Key Points
Single-agent IL-15/IL-15Rα-Fc (ALT-803) therapy was well tolerated and resulted in clinical responses in patients who relapsed post-HCT.
First-in-human use of ALT-803 promoted NK and CD8+ T-cell expansion and activation in vivo without stimulating regulatory T cells.
Abstract
New therapies for patients with hematologic malignancies who relapse after allogeneic hematopoietic cell transplantation (allo-HCT) are needed. Interleukin 15 (IL-15) is a cytokine that stimulates CD8+ T-cell and natural killer (NK) cell antitumor responses, and we hypothesized this cytokine may augment antileukemia/antilymphoma immunity in vivo. To test this, we performed a first-in-human multicenter phase 1 trial of the IL-15 superagonist complex ALT-803 in patients who relapsed >60 days after allo-HCT. ALT-803 was administered to 33 patients via the IV or subcutaneous (SQ) routes once weekly for 4 doses (dose levels of 1, 3, 6, and 10 μg/kg). ALT-803 was well tolerated, and no dose-limiting toxicities or treatment-emergent graft-versus-host disease requiring systemic therapy was observed in this clinical setting. Adverse events following IV administration included constitutional symptoms temporally related to increased serum IL-6 and interferon-γ. To mitigate these effects, the SQ route was tested. SQ delivery resulted in self-limited injection site rashes infiltrated with lymphocytes without acute constitutional symptoms. Pharmacokinetic analysis revealed prolonged (>96 hour) serum concentrations following SQ, but not IV, injection. ALT-803 stimulated the activation, proliferation, and expansion of NK cells and CD8+ T cells without increasing regulatory T cells. Responses were observed in 19% of evaluable patients, including 1 complete remission lasting 7 months. Thus, ALT-803 is a safe, well-tolerated agent that significantly increased NK and CD8+ T cell numbers and function. This immunostimulatory IL-15 superagonist warrants further investigation to augment antitumor immunity alone and combined with other immunotherapies. This trial was registered at www.clinicaltrials.gov as #NCT01885897.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) remains the primary curative option for patients with advanced hematologic malignances. However, disease relapse remains the major cause of treatment failure, with rates approaching 50%, especially after reduced intensity conditioning.1 The prognosis after relapse is poor, and new treatment options are needed.2 Donor lymphocyte infusion has been used to augment alloimmunity; however, long-term efficacy remains disappointing.3 Attempts have been made to enhance the efficacy of this approach using depletion of regulatory T cells (Tregs) and addition of interferon α with variable success.4,5 Use of checkpoint inhibitors in patients relapsed after allo-HCT has been associated with limited remissions and high rates of graft-versus host disease (GVHD).6
Allogeneic graft-versus-leukemia (GVL) is mediated by alloreactive CD8+ T cells and natural killer (NK) cells. NK cells do not express a rearranged clonal antigen-specific receptor but instead recognize targets via a wide array of cytokine, activating, and inhibitory receptors, including the polymorphic killer cell immunoglobulin-like receptors.7 In the allo-HCT setting, NK cells mediate a GVL effect and thereby eliminate leukemia/lymphoma without initiating GVHD.8-11 Adoptively transferred allogeneic NK cells have been investigated safely, without major adverse events (AE), and can induce complete remissions in relapsed or refractory acute myeloid leukemia (AML) patients.12-14 Alloreactive T cells may also mediate GVL via recognition of various allogeneic antigens.
Enhancement of endogenous immune function with cytokines is limited by available pharmaceuticals.15 Recombinant human (rh) interleukin 2 (IL-2) is the only US Food and Drug Administration–approved cytokine available to promote the survival, expansion, and activation of lymphocytes. However, IL-2 preferentially stimulates Tregs that constitutively express the high-affinity IL-2 receptor CD25.13 Thus, IL-15 is an appealing alternative, because under physiologic conditions, IL-15 is trans-presented by IL-15Rα on accessory cells (eg, dendritic cells and macrophages) to the IL-2/15Rβγc receptor expressed on NK and T cells without activating Tregs.16 ALT-803, a superagonist complex of an IL-15 mutein (N72D) bound to the sushi domain of IL-15Rα fused to the immunoglobulin G1 Fc, was developed to extend the in vivo half-life and mimic the physiologic trans-presentation of IL-15.17-19 Preclinical studies show that ALT-803 IL-15/IL15Rα complexes prolong the half-life of IL-15 and promote enhanced immune activation in vivo.16 IV monomeric Escherichia coli–derived rhIL-15 used to treat refractory solid tumors significantly increased proliferation and activation of NK and T cells; however, the short half-life of the drug and the associated dose-limiting hemodynamic instability required inpatient infusion and monitoring.20 We hypothesized that ALT-803 would augment donor NK and T-cell number and function in patients that have relapsed after allo-HCT to enhance donor antitumor immune responses and tested this in a first-in-human, investigator-initiated, multicenter phase 1 clinical trial.
Methods
Study design and participants
We conducted a multicenter (University of Minnesota [UMN] and Washington University School of Medicine [WUSM]) phase 1 clinical trial for patients with hematologic malignancy who relapsed after allo-HCT. Adult patients with adequate organ function, Karnofsky performance scores ≥70% who relapsed ≥60 days from allo-HCT were eligible if they had no active GVHD and a minimum of 10% donor cell chimerism, which varied based on the degree of relapse. Thirty-three patients were enrolled. Starting in November 2013, ALT-803 was administered IV at 1, 3, 6, and 10 μg/kg to 16 patients. In April 2015, the trial was amended to include subcutaneous (SQ) doses at 6 and 10 μg/kg to avoid high fevers related to a high ALT-803 peak serum concentration (Cmax) and to allow up to 2 additional courses; and 17 additional patients were treated. No other concomitant AML therapy was administered. During the escalation phase, the administration of dose 1 was monitored on an inpatient unit with subsequent doses monitored for 2 hours prior to discharge. The study was reviewed and approved by the institutional review boards of the participating institutions and conducted per Declaration of Helsinki and Good Clinical Practice guidelines with US Food and Drug Administration approval (IND 119578, sponsor J. Miller). The study was registered at www.clinicaltrials.gov as #NCT01885897.
The dose-finding phase followed a standard 3+3 design to evaluate safety. AEs were classified according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Dose-limiting toxicities (DLTs) were predefined as (1) any treatment-emergent nonhematologic grade 3 toxicity lasting >48 hours, except for transient constitutional symptoms, diarrhea, or skin rash not requiring systemic steroid therapy; (2) any treatment-emergent, nonhematologic grade 4 or 5 toxicity; or (3) grade III to IV acute GVHD within 6 weeks of the first ALT-803 dose. Response was defined as follows: for AML and myelodysplastic syndromes (MDS) using the International Working Group modified criteria,21 non-Hodgkin lymphoma,22 and multiple myeloma using the International Myeloma Working Group Uniform Response Criteria,23 and acute lymphoblastic leukemia using protocol-specified criteria.
ALT-803 pharmacokinetic and serum cytokine analysis
Serum was frozen and later batch-analyzed for ALT-803 and cytokines. IL-2, IL-4, IL-6, IL-10, tumor necrosis factor, and interferon γ (IFN-γ) concentrations were determined using cytometric bead array Th1/Th2 cytokine Kit II following the manufacturer’s instructions (BD Biosciences).
Immunogenicity assays
Serum samples were analyzed in a dilution series using a qualified anti–ALT-803 bridging enzyme-linked immunosorbent assay with ALT-803 as the capture reagent and HRP-conjugated ALT-803 as the detection reagent. The sample was considered positive for anti–ALT-803 antibodies if the average uncorrected optical density (OD) of the postdose sample was greater than 2 times the average uncorrected OD of the corresponding predose sample. The titer was calculated using the formula: (uncorrected OD sample/2 × [uncorrected OD predose]) × dilution factor.
Flow and mass cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated, stained with monoclonal antibodies (supplemental Table 1, available on the Blood Web site), and acquired on a flow cytometer.24 Mass cytometry was performed on thawed PBMCs stained with a custom NK and T-cell panel (supplemental Table 2), data acquired on a CyTOF Helios instrument, and analyzed as described previously.14 Selected markers that were substantially changed by ALT-803 administration are shown in the figures.
Statistical analysis
We used a 3+3 design to determine the maximum tolerated dose for IV (1, 3, 6, and 10 μg/kg) and subsequent SQ (6 and 10 μg/kg) administration. All patients were evaluable for safety, which was the primary objective for this study. In addition to safety, descriptive statistics such as means and standard errors of the mean were employed to estimate various immunostimulatory measures. Statistical comparisons of normally distributed measures between factors such as IV and SQ over time were carried out with repeated measures 2-way analysis of variance (ANOVA). Tests for measures in change over time employed 1-way repeated-measures ANOVA. The Mann-Whitney-Wilcoxon test was used to compare independent observations. Paired Student t tests were employed for measures with only two time points. All reported P values were 2 sided. GraphPad Prism v7.0 was used for all statistical analyses.
Results
Patients and treatment
Between 2013 and 2016, 33 patients were treated with ALT-803 (16 with IV and 17 with SQ routes) (Table 1). The majority (85%) had AML (n = 22) or MDS (n = 6). Median time from allo-HCT to first dose of ALT-803 was 292 days (range, 88-2843 days), and no patients had active GVHD at the time of enrollment. All subjects were required to be off immune suppression for at least 14 days. Similar numbers of patients received myeloablative (45%) and reduced intensity (55%) conditioning.
Baseline patient characteristics
| . | No. of patients . |
|---|---|
| Total number of patients treated | 33 |
| Sex | |
| Male | 17 (52) |
| Female | 16 (48) |
| Age at study enrollment, median (range), y | 58 (22-74) |
| Diagnosis | |
| AML | 22 (67) |
| MDS | 6 (18) |
| ALL | 2 (1) |
| MM | 2 (1) |
| DLBCL | 1 (0) |
| Time since prior allo-HCT to first dose of ALT-803, median (range), d | 292 (88-2843) |
| Donor relationship | |
| Matched related | 13 (39) |
| Matched unrelated | 7 (21) |
| Unrelated cord blood | 9 (27) |
| Haploidentical related | 4 (12) |
| Conditioning regimen | |
| Myeloablative | 15 (45) |
| Reduced intensity | 18 (55) |
| Prior history of GVHD | |
| Grade I-II, acute | 7 (21) |
| Grade III-IV, acute | 1 (0) |
| All grades, chronic | 1 (0) |
| Moderate to severe, chronic | 0 (0) |
| Donor cell engraftment at the time of enrollment, median (range), % | 57 (25-100) |
| Disease status at enrollment | |
| First relapse | 11 (33) |
| Second relapse | 8 (24) |
| Third relapse | 7 (21) |
| Transplant to time of relapse, median (range), d | 187 (21-2751) |
| Transplant to first dose of ALT-803, median (range), d | 257 (88-2843) |
| . | No. of patients . |
|---|---|
| Total number of patients treated | 33 |
| Sex | |
| Male | 17 (52) |
| Female | 16 (48) |
| Age at study enrollment, median (range), y | 58 (22-74) |
| Diagnosis | |
| AML | 22 (67) |
| MDS | 6 (18) |
| ALL | 2 (1) |
| MM | 2 (1) |
| DLBCL | 1 (0) |
| Time since prior allo-HCT to first dose of ALT-803, median (range), d | 292 (88-2843) |
| Donor relationship | |
| Matched related | 13 (39) |
| Matched unrelated | 7 (21) |
| Unrelated cord blood | 9 (27) |
| Haploidentical related | 4 (12) |
| Conditioning regimen | |
| Myeloablative | 15 (45) |
| Reduced intensity | 18 (55) |
| Prior history of GVHD | |
| Grade I-II, acute | 7 (21) |
| Grade III-IV, acute | 1 (0) |
| All grades, chronic | 1 (0) |
| Moderate to severe, chronic | 0 (0) |
| Donor cell engraftment at the time of enrollment, median (range), % | 57 (25-100) |
| Disease status at enrollment | |
| First relapse | 11 (33) |
| Second relapse | 8 (24) |
| Third relapse | 7 (21) |
| Transplant to time of relapse, median (range), d | 187 (21-2751) |
| Transplant to first dose of ALT-803, median (range), d | 257 (88-2843) |
Values are reported as n (%) of patients unless indicated otherwise.
ALL, acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; MM, multiple myeloma.
AEs and tolerability
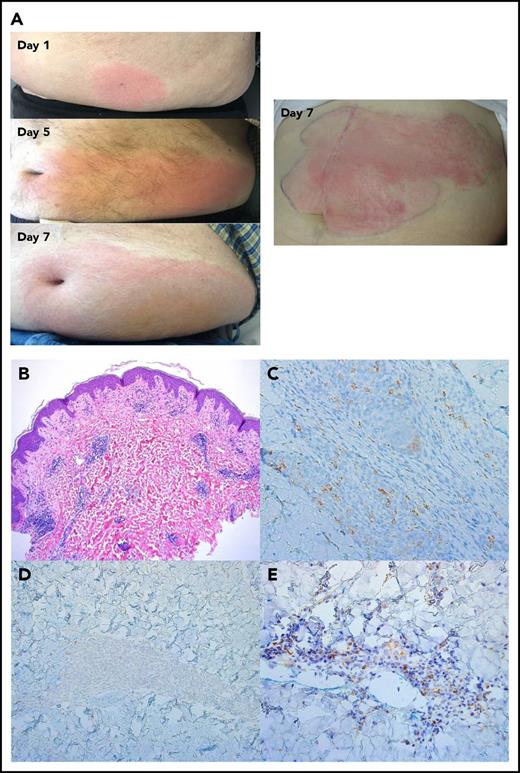
Overall, ALT-803 was well tolerated in both the IV and SQ cohorts, with no DLT identified. Table 2 lists grade 1 to 3 AEs within the 2 highest (6 and 10 μg/kg) IV and SQ cohorts. Distinct AE profiles were observed. IV administration was uniformly associated with infusion-related fever and chills/rigors. This was a result of high peak concentrations of ALT-803, and although the toxicity was not medically serious, the protocol was amended to change from IV to SQ administration to test whether this toxicity could be eliminated without loss of biologic activity. The SQ cohort was uniquely associated with injection site rash (94%). These ALT-803–related, self-limited SQ injection site rashes manifested as macular erythema starting from the injection site and spreading to adjacent areas over the 5 to 10 days. They all resolved without any systemic therapy by day 14 (Figure 1A). Skin biopsy specimens revealed superficial and deep perivascular and either perieccrine or perineural lymphocytic infiltrates (Figure 1B). Immunohistochemical analysis of the infiltrates demonstrated a predominance of CD3+/CD56+/T-BET+/TCRγ3.20+/NKP46− cells consistent with γδ T cells (Figure 1C-E). Hypertension was observed following IV or SQ administration, but it was transient and not clinically serious. None of the patients met criteria for severe cytokine release syndrome or capillary leak syndrome. While on study, 3 patients experienced grade 4 events and 2 progressed to grade 5. Sepsis (n = 2) was associated with neutropenia from active acute leukemia, and 1 of these was fatal after the patient elected comfort care. A fatal intracranial hemorrhage (n = 1) was associated with disease-related thrombocytopenia. These serious AEs were deemed not to be related to ALT-803 and reflected the high-risk, poor-prognosis patients treated in this phase 1 trial.
AEs occurring in more than 1 patient treated on the 6 and 10 μg/kg dose levels
| AE . | IV or SQ (n = 24) . | IV (n = 7) . | SQ (n = 17) . | ||
|---|---|---|---|---|---|
| Grade 1-3 | Grade 1-2 | Grade 3 | Grade 1-2 | Grade 3 | |
| Hypertension | 20 (83) | 3 (43) | 2 (29) | 11 (65) | 4 (24) |
| Injection site reaction* | 19 (79) | 2 (29) | 1 (14) | 14 (82) | 2 (12) |
| Fever* | 18 (75) | 5 (71) | 2 (29) | 2 (12) | 9 (53) |
| Chills | 15 (63) | 7 (100) | 8 (47) | ||
| Hypotension | 15 (63) | 3 (43) | 1 (14) | 10 (59) | 1 (6) |
| GI symptoms | 13 (54) | 2 (29) | 11 (65) | ||
| Fatigue | 12 (50) | 6 (86) | 4 (24) | 2 (12) | |
| Electrolyte abnormalities | 10 (42) | 3 (43) | 5 (29) | 2 (12) | |
| Edema | 9 (38) | 3 (43) | 6 (35) | ||
| Headache | 8 (33) | 4 (57) | 3 (18) | 1 (6) | |
| Dyspnea | 8 (33) | 2 (29) | 5 (29) | 1 (6) | |
| Hypoxia* | 7 (29) | 2 (29) | 3 (43) | 2 (12) | |
| Achiness | 7 (29) | 4 (57) | 3 (18) | ||
| Renal insufficiency | 7 (29) | 2 (29) | 5 (29) | ||
| Hypoalbuminemia | 6 (25) | 1 (14) | 5 (29) | ||
| Abdominal pain | 4 (17) | 3 (18) | 1 (6) | ||
| Abnormal LFT result | 3 (13) | 2 (29) | 1 (6) | ||
| Anorexia | 3 (13) | 1 (14) | 2 (12) | ||
| Confusion | 3 (13) | 1 (14) | 2 (12) | ||
| Mouth sores | 3 (13) | 1 (14) | 2 (12) | ||
| Minor bleeding | 3 (13) | 1 (14) | 2 (12) | ||
| Infections | 3 (13) | 1 (14) | 1 (6) | 1 (6) | |
| Cytopenias | 3 (13) | 1 (14) | 2 (12) | ||
| Bacteremia | 2 (8) | 2 (12) | |||
| Allergic rhinitis | 2 (8) | 2 (29) | |||
| Pemphigus rash | 1 (4) | 1 (6) | |||
| AE . | IV or SQ (n = 24) . | IV (n = 7) . | SQ (n = 17) . | ||
|---|---|---|---|---|---|
| Grade 1-3 | Grade 1-2 | Grade 3 | Grade 1-2 | Grade 3 | |
| Hypertension | 20 (83) | 3 (43) | 2 (29) | 11 (65) | 4 (24) |
| Injection site reaction* | 19 (79) | 2 (29) | 1 (14) | 14 (82) | 2 (12) |
| Fever* | 18 (75) | 5 (71) | 2 (29) | 2 (12) | 9 (53) |
| Chills | 15 (63) | 7 (100) | 8 (47) | ||
| Hypotension | 15 (63) | 3 (43) | 1 (14) | 10 (59) | 1 (6) |
| GI symptoms | 13 (54) | 2 (29) | 11 (65) | ||
| Fatigue | 12 (50) | 6 (86) | 4 (24) | 2 (12) | |
| Electrolyte abnormalities | 10 (42) | 3 (43) | 5 (29) | 2 (12) | |
| Edema | 9 (38) | 3 (43) | 6 (35) | ||
| Headache | 8 (33) | 4 (57) | 3 (18) | 1 (6) | |
| Dyspnea | 8 (33) | 2 (29) | 5 (29) | 1 (6) | |
| Hypoxia* | 7 (29) | 2 (29) | 3 (43) | 2 (12) | |
| Achiness | 7 (29) | 4 (57) | 3 (18) | ||
| Renal insufficiency | 7 (29) | 2 (29) | 5 (29) | ||
| Hypoalbuminemia | 6 (25) | 1 (14) | 5 (29) | ||
| Abdominal pain | 4 (17) | 3 (18) | 1 (6) | ||
| Abnormal LFT result | 3 (13) | 2 (29) | 1 (6) | ||
| Anorexia | 3 (13) | 1 (14) | 2 (12) | ||
| Confusion | 3 (13) | 1 (14) | 2 (12) | ||
| Mouth sores | 3 (13) | 1 (14) | 2 (12) | ||
| Minor bleeding | 3 (13) | 1 (14) | 2 (12) | ||
| Infections | 3 (13) | 1 (14) | 1 (6) | 1 (6) | |
| Cytopenias | 3 (13) | 1 (14) | 2 (12) | ||
| Bacteremia | 2 (8) | 2 (12) | |||
| Allergic rhinitis | 2 (8) | 2 (29) | |||
| Pemphigus rash | 1 (4) | 1 (6) | |||
The number of events and frequency (%) are listed. Pemphigus rash was included as a single grade 2 event, as it was categorized as a serious AE.
GI, gastrointestinal; LFT, liver function test.
P ≤ .01 for differences between IV and SQ administration by Fisher exact test.
ALT-803 SQ injection-site reaction. (A) Shown is a representative injection site rash manifested as pink, edematous, nontender spreading plaque mimicking cellulitis. The sequence of rash expansion from days 1, 5, and 7 from different patients (left) and an additional example of the rash at day 7 (right). (B) Skin biopsy specimen showed a superficial and deep perivascular and perieccrine infiltrate consisting primarily of lymphocytes (hematoxylin and eosin, original magnification ×50). (C-E) Immunohistochemical analysis revealed that a predominance of cells were positive for CD56 (original magnification ×200) (C), negative for NKp46 (original magnification ×200) (D), and positive for TCRγ3.20 (original magnification ×200) (E), indicating presence of γδ T cells. Results are shown for 1 patient and are representative of 4 injection site reactions analyzed.
ALT-803 SQ injection-site reaction. (A) Shown is a representative injection site rash manifested as pink, edematous, nontender spreading plaque mimicking cellulitis. The sequence of rash expansion from days 1, 5, and 7 from different patients (left) and an additional example of the rash at day 7 (right). (B) Skin biopsy specimen showed a superficial and deep perivascular and perieccrine infiltrate consisting primarily of lymphocytes (hematoxylin and eosin, original magnification ×50). (C-E) Immunohistochemical analysis revealed that a predominance of cells were positive for CD56 (original magnification ×200) (C), negative for NKp46 (original magnification ×200) (D), and positive for TCRγ3.20 (original magnification ×200) (E), indicating presence of γδ T cells. Results are shown for 1 patient and are representative of 4 injection site reactions analyzed.
Efficacy
Of 33 patients enrolled, 27 met protocol-defined criteria for evaluation of clinical response, having received at least 3 doses of ALT-803. Four evaluable patients treated with 5 courses of ALT-803 (19%) at first treatment of relapse (without other concomitant treatment) met criteria for clinical benefit, including complete response (CR; n = 1), partial response (n = 1) or stable disease (SD; n = 3). The patient with a CR was a 69 year old with a history of high-risk, complex karyotype MDS enrolled on the 6 μg/kg IV cohort after relapsing 1 year after a second matched unrelated donor allo-HCT. At relapse, the patient had pancytopenia and was packed red blood cell transfusion dependent, with 9% bone marrow (BM) blasts, 46% donor chimerism, and cytogenetics demonstrating monosomy 7 in 5 of 20 metaphases. A cytogenetic CR with 93% donor engraftment was achieved at 2 months (4 doses of ALT-803) with normalization of blood counts and complete (>95%) donor engraftment at 5 months. He remained transfusion independent for 7 months before disease progression. At the time of progression, he was neutropenic with 15% BM myeloblasts and was re-enrolled on the next open cohort of 6 μg/kg SQ. After 4 weeks, his absolute neutrophil count (ANC) improved to 2000/μL, and a BM biopsy showed International Working Group SD with 10% blasts. This patient (during his first course) and an additional patient with progressive disease developed biopsy-proven mild grade II skin GVHD treated with topical steroids without requiring systemic immunosuppression. Thus, after ALT-803 therapy, only 7% of patients experienced treatment-emergent, mild skin GVHD. The partial response patient’s BM blasts decreased from 7% to 3% and remained at this level for 5 months after a total of 12 doses of ALT-803 (6 μg/kg SQ), and the ANC remained normal throughout therapy. Two additional patients had SD. One had no growth of a biopsy-proven myeloid sarcoma on computed tomography scan 2 months after starting ALT-803 therapy (3 μg/kg × 4 doses), and the other had 5% blasts and no measurable neutrophils before treatment and 5% blasts with 600/μL ANC measured 2 months after 4 doses of ALT-803 therapy (6 μg/kg IV × 4 doses). In summary, 4 of 5 responses were at the 6 μg/kg dose and 3 were with IV and 2 were with SQ administration. In this small sample set, there was no correlation between immune biomarkers and clinical response, which will be studied further in larger cohorts. The observation of a cytogenetic CR with resumption of normal hematopoiesis lasting 7 months with single-agent ALT-803 therapy indicates that this IL-15 agent stimulated allogeneic immunity against diseased hematopoietic cells. Although not a definitive clinical end point, the apparent clinical benefit in MDS/AML patients expected to progress without therapy supports the concept that stimulating immunity after allogeneic transplant can mediate activity against malignant hematopoietic cells.
Pharmacokinetics and serum cytokines
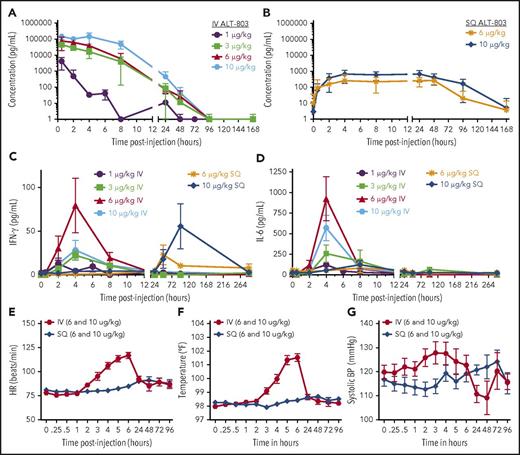
Serum concentrations of IL-15 were measured to define the pharmacokinetics of ALT-803 (Figure 2A-B; supplemental Table 3). Following IV administration, the Cmax occurred at the first (0.5 hour) sampling time point and declined to below the limit of detection by 48 to 96 hours. The IV Cmax exceeded 100 ng/mL at the 10 μg/kg dose level, accompanied by increases of IL-6 and IFN−γ occurring 4 hours postinfusion (Figure 2C-D). Cytokine increases were associated with fever, tachycardia, and relative hypotension (Figure 2E-G). In all cases, these symptoms responded to routine supportive care and resolved within 24 hours. In contrast, ALT-803 peaked 4 hours after SQ injection, with the Cmax 100-fold lower than the IV route (Figure 2B). The serum concentrations were maintained at 96 hours with detectable ALT-803 present 7 days following SQ administration. No changes in IL-6 were evident following SQ injection (Figure 2), and modest increases in IFN-γ were observed much later than IV, peaking at 96 hours postinjection (Figure 2D). Constitutional symptoms were not observed with SQ ALT-803. Although ALT-803 is a chimeric mutein, its immunogenicity was tested at multiple time points (supplemental Table 4), and only a single patient on the 6 μg/kg SQ cohort demonstrated low titer (1:50) reactivity against ALT-803 (7 days after the fourth dose).
ALT-803 pharmacokinetics, serum cytokine levels, and vital sign changes following IV vs SQ administration. (A-B) Measurement of serum ALT-803 protein was performed by enzyme-linked immunosorbent assay with samples collected at various time points following the first ALT-803 injection administered on study. (A) IV ALT-803 administration by dose level, with a dose-dependent increase in ALT-803 concentrations, a high Cmax, and rapid clearance with return to baseline by 96 hours postinjection. (B) SQ ALT-803 administration by dose level, demonstrating a decreased ALT-803 Cmax that peaked after 4 hours, with sustained serum levels through 96 hours and clearance by 168 hours. Complete pharmacokinetic analysis is provided in supplemental Table 3. (C-D). IFN-γ and IL-6 were measured via cytokine bead array at various time points following the first ALT-803 administration. Depending on the route of administration, IFN-γ levels peaked early (IV) or late (SQ). IV ALT-803 resulted in an early IL-6 spike that is absent in SQ ALT-803 administration. Complete cytokine measurements are provided in supplemental Table 2. (E-G) Vital sign assessments immediately following the first dose ALT-803, including heart rate (HR) (E), temperature (F), and systolic blood pressure (G). IV ALT-803 administration resulted in fevers with increased heart rate 3 to 6 hours after administration, concurrent with elevated serum IL-6 levels and IFN-γ levels. SQ ALT-803 did not result in short-term alterations in vital signs.
ALT-803 pharmacokinetics, serum cytokine levels, and vital sign changes following IV vs SQ administration. (A-B) Measurement of serum ALT-803 protein was performed by enzyme-linked immunosorbent assay with samples collected at various time points following the first ALT-803 injection administered on study. (A) IV ALT-803 administration by dose level, with a dose-dependent increase in ALT-803 concentrations, a high Cmax, and rapid clearance with return to baseline by 96 hours postinjection. (B) SQ ALT-803 administration by dose level, demonstrating a decreased ALT-803 Cmax that peaked after 4 hours, with sustained serum levels through 96 hours and clearance by 168 hours. Complete pharmacokinetic analysis is provided in supplemental Table 3. (C-D). IFN-γ and IL-6 were measured via cytokine bead array at various time points following the first ALT-803 administration. Depending on the route of administration, IFN-γ levels peaked early (IV) or late (SQ). IV ALT-803 resulted in an early IL-6 spike that is absent in SQ ALT-803 administration. Complete cytokine measurements are provided in supplemental Table 2. (E-G) Vital sign assessments immediately following the first dose ALT-803, including heart rate (HR) (E), temperature (F), and systolic blood pressure (G). IV ALT-803 administration resulted in fevers with increased heart rate 3 to 6 hours after administration, concurrent with elevated serum IL-6 levels and IFN-γ levels. SQ ALT-803 did not result in short-term alterations in vital signs.
NK cell modulation by ALT-803 in vivo
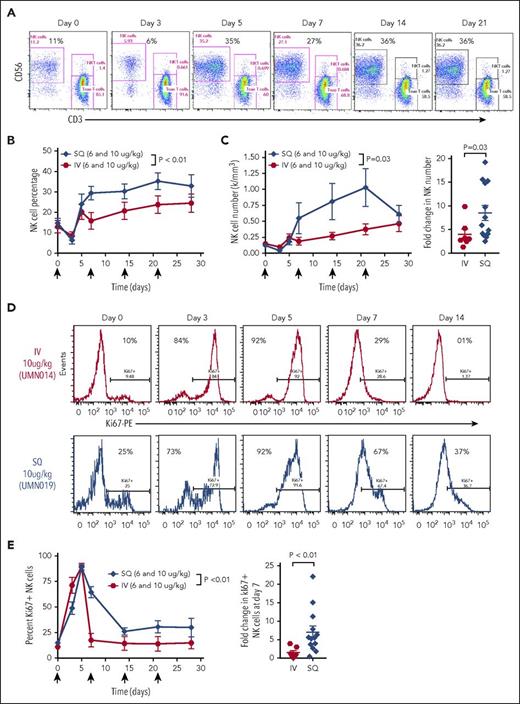
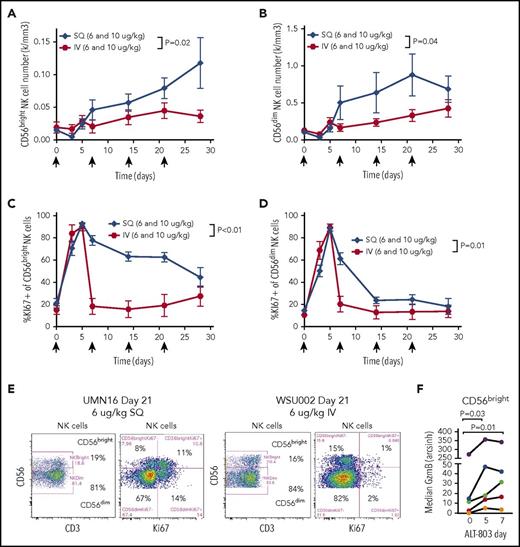
Based on the known biology of IL-15 and IL-15R, we hypothesized that ALT-803 would primarily influence NK cells and CD8+ T cells.25 We evaluated lymphocyte subsets in patients at the 6 and 10 μg/kg dose levels. The number of circulating NK cells increased in a dose-dependent fashion with IV administration and cumulatively increased over time in both the IV and SQ cohorts, with a significantly greater increase following SQ ALT-803 (Figure 3A-B). As observed with rhIL-15, there was an acute decrease in the NK cell frequency and number after IV and SQ administration that rebounded after 5 days.20 Over time, there was also a significant increase in absolute NK cell numbers via both routes, although SQ resulted in even greater expansion over IV. The increase in NK cells following ALT-803 administration could be due to several mechanisms, and ALT-803 markedly increased proliferation (Ki67) following either IV or SQ administration (Figure 3D-E). However, SQ resulted in significantly prolonged Ki67 compared with IV (Figure 3E-F), consistent with proliferation contributing to the in vivo increases after SQ ALT-803 (Figure 2B). Based on their surface expression of the adhesion marker CD56, human NK cells are divided into CD56bright and CD56dim subsets with distinct functional and receptor profiles.7,26 Following ALT-803 administration, both CD56bright and CD56dim NK cells expanded (Figure 4A-B), with both subsets significantly increased in the SQ vs the IV cohort. However, CD56bright NK cells showed markedly prolonged proliferation (Ki67 expression) compared with CD56dim NK cells (Figure 4C-E). Additionally, patient CD56bright NK cells (distinguished clearly from CD56dim NK cells using mass cytometry), not traditionally considered to be highly cytotoxic,27 expressed increased granzyme B (GzmB) following ALT-803 administration in vivo (Figure 4F).
NK cells expand and proliferate after ALT-803 administration. (A) NK cell percentages increase following ALT-803 administration as shown in a representative patient treated at the 10 μg/kg SQ dose level (UMN019). Bivariate flow cytometry plots show CD56 vs CD3 expression, resolving NK cells and T cells. Percentage of NK cells at the indicated time point after the first ALT-803 administration is annotated. (B) Summary data of NK cell percentages during ALT-803 therapy, comparing IV to SQ administration, combining 6 and 10 μg/kg dose levels. Both the IV (P = .04) and SQ (P < .01) cohorts show curves significant increases in NK cell percentage over time compared with time 0 (ANOVA). Comparisons of the curves revealed a significant increase in SQ over IV (P < .01, 2-way ANOVA). (C) NK cell number increases during ALT-803 therapy, comparing IV to SQ administration, combining the 6 and 10 μg/kg dose levels. The IV (P = .03) and SQ (P < .01) curves both significantly increase over time (ANOVA), and comparison of the curves reveals a significant increase in SQ (P = .029, 2-way ANOVA). Fold change comparing IV and SQ demonstrates a significant increase in the peak fold change (Mann-Whitney test) determined as the peak absolute NK cell count during ALT-803 therapy divided by the absolute NK cell count pretherapy. (D) Representative flow cytometry plots demonstrating changes in Ki67 (surrogate for proliferation) over time with IV vs SQ ALT-803. (E) Summary data from panel D. Both the IV (P < .01) and SQ (P < .01) curves significantly increase in Ki67+ NK cell percentage over time compared with time 0 (2-way ANOVA). Comparisons of the curves revealed a significant increase in SQ Ki67+ NK cells that is most apparent at day 7 (2-way ANOVA).
NK cells expand and proliferate after ALT-803 administration. (A) NK cell percentages increase following ALT-803 administration as shown in a representative patient treated at the 10 μg/kg SQ dose level (UMN019). Bivariate flow cytometry plots show CD56 vs CD3 expression, resolving NK cells and T cells. Percentage of NK cells at the indicated time point after the first ALT-803 administration is annotated. (B) Summary data of NK cell percentages during ALT-803 therapy, comparing IV to SQ administration, combining 6 and 10 μg/kg dose levels. Both the IV (P = .04) and SQ (P < .01) cohorts show curves significant increases in NK cell percentage over time compared with time 0 (ANOVA). Comparisons of the curves revealed a significant increase in SQ over IV (P < .01, 2-way ANOVA). (C) NK cell number increases during ALT-803 therapy, comparing IV to SQ administration, combining the 6 and 10 μg/kg dose levels. The IV (P = .03) and SQ (P < .01) curves both significantly increase over time (ANOVA), and comparison of the curves reveals a significant increase in SQ (P = .029, 2-way ANOVA). Fold change comparing IV and SQ demonstrates a significant increase in the peak fold change (Mann-Whitney test) determined as the peak absolute NK cell count during ALT-803 therapy divided by the absolute NK cell count pretherapy. (D) Representative flow cytometry plots demonstrating changes in Ki67 (surrogate for proliferation) over time with IV vs SQ ALT-803. (E) Summary data from panel D. Both the IV (P < .01) and SQ (P < .01) curves significantly increase in Ki67+ NK cell percentage over time compared with time 0 (2-way ANOVA). Comparisons of the curves revealed a significant increase in SQ Ki67+ NK cells that is most apparent at day 7 (2-way ANOVA).
ALT-803 results in expansion of CD56brightand CD56dimNK cell subsets, with sustained proliferation of CD56brightNK cells. (A-B) Absolute numbers of the CD56bright (A) and CD56dim (B) NK cell subsets of peripheral blood. The SQ administration route increases CD56bright NK cell percentages over 4 weeks of therapy. (C-D) Proliferation of CD56bright (C) and CD56dim (D) NK cell subsets measured by intracellular Ki67 expression. CD56bright NK cells exhibit sustained proliferation for weeks during weekly ALT-803 administration via the SQ route (2-way ANOVA). (E) Representative flow bivariate flow cytometry plots showing the NK cell subset flow cytometry gates and expression of Ki67 in CD56bright and CD56dim subsets 21 days after 6 µg/kg ALT-803 administered SQ (UMN16, left) or IV (WSU002, right). (F) CD56bright NK cell expression of GzmB in a subset of patients analyzed by mass cytometry revealed ALT-803 priming of CD56bright NK cells in vivo (Friedman test).
ALT-803 results in expansion of CD56brightand CD56dimNK cell subsets, with sustained proliferation of CD56brightNK cells. (A-B) Absolute numbers of the CD56bright (A) and CD56dim (B) NK cell subsets of peripheral blood. The SQ administration route increases CD56bright NK cell percentages over 4 weeks of therapy. (C-D) Proliferation of CD56bright (C) and CD56dim (D) NK cell subsets measured by intracellular Ki67 expression. CD56bright NK cells exhibit sustained proliferation for weeks during weekly ALT-803 administration via the SQ route (2-way ANOVA). (E) Representative flow bivariate flow cytometry plots showing the NK cell subset flow cytometry gates and expression of Ki67 in CD56bright and CD56dim subsets 21 days after 6 µg/kg ALT-803 administered SQ (UMN16, left) or IV (WSU002, right). (F) CD56bright NK cell expression of GzmB in a subset of patients analyzed by mass cytometry revealed ALT-803 priming of CD56bright NK cells in vivo (Friedman test).
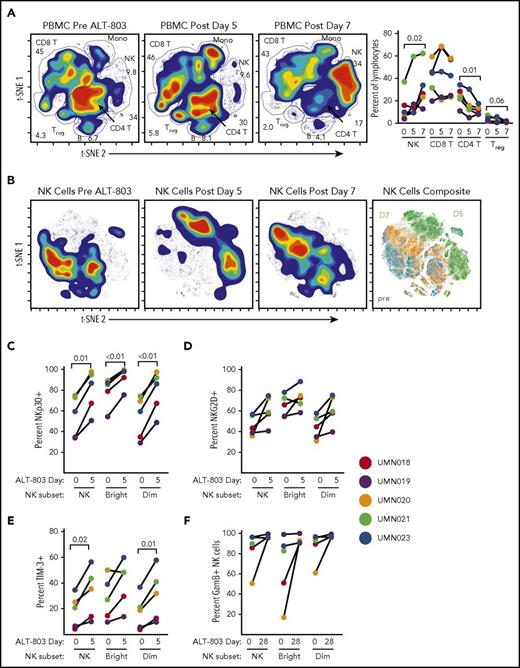
To elucidate ALT-803–induced changes in NK cells, we also performed 40-parameter mass cytometry analysis on 5 patients with available samples from the 10 μg/kg SQ cohort after the first dose of ALT-803. Utilizing viSNE to track major PBMC types,28 the increase in frequency of NK cells was confirmed (Figure 5A). Gating on NK cells and clustering on 20 parameters, it was also evident that ALT-803 SQ administration resulted in profound changes in the conventional NK cell compartment (Figure 5B). This assay includes lineage markers to identify NK cell subsets (supplemental Figure 1) and markers of NK cell activation, functional capacity, and inhibitory/activating receptors (supplemental Table 2). This analysis revealed common changes that occurred with ALT-803 therapy, as well as patient-specific NK cell differences in baseline markers of NK cell functionality, and subsequent modulation with ALT-803. The frequency of NK cells expressing the activating receptors NKp30 and NKG2D increased following ALT-803 expression at day 5 (Figure 5C-D). Consistent induction of the regulatory receptor TIM-3 (Figure 5E) was observed for most patients, but only a single patient demonstrated LAG-3 checkpoint induction (supplemental Figure 2). ALT-803 induced proliferation of all stages of NK cell maturation in the peripheral blood (supplemental Figure 2). Analysis of NK cell GzmB, a key component of NK cell cytotoxic capacity, revealed marked heterogeneity in relapsed patients. In one patient with low GzmB protein levels, ALT-803 restored these levels after 3 doses (Figure 5F). Collectively, these results suggest that IL-15–based immunomodulation has both broad and patient-specific effects on NK cells that warrant ongoing investigation to define biomarkers correlating with clinical efficacy.
Mass cytometry reveals alterations in PBMCs and the NK cell phenotype after ALT-803 therapy. (A) viSNE analysis of PBMCs with CD4, CD8, and Treg and B lymphocytes, NK cells, and monocytes annotated. Numbers represent the percentage of cells within the indicated viSNE cell-type island. At day 7 after ALT-803, there is a significant expansion of NK cells, with a significant decrease in CD4 T-cell frequencies and a trend toward lower Treg frequencies (repeated-measures ANOVA). (B) NK cells demonstrate marked phenotypic changes following ALT-803 therapy. viSNE density map analyses of the NK cells pretherapy and at days 5 and 7 posttherapy clustered on 20 markers. Composite of all time points with island gates shown (right). (C) Changes in the NK receptors NKp30 (C) and NKG2D (D) and TIM-3 (E) on NK cell subsets after ALT-803 SQ administration (paired Student t test). (F) Percentage of NK cells positive for GzmB contrasting pretherapy to posttherapy 3 weekly doses of ALT-803. Experiments were performed on PBMC samples from 5 patients after the first dose of 10 μg/kg SQ ALT-803.
Mass cytometry reveals alterations in PBMCs and the NK cell phenotype after ALT-803 therapy. (A) viSNE analysis of PBMCs with CD4, CD8, and Treg and B lymphocytes, NK cells, and monocytes annotated. Numbers represent the percentage of cells within the indicated viSNE cell-type island. At day 7 after ALT-803, there is a significant expansion of NK cells, with a significant decrease in CD4 T-cell frequencies and a trend toward lower Treg frequencies (repeated-measures ANOVA). (B) NK cells demonstrate marked phenotypic changes following ALT-803 therapy. viSNE density map analyses of the NK cells pretherapy and at days 5 and 7 posttherapy clustered on 20 markers. Composite of all time points with island gates shown (right). (C) Changes in the NK receptors NKp30 (C) and NKG2D (D) and TIM-3 (E) on NK cell subsets after ALT-803 SQ administration (paired Student t test). (F) Percentage of NK cells positive for GzmB contrasting pretherapy to posttherapy 3 weekly doses of ALT-803. Experiments were performed on PBMC samples from 5 patients after the first dose of 10 μg/kg SQ ALT-803.
T-cell modulation by ALT-803
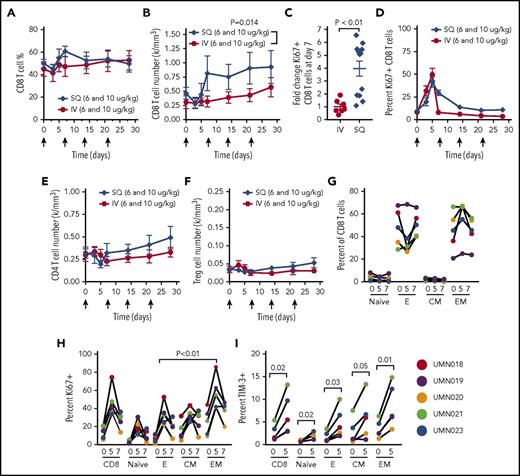
CD8+ T cells also respond to IL-15, which selectively promotes memory T-cell responses.29 CD8+ T-cell numbers increased following ALT-803 administration, although their frequency within PBMCs was unaltered. The SQ route resulted in significantly greater CD8+ T-cell expansion compared with the IV route (Figure 6A-C). Similar to NK cells, both IV and SQ ALT-803 promoted CD8+ T-cell activation and proliferation evidenced by Ki67 induction (Figure 6D). The numbers of CD4+ T cells and CD4+FoxP3+CD25hi Tregs were not significantly altered following ALT-803 administration (Figure 6E-F). To better define the T-cell subsets responding to ALT-803, we analyzed mass cytometry data from the 10 μg/kg SQ patients and categorized CD8+ T cells as naive, effector (TE), effector memory (TEM), or central memory (TCM) (Figure 6G; supplemental Figure 3). A predominance of CD8+ T cells in the peripheral blood of these patients were of the effector or effector memory phenotype (Figure 6G), and a significantly greater Ki67 response was observed in effector memory CD8+ T cells (Figure 6H). Similar to NK cells, CD8+ T cells demonstrated induced expression of TIM-3 (Figure 6I). To address whether γδT cells expanded in the blood concurrent with the SQ injection site reaction, flow cytometry was performed on baseline and day 7 samples. A small but significant increase in circulating γδT cells was observed (1.7-fold increase, P < .05; supplemental Figure 5).
ALT-803 expands and activates CD8+T cells. (A) The percentage of lymphocytes that were CD8+ T cells was not significantly altered following IV or SQ ALT-803 administration. (B) ALT-803 results in a significant increase in CD8+ T-cell numbers following SQ, but not IV, ALT-803 administration. (C) SQ ALT-803 administration resulted in significantly greater fold increase in CD8+ T cells compared with the IV route (Mann-Whitney). (D) ALT-803 resulted in T-cell proliferation detected by Ki67 expression, with a significant increase in SQ vs IV Ki67+ at day 7 (2-way ANOVA with Bonferroni multiple comparisons). (E-F) The number of CD4+ T cells and CD4+FoxP3+CD25hi Treg cells were unchanged with ALT-803 administration. (G) CD8+ T cells were comprised primary of effector (E) and effector memory (EM) phenotype. (H) Frequency of Ki67+ CD8+ T cell subsets. There was a significantly greater frequency of effector memory Ki67+ cells at day 5 after ALT-803 compared with effector CD8+ T cells (paired Student t test). (I) TIM-3 expression is transiently induced on CD8+ T cells after ALT-803 treatment (repeated-measures ANOVA, Fisher least significant difference).
ALT-803 expands and activates CD8+T cells. (A) The percentage of lymphocytes that were CD8+ T cells was not significantly altered following IV or SQ ALT-803 administration. (B) ALT-803 results in a significant increase in CD8+ T-cell numbers following SQ, but not IV, ALT-803 administration. (C) SQ ALT-803 administration resulted in significantly greater fold increase in CD8+ T cells compared with the IV route (Mann-Whitney). (D) ALT-803 resulted in T-cell proliferation detected by Ki67 expression, with a significant increase in SQ vs IV Ki67+ at day 7 (2-way ANOVA with Bonferroni multiple comparisons). (E-F) The number of CD4+ T cells and CD4+FoxP3+CD25hi Treg cells were unchanged with ALT-803 administration. (G) CD8+ T cells were comprised primary of effector (E) and effector memory (EM) phenotype. (H) Frequency of Ki67+ CD8+ T cell subsets. There was a significantly greater frequency of effector memory Ki67+ cells at day 5 after ALT-803 compared with effector CD8+ T cells (paired Student t test). (I) TIM-3 expression is transiently induced on CD8+ T cells after ALT-803 treatment (repeated-measures ANOVA, Fisher least significant difference).
Discussion
This first-in-human phase 1 clinical trial using ALT-803 in post allo-HCT relapsed patients demonstrated that 10 μg/kg ALT-803 was well tolerated via both the IV and SQ routes, with no major unexpected AEs and no cytokine release syndrome or capillary leak syndrome. ALT-803 markedly expanded and activated NK cells, with more modest effects on TE and TEM CD8+ T cells. Minimal changes in CD4+ T cells and Tregs were observed. Despite the impact on CD8+ T-cell activation, no patient developed GVHD requiring systemic immune suppression. This study demonstrates the safety and immune activating potential of SQ ALT-803 dosing amenable to outpatient therapy and a signal of efficacy when used to stimulate allogeneic immunity after HCT. Although IV ALT-803 administered in 4 weekly doses did not result in DLTs, long-term administration would be hampered by high fevers and rigors occurring 4 to 6 hours after infusion.
The previous trial using E coli rhIL-15 administered IV at 3 and 1 μg/kg reported a markedly elevated Cmax, which was associated with increased inflammatory markers, and DLTs including grade 3 hypotension, thrombocytopenia, and elevations of alanine transaminase and aspartate transaminase.20 The maximum tolerable dose of rhIL-15 (0.3 μg/kg IV) resulted in modest increases in NK cells. Therefore, although IL-15 is a promising immunostimulatory agent to activate NK cells and CD8+ T cells, improvements were needed to reduce toxicity while maintaining its immunologic activity. Here, weekly IV administration of ALT-803 at doses of up to 10 μg/kg resulted in elevated Cmax and modest proinflammatory cytokine release, but the associated fevers, chills, and rigors did not result in DLTs. SQ administration of ALT-803 was associated with a lower Cmax, significantly longer half-life, minimal increases in early proinflammatory cytokine levels, and no DLTs. The injection site rashes that developed following SQ administration likely reflect recruitment and/or activation of immune cells (eg, γδ T cells) at the site of the IL-15 depot. Clinically, the injection site rash was self-limited and responded to topical steroids in all the patients. Importantly, despite lymphocyte immunomodulation, treatment-emergent GVHD included only limited skin involvement treated with topical steroids and without systemic immune suppression. We acknowledge that the low frequency and severity of GVHD may be immune-context dependent and impacted by the use of ALT-803 in patients with relapse and only partial donor chimerism. In preclinical studies using xenogeneic transplantation, GVHD was associated with IL-15, but not IL-2.30 Consistent with IL-2 stimulation of Tregs, low-dose IL-2 has been translated to the clinic as GVHD therapy.31 Thus, caution should be used in transplantation settings where full donor chimerism is expected, such as when testing ALT-803 to prevent relapse (NCT02989844), where GVHD will comprise an important stopping rule. In addition, the lack of GVHD in allo-HCT patients could potentially be related to the preferential expansion of NK cells inhibiting GVHD-causing alloreactive T cells, and further study is warranted.32
Tolerable doses of ALT-803 increased absolute NK cell numbers with sustained activation and proliferation to a significantly greater level after SQ vs IV administration. Similar to the study with rhIL-15, early decreases in NK cell numbers were observed and may reflect margination or migration of NK cells from the peripheral blood into tissues.20 Although ALT-803 affected both the CD56bright and CD56dim NK cell subsets,26 the CD56bright NK cells showed more proliferation and pronounced GzmB protein induction. This finding provides additional in vivo support to the recent finding that CD56bright NK cells may be transformed into potent antitumor effectors via IL-15 priming.27 Additional assessment by mass cytometry revealed upregulation of the activating receptors NKG2D and NKp30, demonstrating that IL-15 modulates activating receptor expression transiently in patients. The functional modulator TIM-3 was also transiently increased, which can lead to ligand-induced IFN-γ production33 or may act as a classic inhibitory checkpoint.34 This suggests that the biological impact of TIM-3 expression on NK cells is still controversial as to whether it is a marker of senescence35 or associated with functional responses,33,34 and further study is needed. No changes in killer cell immunoglobulin-like receptor or NKG2A expression were detected, suggesting that ALT-803 does not augment expression of these constitutive inhibitory receptors on NK cells. There were also patient-specific changes induced after ALT-803 (eg, GzmB restoration), suggesting that responses to immunostimulatory agents such as IL-15 by immune cells will vary between individuals. This indicates that individual patient-specific immune monitoring may help to identify combination immunotherapy strategies with the highest impact for a given patient. CD8+ T cells present in patients at post-HCT relapse were primarily TE and TEM, and these cell subsets were activated and proliferated (Ki67+) after ALT-803. Although a minor population, TCM and naive CD8+ T cells also responded to ALT-803 with proliferation and TIM-3 induction. CD4+ T cells and Tregs were not substantially affected by ALT-803 therapy. No immune parameters correlated with response in this small sample set.
These data demonstrate that ALT-803 selectively activates NK cells and CD8+ T cells in patients, providing a clear rationale for testing ALT-803 in other clinical settings and in combination with complementary immunotherapy approaches.36 For example, ALT-803 could complement PD-1 checkpoint blockade by supporting the survival and persistence of the memory CD8+ T-cell pool and stimulation of NK cells. Chimeric antigen receptor T cells and bispecific T-cell engagers are effective against B-cell malignancies, and ALT-803 may provide a unique signal for persistence and functionality of such engineered or redirected T cells. ALT-803 may also augment cancer vaccine approaches seeking to boost T-cell responses to cancer-expressed antigens by enhancing T-cell expansion, response, and memory differentiation. Based on the profound NK cell modulation, combinations of ALT-803 with therapeutic antibodies that rely in part on antibody-dependent cellular cytotoxicity are already being investigated (NCT02384954). Similarly, ALT-803 may support the homeostasis and function of adoptively transferred NK cells, and this strategy is under investigation (NCT01898793 and NCT02782546). For allo-HCT, ALT-803 used prior to relapse may promote the persistence of antileukemic NK or T cells. These concepts and clinical studies are supported by the safety and immunomodulatory results of this study and have led to prospective multicenter relapse prophylaxis trial after reduced intensity conditioning allogeneic transplant where the primary end point will be disease-free survival at 1 year (NCT02989844). Based on this first-in-human study, administration of ALT-803 in AML and transplant settings is recommended at the 6 to 10 μg/kg range. In subjects with solid tumor malignancies and lymphoma, doses up to 20 μg/kg are being tested.
In summary, this first-in-human clinical trial demonstrates the safety of ALT-803, with an excellent safety profile, and provides evidence of activity in patients who have relapsed after allo-HCT. SQ administration of this immunostimulatory agent resulted in more sustained drug levels and better biologic activity on blood NK and CD8+ T cells while avoiding the constitutional toxicities seen with IV administration. This study therefore identifies ALT-803 as a promising and potent immunostimulatory IL-15 agent to promote immune reconstitution that warrants further investigation in multiple cancer immunotherapy settings.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patient volunteers and their families, as well as the dedicated clinical trials staff.
This study was funded by the National Institutes of Health, National Cancer Institute (grants P01CA111412 [J.S.M., S.C., M.R.V., D.J.W., and T.A.F.], P01CA65493 [J.S.M., S.C., M.R.V., J.E.W., and B.R.B.], R01CA205239 [T.A.F.], and F32CA200253 [M.M.B.-E.]), UMN, WUSM Division of Oncology, and Altor BioScience, a Nantworks company (Miramar, FL).
Authorship
Contribution: J.S.M., B.R.B., S.C., and T.E.D. conceived and designed the study; R.R., S.C., P.W., M.R.V., J.E.W., D.J.W., J.F.D., A.F.C., M.M.B.-E., L.P., R.K., A.M., A.S., M.J.A., I.R., and D.M. recruited patients and/or collected data; R.R., S.C., M.M.B.-E., T.E.D., M.J.A., S.V., I.R., T.A.F., and J.S.M. analyzed the data; J.O.E., E.K.J., A.R., and H.C.W. provided critical reagents and contributed to the study design; R.R., T.A.F., and J.S.M. wrote the manuscript; and all authors interpreted the data, were involved in development and review, and approved of the final manuscript. The corresponding authors had full access to all study data and final responsibility for the manuscript, and all authors agreed to accuracy of the data and final submission.
Conflict-of-interest disclosure: J.S.M., S.C., and T.A.F. received partial research support from Altor BioScience, a Nantworks company, but have no financial benefit from the outcome of this trial. J.O.E., E.K.J., A.R., and H.C.W. are employees of Altor BioScience and declare direct financial conflicts. To manage these conflicts, UMN and WUSM investigators led this trial, were sponsors of the IND, managed all the data in the study, and had final responsibility for the manuscript. The study protocol was an investigator-initiated clinical trial. UMN and WUSM investigators performed the clinical trial including data collection, analysis, and interpretation. Altor BioScience performed ALT-803 and cytokine measurements and immunogenicity testing on coded samples. The remaining correlative assays and all statistical analyses were performed by UMN and WUSM. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, Division of Hematology, Oncology and Transplantation, University of Minnesota Cancer Center, MMC806, Harvard St at East River Rd, Minneapolis, MN 55455; e-mail: mille001@umn.edu; and Todd A. Fehniger, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: tfehnige@wustl.edu.
References
Author notes
R.R. and S.C. contributed equally to this study.
T.A.F. and J.S.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal