TO THE EDITOR:
Central nervous system (CNS) involvement in pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is rarely detected at initial presentation.1 Nevertheless, CNS relapse most frequently occurs in children who were initially diagnosed as CNS− and did not have any high-risk characteristics.2 Therefore, all patients receive intensive CNS-directed chemotherapy,3 an approach associated with short- and long-term neurological toxicities.4,5 The CNS microenvironment may contribute to chemoresistance and survival of leukemic cells.6 Interleukin 15 (IL15) was shown to promote ALL survival in the hostile microenvironments of the CNS.7,8 IL7 can be detected in the cerebrospinal fluid (CSF) and high levels have been associated with inflammatory CNS disease,9 which supports that IL7 may be produced by stromal cells in that niche upon different stimuli.10 Also, elevated IL7 plasma levels were detected in BCP-ALL patients.11 Here, we show that IL7R is highly expressed in pediatric BCP-ALL patients who were CNS+ at initial diagnosis, and that an upregulation of IL7R may predict CNS relapse. Using a xenograft model in immunodeficient mice, we show that IL7R is required for leukemic engraftment in vivo, and that targeting IL7R with monoclonal antibody reduces CNS leukemic infiltration.
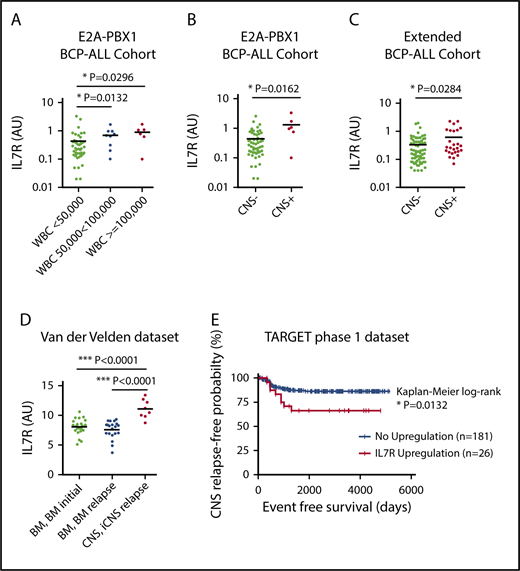
The t(1;19) chromosomal translocation leading to the E2A-PBX1 fusion was shown to be associated with increased IL7R expression,12 and E2A-PBX1–rearranged BCP-ALL cells have a particular propensity to infiltrate the CNS.13,14 Thus, we first analyzed IL7R expression in a cohort of 61 E2A-PBX1+ patients13 and correlated the data with clinical characteristics. IL7R expression was significantly higher in patients with an elevated white blood cell (WBC) count (Figure 1A), which is also a classical risk factor for CNS disease. Importantly, IL7R expression was also significantly higher in CNS+ as compared with CNS− patients (Figure 1B). In contrast, there were no correlations between IL7R expression and sex, age, prednisone response, or minimal residual disease–risk group (supplemental Figure 1, available on the Blood Web site). We next determined IL7R expression in a further cohort of 98 BCP-ALL patients of mixed molecular backgrounds. The cohort contained 26 patients who were initially CNS+ and 72 CNS− patients. There were no statistical differences in sex, age, prednisone response, minimal residual disease–risk groups, and cytogenetics between both groups (supplemental Table 1). Importantly, IL7R expression was found to be significantly elevated in CNS+ compared with CNS− patients (Figure 1C). Multivariate analysis controlling for age and WBC count showed that IL7R expression in the third and fourth quartiles lead to odds ratios of 5.4 (95% confidence interval, 0.997-29.117) and 5.6 (95% confidence interval, 1.023-30.842) for CNS positivity, respectively (supplemental Table 2). These data suggest that increased IL7R expression levels in bone marrow (BM) leukemic cells are associated with and may predict CNS disease at initial diagnosis. IL7R expression also significantly correlated with ζ-chain–associated protein kinase 70 (ZAP70), which is another marker for CNS infiltration15 (supplemental Figure 2A). However, combining both markers did not yield superior correlations (supplemental Figure 2B). The association of IL7R expression with CNS relapse was then explored using 2 publicly available data sets.16-18 ALL cells retrieved from the CSF of children with isolated CNS relapse showed a significantly higher IL7R expression compared with ALL cells from BM at diagnosis and BM at BM relapse without CNS involvement (Figure 1D). Most importantly, high IL7R expression in BCP-ALL cells from BM/peripheral blood at diagnosis was associated with reduced long-term CNS relapse–free probability rates in the TARGET phase 1 data set (Figure 1E). It seems that as IL7R expression increases, reflected by increasing z score, the rate of CNS relapse also increases (supplemental Figure 3A-B; supplemental Table 3). Among different risk factors for CNS relapse, an upregulation of IL7R was a statistically significant predictor of isolated CNS relapse in a Cox proportional hazards model (supplemental Table 4). Nevertheless, increased IL7R expression was not associated with an increased risk for BM relapse or relapses with BM involvement (supplemental Figure 3C-D). Interestingly, there was a significant association between IL7R upregulation and E2A-PBX1 (30% of IL7R overexpressors had this translocation; supplemental Table 5).
IL7R expression is associated with CNS disease and CNS relapse in pediatric BCP-ALL patients. Correlation analysis of IL7R expression in 61 E2A-PBX1+ pediatric patients with white blood cell (WBC) count (A) and CNS status (B). Unpaired Student t test, 2-sided P value. (C) Correlation analysis of IL7R expression in 98 pediatric BCP-ALL patients of mixed cytogenetics and CNS status. Further definitions are provided in supplemental Table 1. Unpaired Student t test, 2-sided P value. (D) IL7R expression in ALL cells retrieved from the CSF of 8 children with CNS relapse of BCP-ALL as well as from the bone marrow (BM) of 22 patients at diagnosis, and cells from the BM of 20 patients at the time of isolated BM relapse (data set, van der Velden et al16 ). Unpaired Student t test, 2-sided P value. (E) Kaplan-Meier survival curve showing reduced isolated CNS (iCNS) relapse-free probability in children with upregulated IL7R gene expression in diagnostic BM (n = 131) or peripheral blood (n = 76) samples of children with high-risk ALL. IL7R upregulation was defined as a z score for gene expression ≥1.2 (TARGET phase 1 data set). AU, arbitrary unit; IL7R, interleukin 7 receptor.
IL7R expression is associated with CNS disease and CNS relapse in pediatric BCP-ALL patients. Correlation analysis of IL7R expression in 61 E2A-PBX1+ pediatric patients with white blood cell (WBC) count (A) and CNS status (B). Unpaired Student t test, 2-sided P value. (C) Correlation analysis of IL7R expression in 98 pediatric BCP-ALL patients of mixed cytogenetics and CNS status. Further definitions are provided in supplemental Table 1. Unpaired Student t test, 2-sided P value. (D) IL7R expression in ALL cells retrieved from the CSF of 8 children with CNS relapse of BCP-ALL as well as from the bone marrow (BM) of 22 patients at diagnosis, and cells from the BM of 20 patients at the time of isolated BM relapse (data set, van der Velden et al16 ). Unpaired Student t test, 2-sided P value. (E) Kaplan-Meier survival curve showing reduced isolated CNS (iCNS) relapse-free probability in children with upregulated IL7R gene expression in diagnostic BM (n = 131) or peripheral blood (n = 76) samples of children with high-risk ALL. IL7R upregulation was defined as a z score for gene expression ≥1.2 (TARGET phase 1 data set). AU, arbitrary unit; IL7R, interleukin 7 receptor.
These findings indicate that IL7R may be used as a diagnostic and prognostic marker without accessing the CNS compartment for diagnosis of CNS leukemia.
We next injected 13 patient samples into NSG mice in duplicates, and mean fluorescence intensity of IL7R was determined. Xenografts were subgrouped into IL7RHi/IL7RLo relative to median mean fluorescence intensity (supplemental Table 6). For 11 xenografts with available histology, CNS infiltration was analyzed.13 Eight of 12 mice (67%) injected with IL7RHi cells were CNS+, whereas only 2 of 10 mice (20%) bearing IL7RLo cells were CNS+ (supplemental Figure 4A). IL7RHi blasts in this experiment showed a tendency to have higher basal levels of extracellular signal-regulated kinase (ERK), phosphorylated extracellular signal-regulated kinase, and phosphorylated AKT compared with IL7RLo (supplemental Figure 4B). To test whether blocking IL7R in vivo can prevent ALL engraftment and homing to CNS, we downregulated IL7R expression by RNA interference using an IL7Rα-specific short hairpin RNA (shRNA) in the human cell line 697, which expresses high levels of IL7R. Downmodulation of IL7R led to a marked decrease of blast percentages in the spleen, BM, and CNS as compared with mice injected with the respective control (Figure 2A-B). To investigate whether inhibition of IL7R signaling using ruxolitinib can interfere with the engraftment of leukemic cells with a high expression of IL7R in vivo, we injected E2A-PBX1+ BCP-ALL cells from 1 pediatric patient into NSG mice and monitored the survival of recipient mice under ruxolitinib treatment with and without concomitant chemotherapy. We found that mice treated with ruxolitinib showed only a minor prolongation in survival in comparison with untreated control and that ruxolitinib was markedly less efficient than standard chemotherapy. In addition, ruxolitinib treatment did not decrease leukemic infiltration in the CNS (data not shown). The combination of ruxolitinib and chemotherapy did not result in additional benefits (Figure 2C). In opposition to previously published data,19 our results indicate that ruxolitinib is not efficient for preventing the engraftment of human ALL cells in vivo. This might be caused by poor bioavailability of ruxolitinib in mice and/or an insufficient inhibition of IL7R signaling, as ruxolitinib inhibits mainly JAK1/2 and not JAK3 that also can be activated by IL7R signaling. Furthermore, the amount of IL7 available in vivo may have overridden the downstream inhibition by ruxolitinib. We therefore next tested whether inhibiting IL7R with a blocking antibody would substantiate our previous findings in a further experiment with an E2A-PBX1+ patient sample in vivo. Antibody treatment significantly prolonged the survival of xenograft mice as compared with treatment with an isotype control antibody (Figure 2D). In addition, IL7R antibody treatment strongly reduced spleen size and leukemic infiltration in spleen, BM, and, most importantly, in the CNS (Figure 2E-G). Ruxolitinib as a single agent or as addition to the antibody treatment had no beneficial effects (Figure 2E-G). We found that in vitro antibody treatment downmodulated IL7R signaling through AKT and induced apoptosis as indicated by an upregulation of cleaved caspase-8 (supplemental Figure 5A-B).
Inhibition of IL7R delays leukemogenesis in xenograft mice. (A) NSG mice were xenografted with 697 cells bearing an shRNA against the IL7Rα (shIL7Rα) or a control shRNA (shGFP). Animals were euthanized at day 26 upon detection of >75% leukemic blasts in the peripheral blood or clinical leukemia (loss of weight or activity, organomegaly, hind-limb paralysis) in first control mice. Spleen (Sp) and BM infiltration by human leukemic blasts in control and treated animals. (B) CNS infiltration as determined by histology (hematoxylin and eosin stain; original magnification ×100 [i,iii] and ×40 [ii]). The arrows indicate human leukemic blasts in an example for the semiquantitative scoring used.13 (C) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with vehicle only, ruxolitinib (Ruxo) only, chemotherapy (Chemo) only (dexamethasone, vincristine, and polyethylene glycol–asparaginase), or a combination of ruxolitinib and chemotherapy (n = 7 per group). Mice were euthanized upon appearance of leukemic symptoms. Statistics for survival were performed according to the Mantel-Cox log-rank method. P1, Control vs Ruxo; P2, Control vs Chemo; P3, Control vs Ruxo/Chemo. (D) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with an anti-IL7R antibody or an isotype antibody (n = 7 and n = 6 per group, as indicated). The experiment was ended on day 135. Statistics for survival were performed according to the Mantel-Cox log-rank method. (E-G) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with control antibody, ruxolitinib, with an anti-IL7R antibody (Ab) or with both ruxolitinib and the antibody (n = 7 per group). One mouse of the Ruxo/anti-IL7R group died during the experiment and accordingly was excluded. The experiment was ended on day 65 and spleen sizes (E), the percentages of Sp and BM blasts (F), and CNS infiltration (G) were assessed (Fisher exact test, 2-sided). Treatment protocol: 60 mg/kg ruxolitinib (LC Laboratories) was administered Monday through Friday by oral gavage. Chemotherapy was administered as previously published.15,22 A total of 1 mg/kg anti-IL7R antibody (monoclonal mouse immunoglobulin G1 [IgG1], clone 40131; R&D Systems) or isotype control antibody were administered on days 0, +3, +7, +21, +35, +48, and +56 postinjection.
Inhibition of IL7R delays leukemogenesis in xenograft mice. (A) NSG mice were xenografted with 697 cells bearing an shRNA against the IL7Rα (shIL7Rα) or a control shRNA (shGFP). Animals were euthanized at day 26 upon detection of >75% leukemic blasts in the peripheral blood or clinical leukemia (loss of weight or activity, organomegaly, hind-limb paralysis) in first control mice. Spleen (Sp) and BM infiltration by human leukemic blasts in control and treated animals. (B) CNS infiltration as determined by histology (hematoxylin and eosin stain; original magnification ×100 [i,iii] and ×40 [ii]). The arrows indicate human leukemic blasts in an example for the semiquantitative scoring used.13 (C) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with vehicle only, ruxolitinib (Ruxo) only, chemotherapy (Chemo) only (dexamethasone, vincristine, and polyethylene glycol–asparaginase), or a combination of ruxolitinib and chemotherapy (n = 7 per group). Mice were euthanized upon appearance of leukemic symptoms. Statistics for survival were performed according to the Mantel-Cox log-rank method. P1, Control vs Ruxo; P2, Control vs Chemo; P3, Control vs Ruxo/Chemo. (D) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with an anti-IL7R antibody or an isotype antibody (n = 7 and n = 6 per group, as indicated). The experiment was ended on day 135. Statistics for survival were performed according to the Mantel-Cox log-rank method. (E-G) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with control antibody, ruxolitinib, with an anti-IL7R antibody (Ab) or with both ruxolitinib and the antibody (n = 7 per group). One mouse of the Ruxo/anti-IL7R group died during the experiment and accordingly was excluded. The experiment was ended on day 65 and spleen sizes (E), the percentages of Sp and BM blasts (F), and CNS infiltration (G) were assessed (Fisher exact test, 2-sided). Treatment protocol: 60 mg/kg ruxolitinib (LC Laboratories) was administered Monday through Friday by oral gavage. Chemotherapy was administered as previously published.15,22 A total of 1 mg/kg anti-IL7R antibody (monoclonal mouse immunoglobulin G1 [IgG1], clone 40131; R&D Systems) or isotype control antibody were administered on days 0, +3, +7, +21, +35, +48, and +56 postinjection.
These findings support the view that targeting IL7/IL7R signaling may be an effective approach in BCP-ALL therapy.20 So far, anti-IL7R antibodies have been investigated in preclinical mouse models of multiple sclerosis to target T cells that require IL7R expression for their homeostasis,21 indicating a toxic effect of the antibody in T cells.
Our study points to IL7R as a main target for BCP-ALL treatment and indicates that further investigation of the anti-IL7R antibodies for immunotherapy of BCP-ALL may lead to improved therapeutic approaches especially for patients with CNS involvement.
Presented orally at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017 (Abstract 479).
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and physicians who contributed samples and data for this study. The authors thank Katrin Timm-Richert, Katrin Neumann, and Omar El Ayoubi for excellent technical assistance.
This work was supported by the Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft (SFB1074; projects A9, A10, B6). This work was also supported by a European Research Council advanced grant (H.J.). This work was also supported by the Wilhelm Sander Stiftung (2016.110.1) and the Deutsche José-Carreras Leukämiestiftung (DJCLS 17 R/2017) (D.M.S.). C.H. was funded by the William and Elizabeth Davies Foundation, Chief Scientist Office (ETM/374).
Authorship
Contribution: A.A. designed experiments, analyzed data, supervised the research direction, and wrote the manuscript; L.L., A.V., A.K., F.V., and C.V. performed experiments and analyzed data; G.C. and M.S. provided ALL materials; F.S., K.-M.D., and L.-H.M. provided materials; A.C. and C.H. provided data set analyses; D.M.S. and E.H. designed experiments and discussed the research direction; D.M.S. wrote the manuscript; H.J. initiated, designed, and discussed the research direction and wrote the manuscript; and all authors discussed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hassan Jumaa, Institute of Immunology, Ulm University Medical Center, Albert-Einstein-Allee 11, D-89081 Ulm, Germany; e-mail: hassan.jumaa@uni-ulm.de.
REFERENCES
Author notes
D.M.S. and H.J. share senior authorship.

![Figure 2. Inhibition of IL7R delays leukemogenesis in xenograft mice. (A) NSG mice were xenografted with 697 cells bearing an shRNA against the IL7Rα (shIL7Rα) or a control shRNA (shGFP). Animals were euthanized at day 26 upon detection of >75% leukemic blasts in the peripheral blood or clinical leukemia (loss of weight or activity, organomegaly, hind-limb paralysis) in first control mice. Spleen (Sp) and BM infiltration by human leukemic blasts in control and treated animals. (B) CNS infiltration as determined by histology (hematoxylin and eosin stain; original magnification ×100 [i,iii] and ×40 [ii]). The arrows indicate human leukemic blasts in an example for the semiquantitative scoring used.13 (C) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with vehicle only, ruxolitinib (Ruxo) only, chemotherapy (Chemo) only (dexamethasone, vincristine, and polyethylene glycol–asparaginase), or a combination of ruxolitinib and chemotherapy (n = 7 per group). Mice were euthanized upon appearance of leukemic symptoms. Statistics for survival were performed according to the Mantel-Cox log-rank method. P1, Control vs Ruxo; P2, Control vs Chemo; P3, Control vs Ruxo/Chemo. (D) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with an anti-IL7R antibody or an isotype antibody (n = 7 and n = 6 per group, as indicated). The experiment was ended on day 135. Statistics for survival were performed according to the Mantel-Cox log-rank method. (E-G) A total of 1 × 106 E2A-PBX1+ patient cells were xenografted into NSG mice. Xenografted mice were treated with control antibody, ruxolitinib, with an anti-IL7R antibody (Ab) or with both ruxolitinib and the antibody (n = 7 per group). One mouse of the Ruxo/anti-IL7R group died during the experiment and accordingly was excluded. The experiment was ended on day 65 and spleen sizes (E), the percentages of Sp and BM blasts (F), and CNS infiltration (G) were assessed (Fisher exact test, 2-sided). Treatment protocol: 60 mg/kg ruxolitinib (LC Laboratories) was administered Monday through Friday by oral gavage. Chemotherapy was administered as previously published.15,22 A total of 1 mg/kg anti-IL7R antibody (monoclonal mouse immunoglobulin G1 [IgG1], clone 40131; R&D Systems) or isotype control antibody were administered on days 0, +3, +7, +21, +35, +48, and +56 postinjection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/15/10.1182_blood-2018-04-844209/4/m_blood844209f2.png?Expires=1769081736&Signature=HyIHVZqCSpmnd~7LrnSz~x2KBRxHIi0lSgv~LulNQIIQq5zYoukUXpmQiqQIbZrHMiLdiIPKTogSd-qYf7c0wcGBdgPLpaWvj7XHrKEyRmEwnGI9O-CK6hcgSBd5lhNYj30zmM7fLA5cQBKCTVueKBhKWYwfZrkTY1cRNobpR7wbghp0zw5KLM8lB-ndDiBi09LF5x51iPmZhOrLctQkqSxRQbqpfwCif6FhAsELcZxoODxGYER36xxzeZk9LVwQ4elT7MaAq5Q0QJpQfFMP3LDz2-ZVGU75426wt4LEJzB~xkjGTWDA38satW4MC4SznpCHGp9DWzrkpJpi5SMLsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal