5-aza-2′-deoxycytidine (Dacogen, DAC) are 2 HMAs currently used for the treatment of AML and myelodysplastic syndromes.13 These HMAs are cytidine nucleoside analogues that incorporate into the DNA during cell replication and division. Although at higher dosages these agents exert direct toxicities toward myeloid cancer cells through DNA damage, at lower dosages they can modulate gene expression due to hypomethylating activity. Particularly, their potential in upregulating NK-activating molecules, such as NKG2D ligands, on tumor cells through their epigenetic modulation and thereby sensitizing tumors to NK-cell–mediated killing, has been reported in several studies and for different cancers, including AML.14-17 Nonetheless, direct impact of HMAs on NK-cell functionalities has not been well established yet. Conflicting data have been reported, describing either advantageous or a deleterious effect on NK cells.15,18-22 Moreover, these data are mostly derived from in vitro studies, often performed at high drug concentrations that do not reflect plasma levels achieved in patients. Therefore, it remains unclear whether application of HMA therapy can augment NK cell–mediated anti-tumor responses in patients with AML.
In this report, we address the possibility to combine HSPC-NK cell therapy with HMAs. Through in vitro and in vivo studies, we performed a head-to-head comparison of AZA and DAC to evaluate their impact on HSPC-NK viability and functionalities, as well as their capacity to potentiate HSPC-NK cell reactivity toward AML. Most importantly, we demonstrate that DAC, but not AZA, positively modulates the in vivo anti-leukemic potential of adoptively transferred HSPC-NK cells through AML cell sensitization, enhancement of NK cell maturation, and cytolytic functions, as well as improves on NK cell trafficking and accumulation at the tumor site. Furthermore, our study reveals that HSPC-NK cells and DAC can potently work together to combat AML, providing a strong rationale to explore this combination strategy in AML treatment.
Methods
HSPC-NK cell generation
NK cell products were generated from CD34+ HSPCs derived from umbilical cord blood obtained after normal full-term delivery and written informed consent (“Commissie Mensgebonden Onderzoek” CMO 2014/226). The culture protocol was adapted from Cany et al2 and Roeven et al23 and combined the use of the aryl hydrocarbon receptor antagonist StemRegenin-1 for the expansion of CD34+ HSPCs, together with interleukin-15 (IL-15) and IL-12 for the differentiation of NK cells. In brief, magnetic-activated cell sorting–isolated CD34+ cells (Miltenyi Biotec) were expanded for 9 to 10 days in the presence of stem cell factor, IL-7, Flt3L, and recombinant human thrombopoietin (all 25 ng/mL, ImmunoTools). Thereafter, recombinant human thrombopoietin was replaced by IL-15 (50 ng/mL, ImmunoTools) for 5 days. From days 14 to 15 onward, cell differentiation and expansion were performed in the presence of stem cell factor (20 ng/mL), IL-7 (20 ng/mL), IL-15 (50 ng/mL), and IL-12 (0.2 ng/mL, Miltenyi Biotech). Medium was supplemented with 2 µM StemRegenin-1 (Cellagen Technology) from day 0 to day 21. All media were prepared with basal CellGro DC medium (CellGenix) and supplemented with 10% or 2% pooled human serum (Sanquin Blood Bank Nijmegen) during the expansion and differentiation phases, respectively. The cell density and CD56 acquisition were checked twice per week by flow cytometry (FCM) and adjusted to 1.5 × 106 to 2.5 × 106 cells/mL by addition of a new medium. HSPC-NK cells were used at the end of the culture process with >80% CD56+ cell purity, which was typically achieved within 5 to 6 weeks of culture.
HMAs
We purchased AZA and DAC from Sigma-Aldrich. For in vitro studies, we dissolved both drugs in NaCl 0.9% at 0.1 to 1 mM, aliquoted them for single use, and stored them at −20°C. Drugs were used immediately after thawing, and treatment of the cell cultures was performed with limited light exposure. In vitro, HMAs were used at concentrations similar to those achieved in plasma of HMA-treated patients,24,25 with daily refreshments due to the short half-life of these agents.26 For in vivo studies, 2 to 4 mg of HMAs was kept on ice, protected from light, and dissolved with NaCl 0.9% just before the mice were injected. We also applied treatments daily, in line with current clinical practice.
In vivo studies
All animal experiments were conducted in immune-deficient NOD/SCID/IL2Rgnull (NSG) mice originally purchased from Jackson Laboratories. Mice were housed and bred in the Radboud University Medical Center (Radboudumc) Central Animal Laboratory and were used in experiments at 6 to 12 weeks of age (20-30 g body weight). Studies were approved by the Animal Experimental Committee of Radboudumc and were conducted in accordance with institutional and national guidelines under the university permit number 10300. The preclinical xenograft model for AML was established by intrafemoral injection of the THP-1 cells, engineered to express the green fluorescent protein (GFP) and luciferase reporter genes (THP-1.LucGFP cells) for longitudinal tumor load monitoring by bioluminescence imaging, as previously described.2 In adoptive transfer studies, HSPC-NK cells were resuspended in phosphate-buffered saline and injected intravenously through the tail vein. Survival of HSPC-NK cells in vivo was supported by subcutaneous administration of recombinant human IL-15 (1 µg per mouse; ImmunoTools), every 2 to 3 days after HSPC-NK cell infusion. Alternatively (for data shown in Figure 5), ALT-803, provided by Hing Wong and Sarah Alter from Altor BioScience, was administered subcutaneously at 0.2 mg/kg every 3 to 4 days. The experimental designs implemented for the treatment of mice with HMAs were based on current clinical practice (dosage, duration, and route of administration). The dosages applied in humans (milligrams per meter squared) were translated for mouse studies (milligrams per kilogram) based on the study by Reagan-Shaw et al27 (see supplemental Figure 5 for calculation, available on the Blood Web site). During treatment with HMA, mice were given wet food to improve feeding and tolerability of the drugs. Mice were carefully monitored for weight and general conditions, and euthanized according to well-defined end points. Ex vivo FCM analysis and gene expression profiling were performed on cells isolated from the spleen, bone marrow, or liver with use of erythrocyte lysis solution or lympholyte-M (Cedarlane).
Statistical analysis
We performed statistical analyses using GraphPad Prism 5 software. Student t-tests and 1- and 2-way analyses of variance (ANOVAs) were used when appropriate, as indicated in the figure legends. Differences were considered to be significant for P < .05.
Supplemental information
Materials and methods implemented for NK/AML cell coculture experiments, FCM-based cellular assays, antibodies, and gene expression analysis are available as supplemental information.
Results
HMAs augment HSPC-NK efficacy against AML in vitro
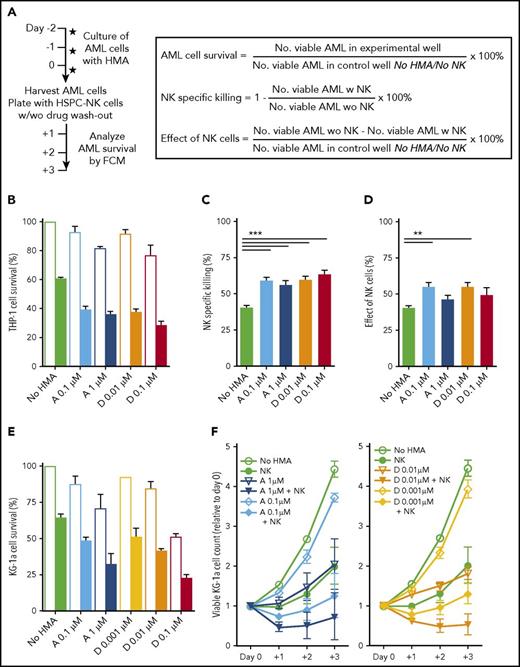
To investigate the possibility of combining HSPC-NK cells with HMA therapy, we first determined the effect of AZA and DAC on 2 AML cell lines. THP-1 and KG1a cells were treated daily with HMAs by use of concentrations similar to those achieved in plasma of treated patients.24,25 As expected, the number of viable AML cells decreased in a time- and dose-dependent manner compared with untreated cells (supplemental Figure 1). AZA at 0.1 to 1 µM and DAC at 0.01 to 0.1 µM had a moderate impact on AML cell viability and proliferation, so these concentrations were selected for subsequent studies.
Next, we tested whether pretreatment of AML cells with HMAs would influence their susceptibility to HSPC-NK cell–mediated killing. Therefore, THP-1 and KG1a cells were cultured in the presence or absence of HMAs for 2 days and were used as targets for HSPC-NK cells with or without washout of the drugs (Figure 1A). At start of the coculture, equal numbers of viable AML cells were plated in each experimental condition. Analysis of AML cell survival showed that the effects of HMAs and HSPC-NK cells were at least additive. After coculture with HSPC-NK cells, the survival of THP-1 cells decreased from 60% without HMA pretreatment to 30% to 40% for pretreated cells (Figure 1B; P < .001 for all HMA groups vs no HMA pretreatment). Because AML cell counts were slightly diminished with the HMAs in the absence of NK cells (Figure 1B), these data translated into a significant increase in NK-specific killing for each treatment condition analyzed independently (Figure 1C). It is remarkable that the effect of HSPC-NK cells, which we defined using the absolute number of AML cells actually killed by NK cells, was significantly increased when THP-1 cells were pretreated with the lower concentrations of HMAs (Figure 1D). These data support that HMAs can sensitize AML cells to HSPC-NK cell–mediated killing. The combination treatment of HMAs and HSPC-NK cells was also studied across time for KG1a cells, without drug washout (Figure 1E-F). Here, the effects of HSPC-NK cells and HMAs were additive, resulting in potent reduction of AML cell numbers when combined together.
HMAs can sensitize AML cells to NK cell–mediated killing. (A) Experimental design: THP-1 and KG1a cells were cultured in the presence of AZA or DAC at the indicated concentrations. Two days later, cells were harvested and used as targets for HSPC-NK cells with or without drug washout. The same numbers of viable AML cells were plated for each condition. The numbers of viable AML cells were determined by FCM after 1 to 3 days of coculture and were used for calculation of AML cell survival, NK-specific killing for each independent treatment, and overall effect of NK cells, as indicated. (B-D) Effect of HMAs pretreatment and HSPC-NK cells on THP-1 cells at day+1. Data were obtained after drug washout and are depicted as the mean ± standard error of the mean (SEM) of 3 independent experiments (1-way ANOVA; **P < .01). (E-F) Effect of HMA pretreatment and HSPC-NK cells on KG1a cells. Data were obtained without drug washout and are depicted as the mean ± SEM of 3 independent experiments. Data shown in panel E were obtained at day+1 and in panel F are depicted the relative numbers of viable AML cells quantified from day 0 to day+3. “No NK” is indicated by open bars; “+NK” is indicated by solid bars (B,E). A, AZA; D, DAC.
HMAs can sensitize AML cells to NK cell–mediated killing. (A) Experimental design: THP-1 and KG1a cells were cultured in the presence of AZA or DAC at the indicated concentrations. Two days later, cells were harvested and used as targets for HSPC-NK cells with or without drug washout. The same numbers of viable AML cells were plated for each condition. The numbers of viable AML cells were determined by FCM after 1 to 3 days of coculture and were used for calculation of AML cell survival, NK-specific killing for each independent treatment, and overall effect of NK cells, as indicated. (B-D) Effect of HMAs pretreatment and HSPC-NK cells on THP-1 cells at day+1. Data were obtained after drug washout and are depicted as the mean ± standard error of the mean (SEM) of 3 independent experiments (1-way ANOVA; **P < .01). (E-F) Effect of HMA pretreatment and HSPC-NK cells on KG1a cells. Data were obtained without drug washout and are depicted as the mean ± SEM of 3 independent experiments. Data shown in panel E were obtained at day+1 and in panel F are depicted the relative numbers of viable AML cells quantified from day 0 to day+3. “No NK” is indicated by open bars; “+NK” is indicated by solid bars (B,E). A, AZA; D, DAC.
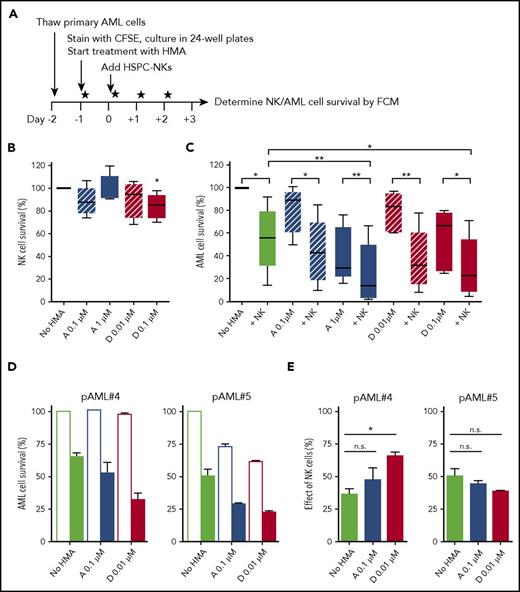
Based on these findings, we next studied the impact of HMAs in HSPC-NK cell cocultures with patient-derived primary AML cells (supplemental Table 1; Figure 2A). Here, we confirmed a robust and dose-dependent effect of HMAs on primary AML cell viability and proliferation (supplemental Figure 2). In contrast, HSPC-NK cell survival was not affected (Figure 2B). It is interesting to note that combined treatment demonstrated sustained killing of primary AML cells by HSPC-NK cells at all drug concentrations tested, and the combination with 1 µM AZA and 0.1 µM DAC showed superior AML-killing efficacy compared with NK cell treatment alone (Figure 2C). As seen with AML cell lines, HSPC-NK cells and HMAs potently worked together in reducing the survival of primary AML blasts (supplemental Figure 3; supplemental Table 2). Although their effects were mostly additive (Figure 2D), the numbers of AML cells killed by NK cells eventually increased in the presence of HMAs, particularly with DAC (as illustrated for patient 4 [pAML#4], Figure 2E). These data demonstrate that HMAs differentially affect AML and HSPC-NK cells in vitro, and that HSPC-NK cells maintain potent anti-leukemic activity during HMA exposure. Most importantly, our findings demonstrate that HMAs can potentiate HSPC-NK cell killing activity, suggesting that the combination of HPSC-NK cells with HMA therapy could result in additive to synergistic effects against AML.
HSPC-NK cells in combination with HMAs potently combat primary AML cells in vitro. (A) Experimental design: Primary AML cells obtained from 5 different patients at diagnosis were stained with carboxyfluorescein diacetate succinimidyl ester and cultured in the presence of AZA or DAC using the indicated concentrations (5 × 104 AML cells per well). One day after, HSPC-NK cells (2.5 × 105 cells per well) were added and the drugs were refreshed daily. The numbers of viable AML cells were determined by FCM at day+3 of coculture. (B) Median HSPC-NK cell survival at day+3 (combined data obtained with 5 different primary AML samples). The number of NK cells quantified without HMAs was set at 100%. (C) Median AML cell survival at day+3 (combined data obtained with 5 different primary AML samples). The number of AML cells quantified without HMAs and NK cells is set at 100%. Data depicted in panels B-C represent combined data obtained with 5 different primary AML samples and were analyzed with 1-way ANOVA. ***P < .001; **P < .01. n.s., not significant. (D-E) The survival of AML cells from 2 different patients (pAML #4 and pAML #5) and corresponding effect of NK cells quantified at day+3 are depicted as the mean ± SEM of data obtained with 4 different HSPC-NK cell donors. Data were analyzed with 2-way ANOVA. ***P < .001; *P < .05. “No NK” is indicated by open bars; “+NK” is indicated by solid bars (D). n.s., not significant.
HSPC-NK cells in combination with HMAs potently combat primary AML cells in vitro. (A) Experimental design: Primary AML cells obtained from 5 different patients at diagnosis were stained with carboxyfluorescein diacetate succinimidyl ester and cultured in the presence of AZA or DAC using the indicated concentrations (5 × 104 AML cells per well). One day after, HSPC-NK cells (2.5 × 105 cells per well) were added and the drugs were refreshed daily. The numbers of viable AML cells were determined by FCM at day+3 of coculture. (B) Median HSPC-NK cell survival at day+3 (combined data obtained with 5 different primary AML samples). The number of NK cells quantified without HMAs was set at 100%. (C) Median AML cell survival at day+3 (combined data obtained with 5 different primary AML samples). The number of AML cells quantified without HMAs and NK cells is set at 100%. Data depicted in panels B-C represent combined data obtained with 5 different primary AML samples and were analyzed with 1-way ANOVA. ***P < .001; **P < .01. n.s., not significant. (D-E) The survival of AML cells from 2 different patients (pAML #4 and pAML #5) and corresponding effect of NK cells quantified at day+3 are depicted as the mean ± SEM of data obtained with 4 different HSPC-NK cell donors. Data were analyzed with 2-way ANOVA. ***P < .001; *P < .05. “No NK” is indicated by open bars; “+NK” is indicated by solid bars (D). n.s., not significant.
Exposure to low-dose HMA concentrations does not impair HSPC-NK cell cytolytic activity in vitro
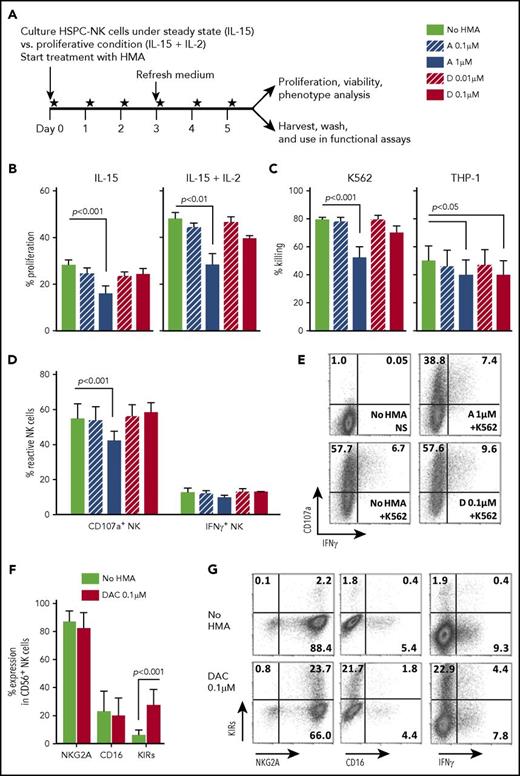
Thereafter, we aimed to confirm that HMAs did not impair HSPC-NK cell functionalities. To do this, HSPC-NK cells were cultured either under proliferative (ie, 20 ng/mL of IL-15 plus 1000 IU/mL of IL-2) or steady-state (ie, 5 ng/mL of IL-15) conditions and treated with HMAs for 6 consecutive days (Figure 3A). HMA treatment did not affect HSPC-NK cell viability (data not shown); however, 1 µM AZA significantly decreased NK cell proliferation (Figure 3B). DAC had a minor impact, even at the highest concentration tested.
Low-dose HMAs do not impair HSPC-NK cell viability, proliferation, and cytolytic functions. (A) Experimental design: Carboxyfluorescein diacetate succinimidyl ester–labeled HSPC-NK cells were cultured under proliferative (high-dose IL-15 and IL-2) or steady-state (low-dose IL-15) conditions in the presence of AZA or DAC refreshed daily at the indicated concentrations. Cell proliferation, viability, and absolute numbers, as well as functionality and phenotype, were analyzed by FCM after 6 days of treatment. (B) Percentages of proliferating HSPC-NK cells under steady-state (left panel) and proliferative (right panel) conditions. Combined data from 3 independent experiments (mean ± SEM) are shown. (C) Specific killing of K562 and THP-1 cells by HSPC-NK cells pretreated with HMAs. The same numbers of viable NK cells were plated in each experimental well after washout of the drug, and the killing of K562 and THP-1 cells was determined after overnight coculture using 1:1 E:T ratio. Data obtained with NK cells that were treated either under proliferative or steady-state conditions and performed with 6 different HSPC-NK cell donors are combined and depicted as mean ± SEM. (D-E) NK cell reactivity upon K562 stimulation and analyzed at the single-cell level by FCM. Combined data from 4 experiments using proliferative (n = 2) or steady-state (n = 2) conditions (D), and representative dot plots of HSPC-NK cells cultured under proliferative conditions and treated with AZA 1 µM, DAC 0.1 µM, or without HMAs (E) are shown. (F) Expression level of the maturation markers NKG2A, CD16, and killer immunoglobulin-like receptor-positive (KIR) cells on HSPC-NK cells following culture upon proliferative conditions in the presence of DAC 0.1 µM, or without HMAs. Mean ± standard deviation (SD) of 6 HSPC-NK cell donors is shown. (G) Representative dot plots of HSPC-NK cell IFN-γ production capacity with respect to KIR expression following DAC 0.1 µM or no HMA treatment under proliferative conditions. Statistical analyses were performed with 1-way (B-D) and 2-way ANOVA (E). NS, not stimulated.
Low-dose HMAs do not impair HSPC-NK cell viability, proliferation, and cytolytic functions. (A) Experimental design: Carboxyfluorescein diacetate succinimidyl ester–labeled HSPC-NK cells were cultured under proliferative (high-dose IL-15 and IL-2) or steady-state (low-dose IL-15) conditions in the presence of AZA or DAC refreshed daily at the indicated concentrations. Cell proliferation, viability, and absolute numbers, as well as functionality and phenotype, were analyzed by FCM after 6 days of treatment. (B) Percentages of proliferating HSPC-NK cells under steady-state (left panel) and proliferative (right panel) conditions. Combined data from 3 independent experiments (mean ± SEM) are shown. (C) Specific killing of K562 and THP-1 cells by HSPC-NK cells pretreated with HMAs. The same numbers of viable NK cells were plated in each experimental well after washout of the drug, and the killing of K562 and THP-1 cells was determined after overnight coculture using 1:1 E:T ratio. Data obtained with NK cells that were treated either under proliferative or steady-state conditions and performed with 6 different HSPC-NK cell donors are combined and depicted as mean ± SEM. (D-E) NK cell reactivity upon K562 stimulation and analyzed at the single-cell level by FCM. Combined data from 4 experiments using proliferative (n = 2) or steady-state (n = 2) conditions (D), and representative dot plots of HSPC-NK cells cultured under proliferative conditions and treated with AZA 1 µM, DAC 0.1 µM, or without HMAs (E) are shown. (F) Expression level of the maturation markers NKG2A, CD16, and killer immunoglobulin-like receptor-positive (KIR) cells on HSPC-NK cells following culture upon proliferative conditions in the presence of DAC 0.1 µM, or without HMAs. Mean ± standard deviation (SD) of 6 HSPC-NK cell donors is shown. (G) Representative dot plots of HSPC-NK cell IFN-γ production capacity with respect to KIR expression following DAC 0.1 µM or no HMA treatment under proliferative conditions. Statistical analyses were performed with 1-way (B-D) and 2-way ANOVA (E). NS, not stimulated.
Next, we analyzed the cytolytic activity of HMA-treated HSPC-NK cells against MHC-Ineg K562 cells and MHC-Ipos THP-1 cells. Target-cell killing was only diminished after HSPC-NK pretreatment with the highest dosages of AZA and, to a lesser extent, with DAC (Figure 3C). These observations were confirmed at the single-cell level with use of CD107a degranulation and intracellular interferon-gamma (IFN-γ) staining. As depicted in Figure 3D-E, the proportion of CD107a+ HSPC-NK cells on K562 stimulation was only negatively affected with 1-µM AZA but not with 0.1-µM DAC. The percentages of IFN-γ+ HSPC-NK cells remained similar.
In addition, we examined the phenotype of HSPC-NK cells cultured in the presence of HMAs. Only the frequency of killer cell immunoglobulin-like receptor (KIR)–positive HSPC-NK cells increased, particularly after exposure to HMAs under proliferative conditions (Figure 3F). We showed previously that NKG2A+KIR+ HSPC-NK cells display higher IFN-γ production capacity compared with NKG2A+KIR− cells.2 Nonetheless, the increase in KIR expression after HMA treatment does not correlate with IFN-γ responses (Figure 3G). Expression of other maturation markers (NKG2A and CD16), as well as activating receptors, adhesion molecules, and death receptors, remained unchanged after exposure to HMAs in vitro (supplemental Figure 4). These data confirm that AML and HSPC-NK cells are differently affected by HMAs, and that low drug concentration preserves HSPC-NK cell anti-leukemic activity.
DAC, but not AZA, potentiates HSPC-NK cell anti-leukemic efficacy in vivo
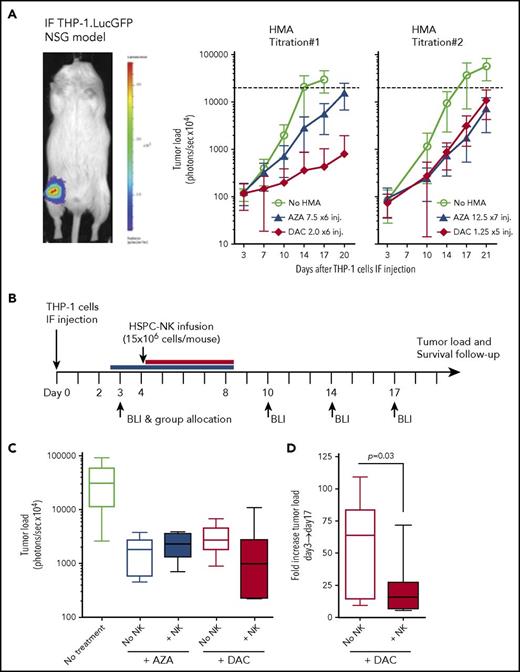
To investigate combinatorial HMA and HSPC-NK cell therapy against AML in vivo, we conducted studies using our established intrafemoral THP-1 mouse model.2 First, we tested the tolerability of HMA treatment in mice by daily application of de-escalating dosages, which were defined based on current practice in patients (ie, 75 mg/m2 for AZA and 20 mg/m2 for DAC). Here, we identified 12.5 mg/m2 of AZA × 7 injections and 5 mg/m2 of DAC × 5 injections as the highest tolerated dose in NSG mice based on body weight loss and reduced THP-1 cell progression (supplemental Figure 5). To obtain a moderate effect of HMAs alone in this model, we subsequently tested 7.5 mg/m2 of AZA and 2.0 mg/m2 of DAC, which are 10 times lower than current dosages applied in patients (Figure 4A, HMA titration #1). These dosages were well tolerated and still reduced THP-1 growth. However, at these dosages, AZA exerted a limited anti-leukemic effect, whereas DAC strongly reduced tumor growth. Based on these observations, we further refined the treatment schedule to give the highest tolerated dose of AZA (12.5 mg/m2) for 7 consecutive days and lowered the dosage of DAC to 1.25 mg/m2 during 5 days (Figure 4A, HMA titration #2). Finally, these dosages resulted in a similar and intermediate effect on THP-1 cells in vivo and were used to test the efficacy of combination therapy with HSPC-NK cells.
DAC, but not AZA, potentiates HSPC-NK cell anti-leukemic effect in vivo. (A) Adult NSG mice that were injected with luciferase-expressing THP-1 cells in their femur were treated with HMAs with use of dosages as indicated in the figure (in milligrams per millimeter squared) and monitored for tumor load progression every 3 to 4 days by bioluminescence imaging (BLI). Treatment was applied daily from days 4 to 9 in titration #1, and from days 2 to 8 and days 4 to 8 for AZA and DAC, respectively, in titration #2. AZA was injected subcutaneously and DAC intravenously based on current clinical practices. Data are depicted as mean ± SD including 8 to 10 mice per group. Dotted lines indicate upper detection limit for tumor load monitoring (signal saturation). (B-D) THP-1–bearing mice were treated with HMAs with use of the same dosages as described in titration #2, and with a single infusion of HSPC-NK cells, applied at day 4. Survival of NK cells in vivo was supported by recombinant human IL-15, given subcutaneously every 2 to 3 days. (B) Experimental design. (C) Median tumor load at day 17. (D) Fold increase in tumor load after 2 weeks of treatment with DAC alone or in combination with HSPC-NK cells (calculated as the ratio between day 17 and day 3 signals). Data were analyzed with an unpaired, 2-tailed Student t test. IF, intrafemoral.
DAC, but not AZA, potentiates HSPC-NK cell anti-leukemic effect in vivo. (A) Adult NSG mice that were injected with luciferase-expressing THP-1 cells in their femur were treated with HMAs with use of dosages as indicated in the figure (in milligrams per millimeter squared) and monitored for tumor load progression every 3 to 4 days by bioluminescence imaging (BLI). Treatment was applied daily from days 4 to 9 in titration #1, and from days 2 to 8 and days 4 to 8 for AZA and DAC, respectively, in titration #2. AZA was injected subcutaneously and DAC intravenously based on current clinical practices. Data are depicted as mean ± SD including 8 to 10 mice per group. Dotted lines indicate upper detection limit for tumor load monitoring (signal saturation). (B-D) THP-1–bearing mice were treated with HMAs with use of the same dosages as described in titration #2, and with a single infusion of HSPC-NK cells, applied at day 4. Survival of NK cells in vivo was supported by recombinant human IL-15, given subcutaneously every 2 to 3 days. (B) Experimental design. (C) Median tumor load at day 17. (D) Fold increase in tumor load after 2 weeks of treatment with DAC alone or in combination with HSPC-NK cells (calculated as the ratio between day 17 and day 3 signals). Data were analyzed with an unpaired, 2-tailed Student t test. IF, intrafemoral.
To assess the impact of HMAs on NK cells in vivo, we applied infusions of HSPC-NK cells plus rhIL-15 at the start of HMA treatment (Figure 4B). Of note, treatment with only HSPC-NK cells was not included because such treatment is ineffective in this highly stringent THP-1 AML mouse model.2 Bioluminescence imaging revealed lower tumor load in mice treated with HSPC-NK cells in combination with DAC, compared with DAC monotherapy (Figure 4C). Although transient, this reduction in tumor progression was significant at 2 weeks after treatment (Figure 4D). In contrast, no difference was observed in AZA-treated mice. This did not rely on a defective HSPC-NK cell persistence on AZA treatment, as the numbers of HSPC-NK cells present in the blood and spleen of treated mice were comparable to those seen in control animals (supplemental Figure 6).
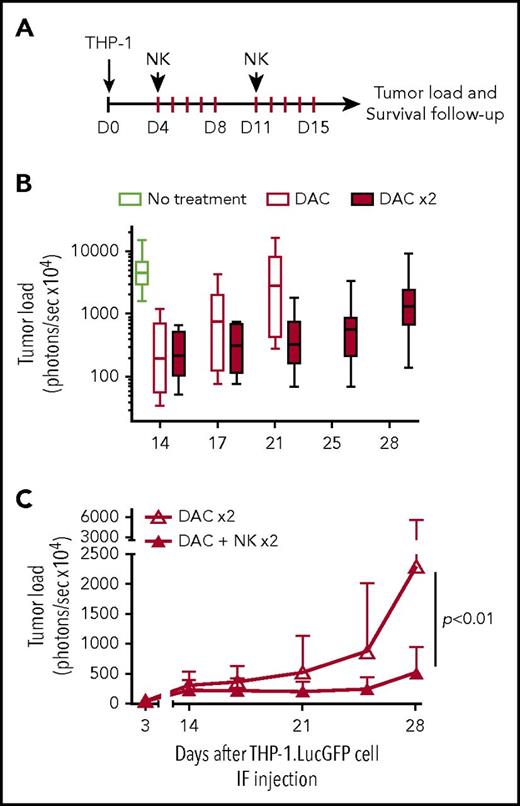
To validate these findings and further improve anti-leukemic potency, we performed a new experiment applying the same approach but with 2 treatment cycles (Figure 5A). In addition, we used ALT-803 instead of rhIL-15 to support NK cell persistence in vivo. ALT-803 is an IL-15 superagonist complex composed of an IL-15 mutant (N72D) bound to sushi domain of IL-15Rα fused to IgG1 Fc. This complex has been shown to display higher stability and enhanced biological activity on NK cells in vivo as well as superior localization to the lymphoid organs when compared with rhIL-15.28,29 The effects of ALT-803 on HSPC-NK cells were validated in vitro (supplemental Figure 7). In this experimental model, treatment with 2 DAC cycles further slowed down THP-1 progression (Figure 5B). Notably, we showed that 2 cycles of combined DAC, HSPC-NK cells, and ALT-803 treatment resulted in significantly reduced THP-1 progression for up to 4 weeks compared with DAC treatment only (Figure 5C). These data demonstrate that DAC does not impair HSPC-NK cells in vivo, and that this combination strategy can boost the reactivity of adoptively transferred HSPC-NK cells against AML.
Treatment with HSPC-NK cell infusions and DAC improves control of AML in vivo. (A) Experimental design: THP-1–bearing mice received 1 or 2 cycles of DAC (1.25 mg/m2), with or without HSPC-NK cells, which were infused on the first day from each cycle. Survival of HSPC-NK cells in vivo was supported by ALT-803 (an IL-15 superagonist complex), which was given subcutaneously every 3 to 4 days until day 35 (0.2 mg/kg per injection). Groups that were not treated with NK cells were also given ALT-803 as a control. (B) Impact of DAC on tumor load progression. Median tumor load from untreated mice and mice treated with 1 or 2 cycles of DAC is shown. (C) Impact of HSPC-NK cell infusions on tumor load progression in mice cotreated with 2 cycles of DAC. Data are shown as mean ± SD and were analyzed with 2-way ANOVA. One mouse in each DAC × 2 and DAC+NK × 2 group died at day 17 and day 15, respectively, likely due to DAC-related toxicities (weight loss >20% after second treatment cycle). These mice were excluded from the complete data set shown in this figure.
Treatment with HSPC-NK cell infusions and DAC improves control of AML in vivo. (A) Experimental design: THP-1–bearing mice received 1 or 2 cycles of DAC (1.25 mg/m2), with or without HSPC-NK cells, which were infused on the first day from each cycle. Survival of HSPC-NK cells in vivo was supported by ALT-803 (an IL-15 superagonist complex), which was given subcutaneously every 3 to 4 days until day 35 (0.2 mg/kg per injection). Groups that were not treated with NK cells were also given ALT-803 as a control. (B) Impact of DAC on tumor load progression. Median tumor load from untreated mice and mice treated with 1 or 2 cycles of DAC is shown. (C) Impact of HSPC-NK cell infusions on tumor load progression in mice cotreated with 2 cycles of DAC. Data are shown as mean ± SD and were analyzed with 2-way ANOVA. One mouse in each DAC × 2 and DAC+NK × 2 group died at day 17 and day 15, respectively, likely due to DAC-related toxicities (weight loss >20% after second treatment cycle). These mice were excluded from the complete data set shown in this figure.
DAC enhances HSPC-NK cell cytolytic functions and trafficking to the bone marrow in vivo
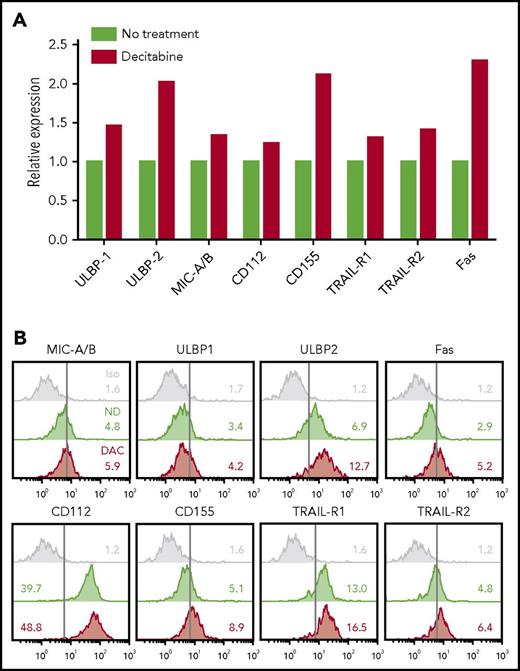
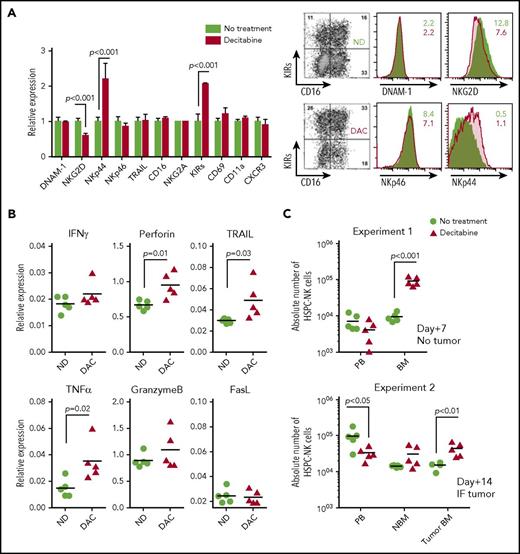
To understand how NK cell potentiation by DAC occurred in vivo, we analyzed the phenotype of THP-1 cells and HSPC-NK cells after treatment of the mice with DAC. First, we examined activating ligands and death receptors on THP-1 cells by FCM (Figure 6). Although only slight differences were seen for ULBP1, MIC-A/B, CD112, TRAIL receptors, and Fas, the expression levels of ULBP2 and CD155 were evidently increased with DAC, which supports our finding that HMAs can sensitize AML cells to HSPC-NK cell–mediated killing. On HSPC-NK cells, we observed sustained expression of DNAM-1, NKp46, TRAIL, and CD69 (Figure 7A). The level of NKG2D was diminished but was still present on NK cells, and expression of NKp44 was significantly increased. Similar phenotypical changes were observed with AZA (supplemental Figure 8). Furthermore, HSPC-NK cells maintained high expression of NKG2A following adoptive transfer into NSG mice, and acquired CD16 and KIR expression as compared with the cell product before infusion (data not shown). This finding is in line with our previous report,2 but treatment with DAC further increased the frequency of KIR+ HPSC-NK cells. By quantitative reverse transcription polymerase chain reaction, we also demonstrated enhanced gene expression of inflammatory cytokines, perforin, and TRAIL after DAC treatment, which are important players in NK cell–mediated killing (Figure 7B). It is notable that no regulation, or less upregulation, of these inflammatory and cytolytic mediators was seen with AZA (supplemental Figure 8). Finally, we examined HPSC-NK cell counts in the bone marrow compartment. In 3 independent experiments (data of 2 independent experiments shown in Figure 7C), we repeatedly found increased numbers of HSPC-NK cells in mouse bone marrow after DAC treatment, whereas the numbers of circulating NK cells were either similar (day+7) or lower (day+14) in peripheral blood compared with control mice. Altogether, these data strongly support that low-dose DAC can potentiate the anti-leukemic reactivity of HSPC-NK cells toward AML through modulation of gene expression and phenotype in AML and NK cells, and by influencing cell trafficking and tumor targeting in vivo.
DAC treatment upregulates NK-inducing ligands on THP-1 cells in vivo. (A-B) THP-1–bearing mice were treated with DAC (1.25 mg/m2) for 5 consecutive days. One week after the start of treatment, mice were euthanized and bone marrow cells were isolated for ex vivo analysis of THP-1 cells. Data obtained from 5 individual mice per treatment group were pooled to reach enough events by FCM (500-900 single and viable THP-1 cells acquired per test). The relative expression level of NKG2D and DNAM-1 ligands, as well as death receptors, are depicted in panel A, and overlay plots with mean fluorescence intensities are shown in panel B. Iso, isotype control.
DAC treatment upregulates NK-inducing ligands on THP-1 cells in vivo. (A-B) THP-1–bearing mice were treated with DAC (1.25 mg/m2) for 5 consecutive days. One week after the start of treatment, mice were euthanized and bone marrow cells were isolated for ex vivo analysis of THP-1 cells. Data obtained from 5 individual mice per treatment group were pooled to reach enough events by FCM (500-900 single and viable THP-1 cells acquired per test). The relative expression level of NKG2D and DNAM-1 ligands, as well as death receptors, are depicted in panel A, and overlay plots with mean fluorescence intensities are shown in panel B. Iso, isotype control.
DAC enhances the anti-leukemic potential of HSPC-NK cells through modulation of their maturation, activation, cytolytic functions, and trafficking to the bone marrow. (A-C) Adult NSG mice were infused with HSPC-NK cells and treated with DAC (1.25 mg/m2) for 5 consecutive days. Persistence of NK cells in vivo was supported by subcutaneous administration of IL-15 (1 µg/injection) every 2 to 3 days. Mice were euthanized 1 or 2 weeks after NK cell infusion for detailed ex vivo analysis. (A) Phenotype of HSPC-NK cells analyzed 1 week after the start of DAC treatment. Analysis was performed on cells isolated from the spleen, including 5 mice per treatment group. The relative expression of various maturation and activation markers, as well as adhesion molecules and homing receptor (right panel) and representative dot plots (left panel), is shown. (B) Gene expression profiling for the cytolytic machinery of NK cells, analyzed by quantitative reverse transcription polymerase chain reaction on cells isolated from livers, including 5 mice per treatment group. Data were normalized to human β-actin. (C) Absolute numbers of HSPC-NK cells were determined in peripheral blood (absolute number per milliliter) and mouse bone marrow either 1 week (experiment #1) or 2 weeks (experiment #2) after the start of treatment. Two femurs per mouse were combined in experiment #1, whereas experiment #2 was performed in IF THP-1–bearing mice and absolute NK cell counts were determined in each femur, with (Tumor BM) or without (NBM) tumor. Data shown in panel A were analyzed with 2-way ANOVA and data from panels B and C with an unpaired, 2-tailed Student t test. BM, bone marrow; IF, intrafemoral; ND, no drug; PB, peripheral blood.
DAC enhances the anti-leukemic potential of HSPC-NK cells through modulation of their maturation, activation, cytolytic functions, and trafficking to the bone marrow. (A-C) Adult NSG mice were infused with HSPC-NK cells and treated with DAC (1.25 mg/m2) for 5 consecutive days. Persistence of NK cells in vivo was supported by subcutaneous administration of IL-15 (1 µg/injection) every 2 to 3 days. Mice were euthanized 1 or 2 weeks after NK cell infusion for detailed ex vivo analysis. (A) Phenotype of HSPC-NK cells analyzed 1 week after the start of DAC treatment. Analysis was performed on cells isolated from the spleen, including 5 mice per treatment group. The relative expression of various maturation and activation markers, as well as adhesion molecules and homing receptor (right panel) and representative dot plots (left panel), is shown. (B) Gene expression profiling for the cytolytic machinery of NK cells, analyzed by quantitative reverse transcription polymerase chain reaction on cells isolated from livers, including 5 mice per treatment group. Data were normalized to human β-actin. (C) Absolute numbers of HSPC-NK cells were determined in peripheral blood (absolute number per milliliter) and mouse bone marrow either 1 week (experiment #1) or 2 weeks (experiment #2) after the start of treatment. Two femurs per mouse were combined in experiment #1, whereas experiment #2 was performed in IF THP-1–bearing mice and absolute NK cell counts were determined in each femur, with (Tumor BM) or without (NBM) tumor. Data shown in panel A were analyzed with 2-way ANOVA and data from panels B and C with an unpaired, 2-tailed Student t test. BM, bone marrow; IF, intrafemoral; ND, no drug; PB, peripheral blood.
Discussion
Here, we investigated the capacity of AZA and DAC to enhance HSPC-NK cell reactivity against AML. To the best of our knowledge, our study is the first head-to-head comparison of AZA and DAC in promoting NK cell–mediated anti-leukemic reactivity in vivo. In line with previous reports, we showed that these HMAs exert direct effects on AML cell viability, proliferation, and phenotype that eventually result in a greater susceptibility to NK cell–mediated killing. Furthermore, we also found increased expression of NKG2D and DNAM-1 ligands on THP-1 AML cells after treatment with HMAs. In contrast to AML cells, exposure to HMAs has a minimal impact on HSPC-NK cell viability and proliferation. Moreover, our data demonstrated, in vitro and in vivo, that DAC can potentiate HSPC-NK cell functionalities and anti-leukemic activity. Notable is that combined HSPC-NK cell and DAC treatment resulted in improved control of THP-1 AML in NSG mice, whereas the combination with AZA did not yield an additive anti-leukemic effect. Multiple factors can explain the difference between both HMAs, including reduced NK cell proliferation and degranulation capacity upon AZA treatment, or a lower impact of AZA on NK cell cytolytic machinery, in vivo trafficking, and accumulation in the bone marrow compartment compared with DAC. We believe this difference is not due to different dosing of the HMAs in our mouse studies, because we carefully titrated AZA and DAC for exerting the same direct effect on THP-1 cells in vivo.
It is interesting that increased anti-leukemic activity of NK cells could already be demonstrated in DAC-treated mice after a single infusion of HSPC-NK cells but was further enhanced by the application of 2 treatment cycles. It is important to underline that this intrafemoral THP-1 AML model is very stringent: on the one hand, because of the fast and aggressive THP-1 cell progression in vivo,2 and on the other hand, because it requires effective NK homing to the tumor site after intravenous infusion.30 In this model, treatment with DAC did not eradicate THP-1 cells in vivo but allowed indolent disease progression, thereby favoring HSPC-NK cell responses to less bulky and residual disease. In addition, increased HSPC-NK cell numbers were seen in the bone marrow of particularly DAC-treated mice. Shortly after infusion, NK cell homing to the bone marrow may be favored as a result of transient leukopenia occurring in HMA-treated mice. Moreover, at 2 weeks after NK cell transfer to tumor-bearing mice, HSPC-NK cell numbers were particularly increased within the tumor bed rather than in normal bone marrow, which suggests a specific NK cell homing to the tumor site. Therefore, we showed that HSPC-NK cells express and maintain high levels of the chemokine receptor CXCR3 in vivo and display a higher capacity of inflammatory cytokine production after DAC treatment. These observations are in line with a recent publication by Wang et al,31 which described a dual effect of DAC on inflammation and lymphocyte trafficking at the tumor site in a mouse ovarian cancer model. They showed that treatment with low-dose DAC increases the expression of chemokines that recruit NK cells and CD8+ T cells as well as promotes their production of IFN-γ and tumor necrosis factor alpha (TNF-α). It is interesting to note that we also observed that AML cells, including THP-1, can secrete the inflammatory chemokines CXCL9, CXCL10, and CXCL11 upon exposure to IFN-γ and TNF-α (data not shown). In a likewise manner, it was recently shown that HMAs can reactivate expression of endogenous retroviral elements, thereby eliciting type I and III IFN response, as well as induce expression of CXCL9 and CXCL10 by tumor cells.32 The DAC-induced IFN response may positively affect HSPC-NK cell activity and function as seen by the upregulation of immune mediators such as IFN-γ, TNF-α, and perforin. All of these findings bring evidence that DAC can modulate tumor environments and immune cell trafficking in vivo. Therefore, we believe that, besides its direct anti-leukemic activity, DAC can potently maximize HSPC-NK cell responses presumably through epigenetic modulation. Besides sensitizing AML cells to NK cell–mediated killing, our data support that DAC can boost HSPC-NK cell cytolytic functions, enhance inflammatory responses, and upregulate expression of the activating receptor NKp44. This mechanism could result in a self-stimulatory loop, further promoting NK cell recruitment to the tumor site and sustained control of AML.
Altogether, our findings provide a strong rationale to investigate HMA therapy in patients not only prior to adoptive NK cell immunotherapy, but also concomitantly with HSPC-NK cell infusion to exploit superior efficacy of these 2 treatment regimens. Potential clinical applications are broad. For AML, current remission induction and consolidation regimens could be complemented with adoptive transfer of allogeneic HSPC-NK cells combined with low or increasing doses of DAC as an adjuvant consolidation or a bridge-to-transplant strategy. Furthermore, combining DAC and HSPC-NK cell infusion with the IL-15 superagonist ALT-803, which has shown enhanced biological activity and a better half-life in vivo compared with rhIL-15, is a promising combinatorial approach to maximizing the anti-leukemic effect. This combination could further eradicate MRD and ultimately improve outcome of allogeneic stem cell transplantation.33,34 We cannot affirm that the same findings will occur in patients, and treatment dosages are particularly difficult to translate from humans to animals and vice versa. In our model, we applied 5-day treatment with DAC, whereas in patients it has been observed that 10-day treatment is more efficacious.35-37 More investigations are needed regarding DAC-mediated changes in the tumor environment, inflammation, and chemokine and cytokine levels in diseased bone marrow. Because cell trafficking is a tightly regulated process, we believe that this strategy should be further addressed in patients rather than in xenograft mouse models. Moreover, our study highlights a possible synergy when combining HSPC-NK cells with DAC as demonstrated by the capacity to potentiate HSPC-NK cell reactivity toward AML. This action was seen in coculture experiments, whereas exposure to HMAs had a minor influence on the phenotype of THP-1 cells (supplemental Figure 9) or primary AML blasts (data not shown) in vitro, so other factors might be implicated in modulating NK cell reactivity. One explanation could be changes in inhibitory pathways. We observed higher CD155 expression on THP-1 cells after treatment with DAC, but this molecule also binds to CD96 and TIGIT, which compete with DNAM-1 on NK cells. On HSPC-NK cells, TIGIT is not expressed at the end of the culture process and is not upregulated in vivo regardless of DAC treatment (data not shown). In contrast, CD96 is highly expressed on HSPC-NK cells. However, its function is more controversial with demonstration of activating as well as inhibitory activity for NK cells.38-40 We have not yet investigated its effect on HSPC-NK cells, but this pathway is currently being studied in our laboratory in the context of AML attack by HSPC-NK cells. Screening of primary AML blasts with respect to risk group, cytogenetic abnormalities, or FAB classification could help identify best responders to combined DAC and HSPC-NK cell therapy. Finally, our mouse studies revealed that 2 cycles with combined DAC, HSPC-NK cells, and ALT-803 treatment is better, but the effect seems to decrease at 28 days. Therefore, further improvement with longer duration of DAC treatment of 10 days in each cycle and/or more treatment cycles should be tested to maximize the combined effect of NK cells, DAC, and ALT-803. Opportunities also could be considered in the post-transplant setting, where low-dose maintenance therapy with HMAs has been shown to be feasible.13 In this situation, using HSPC-NK cells generated from the donor stem cell graft would be beneficial to allow longer persistence of adoptively transferred NK cells.23 Furthermore, the influence of DAC on inflammation and immune-cell trafficking to the tumor site, epigenetic modulation leading to tumor-associated antigen upregulation, and further stimulation of NK and dendritic cell cross-talk could also boost anti-leukemic T-cell responses.41-43 Therefore, our study, which shows that HSPC-NK cells and DAC can potently work together to eradicate AML, provides a strong rationale to explore this combination strategy in the treatment of AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the team of the Radboudumc Central Animal Laboratory and all colleagues from the Laboratory of Hematology for assistance and constructive discussion on this project. The authors also thank Hing Wong and Sarah Alter from Altor BioScience, who provided ALT-803 and advised the authors on its application in this animal study.
This work was supported by the Dutch Cancer Society (KWF#KUN2014-6701) and Radboudumc.
Authorship
Contribution: J.C. designed and performed the research, analyzed data, and wrote the manuscript; M.W.H.R. performed experiments and analyzed data; J.S.H.-v.E. coordinated UCB units’ availability and participated in experiments; F.M. assisted in the research; R.F.F. performed experiments; W.H., N.M.A.B., W.J.v.d.V., G.H., J.H.J., and N.P.M.S. provided advice; H.D. designed and supervised the research; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harry Dolstra, Department of Laboratory Medicine–Laboratory of Hematology, Radboudumc, Geert Grooteplein 8, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: harry.dolstra@radboudumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal