Key Points
miR-150 is downmodulated with transformation of FL leading to upregulation of FOXP1 protein.
Upregulation of MYC protein is responsible for the repression of miR-150 in transformed FL.
Follicular lymphoma (FL) is a common indolent B-cell malignancy with a variable clinical course. An unfavorable event in its course is histological transformation to a high-grade lymphoma, typically diffuse large B-cell lymphoma. Recent studies show that genetic aberrations of MYC or its overexpression are associated with FL transformation (tFL). However, the precise molecular mechanisms underlying tFL are unclear. Here we performed the first profiling of expression of microRNAs (miRNAs) in paired samples of FL and tFL and identified 5 miRNAs as being differentially expressed. We focused on one of these miRNAs, namely miR-150, which was uniformly downmodulated in all examined tFLs (∼3.5-fold), and observed that high levels of MYC are responsible for repressing miR-150 in tFL by binding in its upstream region. This MYC-mediated repression of miR-150 in B cells is not dependent on LIN28A/B proteins, which influence the maturation of miR-150 precursor (pri-miR-150) in myeloid cells. We also demonstrated that low miR-150 levels in tFL lead to upregulation of its target, namely FOXP1 protein, which is a known positive regulator of cell survival, as well as B-cell receptor and NF-κB signaling in malignant B cells. We revealed that low levels of miR-150 and high levels of its target, FOXP1, are associated with shorter overall survival in FL and suggest that miR-150 could serve as a good biomarker measurable in formalin-fixed paraffin-embedded tissue. Overall, our study demonstrates the role of the MYC/miR-150/FOXP1 axis in malignant B cells as a determinant of FL aggressiveness and its high-grade transformation.
Introduction
Follicular lymphoma (FL) is the second most common indolent B-cell malignancy, with a variable clinical course. An adverse event in the course of FL is histological transformation to a high-grade lymphoma, which is associated with clinically aggressive behavior and poor patient prognosis.1,,-4 FL most frequently transforms to diffuse large B-cell lymphoma (DLBCL), with the continuous risk of 2% to 3% per year.2,5 The effort to understand and predict histological transformation in FL is an active area of research, and several aberrations have been described as potential drivers of this process.6,,,,,,,,,-16 The most common genomic aberrations acquired during transformation include genetic lesions activating MYC protooncogene and mutations resulting in the inactivation of the cell-cycle regulators CDKN2A/B, p53, and MDM2.6,7,10,15,17 Changes in malignant B-cell gene expression and/or lymphoma microenvironment have also been associated with FL progression and transformation.10,17,,,-21 Gene expression profiling studies revealed that a MYC pathway “proliferation signature” is associated with >70% of high-grade FL transformations.10,17 However, the precise molecular mechanisms underlying the transformation of FL to DLBCL remain largely unknown.
Factors that might regulate the expression of protein-coding genes involved in FL transformation are microRNAs (miRNAs). These short noncoding RNAs can posttranscriptionally regulate expression of variety of genes and thus fine tune essential cell functions. We and others have shown that this is needed for the survival and normal maturation of B cells, immunoglobulin production, and B-cell receptor (BCR) signaling regulation.22,,-25 Aberrations in such miRNA-mediated regulation directly influence malignant B-cell biology, as well as the prognosis of patients (reviewed by Musilova and Mraz24 ). The miRNA role in lymphomagenesis was directly demonstrated in studies generating miRNA mouse models, with an impact on B-cell lymphoma development.24,26,-28 However, the role of miRNA deregulation in tFL is unclear.
Here we performed the first complex profiling of miRNA expression in paired samples of FL before and after its transformation to DLBCL. We identified 5 miRNAs as being differentially expressed between FL and transformed FL (tFL). In our study, we focused on one of these miRNAs, namely miR-150, which was downmodulated in tFL. We show that low levels of miR-150 in tFL lead to upregulation of FOXP1 protein, which is a direct target of miR-150. We have previously shown that high-level expression of FOXP1 increases BCR signaling propensity in chronic lymphocytic leukemia (CLL).22 FOXP1 has been extensively studied in DLBCL, where it is known to regulate survival and aggressiveness of B cells (reviewed by Gascoyne and Banham29 ).30,,-33 High FOXP1 levels are associated with worse prognosis in DLBCL,34,,-37 and FOXP1 is used in numerous algorithms (such as Choi, Tally, and Visco-Young) that distinguish the non–germinal center (non-GC) vs GC subtypes of DLBCL.29,38,,,,-43 We further show that upregulation of MYC protein is responsible for the repression of miR-150 in tFL. Additionally, we reveal that miR-150 and its target FOXP1 are strong prognostic markers in FL, which further supports the relevance of the MYC/miR-150/FOXP1 axis in malignant B-cell biology.
Methods
Patient samples and lymphoma cell lines
Formalin-fixed paraffin-embedded (FFPE) tissue or freshly frozen lymph node biopsy material was obtained from the contributing institutions, and the study was approved by the respective institutional review boards. The samples were obtained with written informed consent. Patient characteristics are described in supplemental Methods and supplemental Tables 1 and 2, available on the Blood Web site. The samples were processed for gene expression analysis and immunohistochemistry (IHC; supplemental Methods).
RNA isolation and mRNA/miRNA expression analyses
Total RNA was isolated from 10- to 20-μm FFPE tissue sections using a High Pure miRNA Isolation Kit (Roche) according to the manufacturer’s protocol. Total RNA from freshly frozen tissue or cell lines was isolated with TRI-Reagent (Sigma-Aldrich) as described previously.44 MiRNA expression profiling was performed using TaqMan Array Human MicroRNA A Cards v2.0 (377 miRNAs; Thermo Fisher Scientific) and individual TaqMan microRNA Assays (detailed description provided in supplemental Methods).
Luciferase assay
Luciferase reporter assay was performed using psiCHECK2 reporter plasmid and the Promega Dual-Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol (supplemental Methods).
Transient miRNA or siRNA transfection and cell-cycle analysis
A WSU-NHL B-cell line was obtained from the German Collection of Microorganisms and Cell Cultures and cultured in RPMI 1640 supplemented with 10% fetal bovine serum in 5% carbon dioxide at 37°C. B cells (0.75 × 106) were electroporated (Neon Transfection System; Thermo Fisher Scientific; program 1500 V per 20 ms per 1 pulse) with a short artificial miR-150 (1000 nM; MISSION miRNA Mimic; Sigma-Aldrich), control miRNA (1000 nM; MISSION miRNA Mimic Negative Control), short interfering RNA (siRNA; 1000 nM; On-TargetPlus Smartpool siRNAs for FOXP1; GE Dharmacon; Silencer Select Pre-Designed siRNA for MYC; Thermo Fisher Scientific; or 1000 nM; On-Target Plus Smartpool Negative Control). The WSU-NHL cell line was used for the transfection experiments because of its FL-like features (GC origin and presence of t[14;18]), relatively low-level expression of miR-150, relatively high levels of FOXP1 protein, and sufficient silencing efficiency and viability after transfection. After the indicated period of time, all cells in the well were harvested for cell-cycle and viability analyses and immunoblotting (supplemental Methods).
Statistical analysis
Univariate and multivariate Cox regression analyses of overall survival (OS) were computed using Statistica 13.2 (TIBCO Software Inc.; supplemental Methods). All other statistical analyses were performed with GraphPad Prism software v5.0 (GraphPad Software). All statistical tests were 2 sided, and P values <.05 were considered significant.
Results
miR-150 is downregulated after transformation of FL to DLBCL
To identify miRNAs that are changed upon FL transformation, we analyzed the global expression of 377 miRNAs in 8 pairs of samples (n = 16) from patients with FL that subsequently transformed to DLBCL. The analyzed miRNAs contained most of the miRNAs known to be associated with normal or malignant B-cell biology (supplemental Table 3). This identified 4 miRNAs (miR-150, miR-31, miR-222, and miR-517b) as downregulated and 1 miRNA (miR-106b) as upregulated in tFL (P < .05; fold-change ≥2; Figure 1A; supplemental Table 3). We further focused on miR-150 studies, because we have previously shown that miR-150 plays an important role in CLL biology by regulating transcription factor FOXP1 known to be of biological relevance in multiple B-cell lymphomas.22,29,34,35,37 miR-150 was the most constantly changed miRNA, with ∼3.5-fold downmodulation in FL (Figure 1A). To validate this, we analyzed miR-150 expression by individual qRT-PCRs in an extended cohort of 26 paired FL-tFL samples (13 pairs, including the original 8 pairs) and confirmed reduced miR-150 levels in all examined tFL samples (Figure 1B; supplemental Figure 1A). Additional miR-150 expression analyses in an extended cohort of tFLs (n = 18), nontransformed FL (n = 66), and de novo DLBCLs (n = 41) validated that miR-150 is consistently less expressed in tFL and DLBCL than in FL (Figure 1C; supplemental Figure 2). The non-B cells in the non-Hodgkin lymphoma samples (eg, T cells or stromal cells) did not significantly affect the measurement of miR-150 levels (supplemental Figure 3A), which was further supported by lack of any correlation between miR-150 levels and the percentage of T-cell infiltration in FL samples (supplemental Figure 3B).
MiRNAs are differentially expressed in tFL. (A) miRNAs were quantified in paired FL-tFL samples from 8 patients (analyzed by TaqMan Array Human MicroRNA Cards; supplemental Methods). Expression of each miRNA was centered on the median miRNA expression in all samples and visualized in a heatmap (MeV software). Lower expression is indicated in blue, and higher expression in yellow (linear scale). The differences in miRNA expression between FL vs tFL were tested by paired t test (P < .05; fold change ≥2). Patient characteristics are listed in supplemental Table 1 (FL patients 1-8). All tFLs were transformations (transf) to histologically verified DLBCL. All RNA samples in the analyses were isolated from FFPE tissue. (B) Validation of miR-150 levels in paired FL and tFL samples from 13 patients. The same 16 samples from panel A and additional 10 samples (5 pairs) were analyzed by individual quantitative reverse transcription polymerase chain reaction (qRT-PCR; tested by paired t test; patient characteristics listed in supplemental Table 1 [FL patients 1-13]). (C) miR-150 expression in patients who did not experience transformation during follow-up (FL non-transf) and those with FL before transformation (FL before transf; patient later transformed to DLBCL), tFL (at sampling), and primary DLBCL (tested by Mann-Whitney test). The median follow-up for patients in the nontransforming FL group was 131 months (range, 2-276 months), the median follow-up for those in the FL before transformation group was 95 months (range, 25-231 months), and the median follow-up for those in the tFL group was 98 months (range, 5-231 months). NS, not significant.
MiRNAs are differentially expressed in tFL. (A) miRNAs were quantified in paired FL-tFL samples from 8 patients (analyzed by TaqMan Array Human MicroRNA Cards; supplemental Methods). Expression of each miRNA was centered on the median miRNA expression in all samples and visualized in a heatmap (MeV software). Lower expression is indicated in blue, and higher expression in yellow (linear scale). The differences in miRNA expression between FL vs tFL were tested by paired t test (P < .05; fold change ≥2). Patient characteristics are listed in supplemental Table 1 (FL patients 1-8). All tFLs were transformations (transf) to histologically verified DLBCL. All RNA samples in the analyses were isolated from FFPE tissue. (B) Validation of miR-150 levels in paired FL and tFL samples from 13 patients. The same 16 samples from panel A and additional 10 samples (5 pairs) were analyzed by individual quantitative reverse transcription polymerase chain reaction (qRT-PCR; tested by paired t test; patient characteristics listed in supplemental Table 1 [FL patients 1-13]). (C) miR-150 expression in patients who did not experience transformation during follow-up (FL non-transf) and those with FL before transformation (FL before transf; patient later transformed to DLBCL), tFL (at sampling), and primary DLBCL (tested by Mann-Whitney test). The median follow-up for patients in the nontransforming FL group was 131 months (range, 2-276 months), the median follow-up for those in the FL before transformation group was 95 months (range, 25-231 months), and the median follow-up for those in the tFL group was 98 months (range, 5-231 months). NS, not significant.
We also analyzed miR-150 levels in FL patient samples that later developed transformation (n = 21) and those FL samples that did not develop transformation during follow-up (n = 45). This showed that there is no difference in miR-150 levels in cases that later develop transformation (Figure 1C), and miR-150 is only downmodulated after transformation to DLBCL. The miR-150 levels also did not significantly decrease with disease progression in FL (diagnosis vs relapse [transformation excluded]; supplemental Figure 4). Altogether these data demonstrate that miR-150 is consistently and specifically downmodulated in tFL when compared with FL.
High levels of MYC repress miR-150 expression in transformed FL
We further investigated the possible mechanisms for miR-150 downmodulation in tFL. MYC was previously reported to downregulate miR-150 in acute myeloid leukemia,45 and MYC mutations/translocations (∼40% of tFL cases) and overexpression are among the most frequent aberrations in tFL.7,10,17 We detected reduced miR-150 expression in splenic B cells from transgenic MYC-overexpressing mice (MYC transgene controlled by the immunoglobulin α heavy-chain enhancer) compared with wild-type mice (Figure 2A-B). Furthermore, the factors known to induce MYC and activate B cells such as CD40 and interleukin-4 signaling,46,47 also lead to downmodulation of miR-150 levels in malignant B cells (supplemental Figure 5A-B). Importantly, silencing MYC in B cells by siRNA led to upregulation of miR-150 expression in B cells (Figure 2C).
MYC represses miR-150 expression in tFL. (A-B) The differences in MYC messenger RNA (mRNA) and miR-150 levels in B cells from transgenic iMycCα mice (MYC transgene controlled by the immunoglobulin [Ig] α heavy-chain enhancer) compared with wild-type (WT) mice. The splenic B cells were purified from MYC hemizygous and control (ctrl) spleens of relatively young mice before the development of any malignancy, which was verified by flow cytometry together with the sample purity (>95% B cells postpurification; supplemental Methods). The differences between groups were tested by unpaired t test. (C) miR-150 expression in WSU-NHL B-cell line transfected with siRNA against MYC (siMYC) or siRNA negative control (siNeg. Ctrl) (n = 4). Statistical differences were tested by paired t test. (D) Intensity of MYC IHC staining in paired FL-tFL samples from all 14 patients with available material (patient characteristics listed in supplemental Table 1 [FL patients 5-10 and 12-19]). Percentages of FL-tFL samples are shown, which were scored as MYC− (0% to 1% positive cells), mostly MYC− (1% to 5% positive cells), MYC+ (10% to 40% positive cells), strongly MYC+ (40% to 70% positive cells), and very strongly MYC+ (>70% positive cells). The number in each segment of the column indicates the number of samples with the corresponding intensity of IHC staining. Each arrow indicates the change in the IHC staining category in each of the analyzed pairs (note that the angle of the line does not show the trend of increase/decrease in percentage of positive cells). Representative images of the scoring of MYC staining in FL are shown in panel E and in supplemental Figure 7. The statistical differences between 13 paired FL-tFL samples were tested by Wilcoxon matched-pairs test. For 1 patient, only the material from the tFL sample was available for IHC (included in the graph but not in the paired statistical analysis). (E) Representative examples of differences in IHC staining for MYC between paired samples of FL (upper) and tFL (lower) from 3 patients. Original magnification ×400. (F) The miR-150 expression levels in FL samples scored as MYC− (0% to 1% positive cells), mostly MYC− (1% to 5% positive cells), weakly MYC+ (5% to 10% positive cells), or MYC+ (10% to 40% positive cells) for MYC staining. P values were assessed by unpaired t test. Representative images of the scoring of MYC staining in FL are shown in supplemental Figure 7 (MYC staining performed as tissue microarray blocks; supplemental Methods). (G) Immunoblot analysis of LIN28 protein (LIN28A and LIN28B) in B-cell non-Hodgkin lymphoma (B-NHL) cell lines and primary samples. The membrane was overexposed while detecting immunocomplexes, and no band(s) appeared in any B-NHL samples. Protein lysates from human embryonic stem cells (hESCs) were used as a positive control for LIN28A/B. *Two bands identified by the anti-LIN28B antibody represent two isoforms of LIN28B protein. (H) ChIP analysis for MYC binding in the region upstream of the MIR150 transcription start site in WSU-NHL cells. DNA from immunoprecipitates was assayed by qPCR on the independent amplicons upstream of the MIR150 gene (−12.7 to −0.35 kb relative to the MIR150 transcription start site [TSS]; supplemental Figure 12). The results are represented as percentage of DNA input; the error bars indicate the standard error of the mean. Enrichment quantitation of immunoprecipitates with control IgG antibody was calculated to equal 1, and MYC IP was normalized accordingly. NS, not significant.
MYC represses miR-150 expression in tFL. (A-B) The differences in MYC messenger RNA (mRNA) and miR-150 levels in B cells from transgenic iMycCα mice (MYC transgene controlled by the immunoglobulin [Ig] α heavy-chain enhancer) compared with wild-type (WT) mice. The splenic B cells were purified from MYC hemizygous and control (ctrl) spleens of relatively young mice before the development of any malignancy, which was verified by flow cytometry together with the sample purity (>95% B cells postpurification; supplemental Methods). The differences between groups were tested by unpaired t test. (C) miR-150 expression in WSU-NHL B-cell line transfected with siRNA against MYC (siMYC) or siRNA negative control (siNeg. Ctrl) (n = 4). Statistical differences were tested by paired t test. (D) Intensity of MYC IHC staining in paired FL-tFL samples from all 14 patients with available material (patient characteristics listed in supplemental Table 1 [FL patients 5-10 and 12-19]). Percentages of FL-tFL samples are shown, which were scored as MYC− (0% to 1% positive cells), mostly MYC− (1% to 5% positive cells), MYC+ (10% to 40% positive cells), strongly MYC+ (40% to 70% positive cells), and very strongly MYC+ (>70% positive cells). The number in each segment of the column indicates the number of samples with the corresponding intensity of IHC staining. Each arrow indicates the change in the IHC staining category in each of the analyzed pairs (note that the angle of the line does not show the trend of increase/decrease in percentage of positive cells). Representative images of the scoring of MYC staining in FL are shown in panel E and in supplemental Figure 7. The statistical differences between 13 paired FL-tFL samples were tested by Wilcoxon matched-pairs test. For 1 patient, only the material from the tFL sample was available for IHC (included in the graph but not in the paired statistical analysis). (E) Representative examples of differences in IHC staining for MYC between paired samples of FL (upper) and tFL (lower) from 3 patients. Original magnification ×400. (F) The miR-150 expression levels in FL samples scored as MYC− (0% to 1% positive cells), mostly MYC− (1% to 5% positive cells), weakly MYC+ (5% to 10% positive cells), or MYC+ (10% to 40% positive cells) for MYC staining. P values were assessed by unpaired t test. Representative images of the scoring of MYC staining in FL are shown in supplemental Figure 7 (MYC staining performed as tissue microarray blocks; supplemental Methods). (G) Immunoblot analysis of LIN28 protein (LIN28A and LIN28B) in B-cell non-Hodgkin lymphoma (B-NHL) cell lines and primary samples. The membrane was overexposed while detecting immunocomplexes, and no band(s) appeared in any B-NHL samples. Protein lysates from human embryonic stem cells (hESCs) were used as a positive control for LIN28A/B. *Two bands identified by the anti-LIN28B antibody represent two isoforms of LIN28B protein. (H) ChIP analysis for MYC binding in the region upstream of the MIR150 transcription start site in WSU-NHL cells. DNA from immunoprecipitates was assayed by qPCR on the independent amplicons upstream of the MIR150 gene (−12.7 to −0.35 kb relative to the MIR150 transcription start site [TSS]; supplemental Figure 12). The results are represented as percentage of DNA input; the error bars indicate the standard error of the mean. Enrichment quantitation of immunoprecipitates with control IgG antibody was calculated to equal 1, and MYC IP was normalized accordingly. NS, not significant.
We next assessed MYC protein levels by IHC in paired FL-tFL samples from 14 patients for whom material was available. tFL samples were in great majority strongly positive for MYC staining in a majority of B cells, and all had lower miR-150 levels compared with FL samples, which were mostly weakly positive for MYC (≤5% MYC+ cells); (Figures 1 and 2D-E; supplemental Figure 6A-B). Study of additional 30 FL samples using tissue microarrays showed that the MYC+ (>5% positive cells) FL cases, although rare (n = 7), had significantly lower miR-150 levels (P < .05; Figure 2F). Similarly, IHC staining of de novo DLBCL showed significantly lower miR-150 expression in highly MYC+ cases in comparison with MYC− cases (supplemental Figure 8). Additionally, the study of a cohort of rare samples of CLL cells with MYC aberration (n = 29) also showed significantly lower miR-150 levels compared with CLL samples that did not harbor MYC aberration (supplemental Figure 5C). These data suggest that miR-150 is transcriptionally repressed by MYC and that this could be an important feature of high-grade transformation of FL.
miR-150 is directly repressed by MYC and its maturation is not regulated by LIN28A/B proteins
It has previously been shown in AML cells that MYC upregulates LIN28, which then inhibits the maturation of miR-150 from its precursor.45 However, the regulation of miR-150 expression could be cell type and context specific. Therefore, we analyzed the expression of LIN28 family members (LIN28A and LIN28B) in FL/tFL. The LIN28B mRNA was undetectable by qRT-PCR in most FL specimens (94% of cases had cycle threshold [Ct] value >40), and LIN28A mRNA had median Ct value of 38.0 and was not reliably detectable in most FL specimens. There was no anticorrelation of LIN28A mRNA with miR-150 levels (supplemental Figure 9). Immunoblot analysis of LIN28A/B protein in B-cell lymphoma cell lines and primary FL and CLL samples revealed the absence of this protein in B cells (Figure 2G). The expression of primary miR-150 transcript (pri-miR-150) in FL and DLBCL strongly correlated with mature miR-150 levels (supplemental Figure 10). pri-miR-150 was expressed at relatively low levels (median Ct value, 32.7) when compared with mature miR-150 (median Ct value, 21.7), suggesting that miR-150 is efficiently processed from its precursor (pri-miR-150) in FL B cells. We also did not find any association between the miR-150 levels in B cells and functionality of p53 protein (supplemental Figure 11), which is frequently affected by aberrations in tFL. We hypothesized that MYC regulates miR-150 expression by binding directly to its upstream regions. Indeed, our chromatin immunoprecipitation data for MYC have shown that MYC directly binds in the region upstream of MIR150 transcription start site in malignant B cells (Figure 2H; supplemental Figure 12).
Low levels of miR-150 lead to high levels of FOXP1 transcription factor
We have previously shown that miR-150 regulates the levels of FOXP1 transcription factor in CLL cells.22 Similarly to CLL cells, the transfection of the non-Hodgkin lymphoma B-cell line WSU-NHL with synthetic miR-150 reduced the FOXP1 levels by ∼33% (P < .05; Figure 3A). A luciferase assay with cloned 3′UTR region of FOXP1 gene containing the putative miR-150 binding site confirmed a direct physical binding of miR-150 to FOXP1, and this was interrupted by introducing mutations in the region encoding the miR-150 binding side (Figure 3B). This suggests that FOXP1 is a relevant target for miR-150 in FL/tFL.
miR-150 regulates FOXP1 levels in FL/tFL. (Ai) Statistical analysis of the effect of synthetic miR-150 on FOXP1 levels in WSU-NHL cells (48 hours; n = 3). WSU-NHL cells were transfected with siRNA against FOXP1 (siFOXP1) or siRNA negative control (siNeg. Ctrl) or miR-150 mimic (miR-150 MIMIC) or miRNA negative control (miR Neg. Ctrl) and harvested after 48 hours. The siRNA against FOXP1 serves as a positive control. The blot images were quantified with UVItec Alliance 4.7, and the FOXP1/β-actin ratio in the first sample (siNeg. Ctrl) was arbitrarily set at 1. The differences were tested by paired t test. (Aii) Representative immunoblot of FOXP1 levels in WSU-NHL cells transfected with siRNA against FOXP1 or miR-150 mimic as described in panel Ai. *Two bands identified by the anti-FOXP1 antibody represent two isoforms of FOXP1 protein. (B) The luciferase activity in HEK293T cells cotransfected with miR-150 mimic (400 nM) and luciferase reporter construct (15 ng), containing the 3′UTR region of wild-type or mutated FOXP1 (supplemental Methods). As a negative control, we used a control miRNA mimic (miR Neg. Ctrl; 400 nM). Luciferase activity was measured 24 hours posttransfection and compared by paired t test. (C) Statistical analysis of FOXP1 staining in paired FL-tFL samples from all 14 patients with available material (patient characteristics listed in supplemental Table 1 [FL patients 5-10 and 12-19]). Percentages of samples with low (≤30%), intermediate (inter; 30%-70%), and high (≥70%) percentages of FOXP1+ cells are shown. The number in each segment of the column indicates the number of samples with the corresponding intensity of IHC staining. Each arrow indicates the change in the IHC staining category in each of the analyzed pairs (note that the angle of the line does not show the trend of increase/decrease in percentage of positive cells). The statistical differences were tested by Wilcoxon matched-pairs test. Representative images of the scoring of FOXP1 staining in FL are shown in panel D and in supplemental Figure 14. (D) Representative examples of FOXP1 IHC staining between paired samples of FL (upper) and tFL (lower). Original magnification ×400. (E) Differences in miR-150 expression levels between FL samples with low (≤30%), intermediate (30%-70%), and high (≥70%) percentages of FOXP1+ cells from 30 FL patients with available material (FOXP1 staining performed as tissue microarray blocks; supplemental Methods). P values were assessed by unpaired t test. Representative images of the scoring of FOXP1 staining in FL are shown in supplemental Figure 14. (F) Cell-cycle analysis of WSU-NHL cells transfected with synthetic miR-150 mimic or siRNA against FOXP1 or negative controls for miRNA or siRNA. Cells were harvested after 24 hours (n = 10 independent experiments) and analyzed for cell cycle (supplemental Methods; supplemental Figure 16). P values were assessed by paired t test. (G) The Ki67 proliferation index in FL samples with low (≤30%), intermediate (30%-70%), or high (≥70%) percentage of FOXP1+ cells from 27 FL patients with available material (FOXP1 staining performed as tissue microarray blocks; supplemental Methods). P values were assessed by unpaired t test. NS, not significant.
miR-150 regulates FOXP1 levels in FL/tFL. (Ai) Statistical analysis of the effect of synthetic miR-150 on FOXP1 levels in WSU-NHL cells (48 hours; n = 3). WSU-NHL cells were transfected with siRNA against FOXP1 (siFOXP1) or siRNA negative control (siNeg. Ctrl) or miR-150 mimic (miR-150 MIMIC) or miRNA negative control (miR Neg. Ctrl) and harvested after 48 hours. The siRNA against FOXP1 serves as a positive control. The blot images were quantified with UVItec Alliance 4.7, and the FOXP1/β-actin ratio in the first sample (siNeg. Ctrl) was arbitrarily set at 1. The differences were tested by paired t test. (Aii) Representative immunoblot of FOXP1 levels in WSU-NHL cells transfected with siRNA against FOXP1 or miR-150 mimic as described in panel Ai. *Two bands identified by the anti-FOXP1 antibody represent two isoforms of FOXP1 protein. (B) The luciferase activity in HEK293T cells cotransfected with miR-150 mimic (400 nM) and luciferase reporter construct (15 ng), containing the 3′UTR region of wild-type or mutated FOXP1 (supplemental Methods). As a negative control, we used a control miRNA mimic (miR Neg. Ctrl; 400 nM). Luciferase activity was measured 24 hours posttransfection and compared by paired t test. (C) Statistical analysis of FOXP1 staining in paired FL-tFL samples from all 14 patients with available material (patient characteristics listed in supplemental Table 1 [FL patients 5-10 and 12-19]). Percentages of samples with low (≤30%), intermediate (inter; 30%-70%), and high (≥70%) percentages of FOXP1+ cells are shown. The number in each segment of the column indicates the number of samples with the corresponding intensity of IHC staining. Each arrow indicates the change in the IHC staining category in each of the analyzed pairs (note that the angle of the line does not show the trend of increase/decrease in percentage of positive cells). The statistical differences were tested by Wilcoxon matched-pairs test. Representative images of the scoring of FOXP1 staining in FL are shown in panel D and in supplemental Figure 14. (D) Representative examples of FOXP1 IHC staining between paired samples of FL (upper) and tFL (lower). Original magnification ×400. (E) Differences in miR-150 expression levels between FL samples with low (≤30%), intermediate (30%-70%), and high (≥70%) percentages of FOXP1+ cells from 30 FL patients with available material (FOXP1 staining performed as tissue microarray blocks; supplemental Methods). P values were assessed by unpaired t test. Representative images of the scoring of FOXP1 staining in FL are shown in supplemental Figure 14. (F) Cell-cycle analysis of WSU-NHL cells transfected with synthetic miR-150 mimic or siRNA against FOXP1 or negative controls for miRNA or siRNA. Cells were harvested after 24 hours (n = 10 independent experiments) and analyzed for cell cycle (supplemental Methods; supplemental Figure 16). P values were assessed by paired t test. (G) The Ki67 proliferation index in FL samples with low (≤30%), intermediate (30%-70%), or high (≥70%) percentage of FOXP1+ cells from 27 FL patients with available material (FOXP1 staining performed as tissue microarray blocks; supplemental Methods). P values were assessed by unpaired t test. NS, not significant.
We further examined FOXP1 protein levels by IHC in FL and tFL. The immunostaining of these paired FL-tFL samples showed significantly stronger FOXP1 staining in tFL (P < .05; Figure 3C-D; supplemental Figure 6C). We also assessed the protein levels of FOXP1 in the other 30 FL samples (in a tissue microarray format). This revealed significantly lower miR-150 levels in FL samples with high FOXP1 positivity (Figure 3E). These data suggest that miR-150 contributes to the regulation of FOXP1 levels in both FL and tFL.
Impact of miR-150 on the cell cycle
We and others have previously shown that FOXP1 can directly influence normal and malignant B-cell BCR signaling,22 positively regulate NF-κB signaling activity,33 and bind ∼300 gene promotors in malignant B cells.48 These data suggest that the miR-150/FOXP1 axis might influence malignant B-cell behavior on multiple levels. Additionally, several publications have reported the role of FOXP1 in the proliferation and survival of activated B-cell (ABC) DLBCL B cells.33 To examine this, we transfected B-cell line WSU-NHL with artificial miR-150 or siRNA against its target FOXP1 (Figure 3A). Transfection with miR-150 or siRNA against FOXP1 did not have any direct impact on cell viability (supplemental Figure 15). However, the miR-150 transfection resulted in a significantly increased percentage of cells in the G1 phase and a reduced percentage of cells in the S phase, which corresponded to reduced cell proliferation (Figure 3F; supplemental Figure 16). However, we did not observe changes in cell cycle or viability upon transfecting B cells with siRNA for FOXP1 (Figure 3F; supplemental Figures 15 and 16). This suggests that miR-150 might regulate other targets besides FOXP1 that are influencing the cell cycle independently or together with FOXP1. This is also in line with other reports showing that FOXP1 affects cell proliferation and survival in ABC DLBCL, but not GC DLBCL, cell lines such as WSU-NHL used in our study.29,30,33 The effect of FOXP1 on cell proliferation is likely to depend on the specific context of other signaling pathways in individual patients with FL. Importantly, primary FL samples with high FOXP1 levels have a higher percentage of Ki67+ B cells (Figure 3G), suggesting FOXP1 relevance in proliferation in vivo.
Prognostic significance of miR-150 and FOXP1 in FL
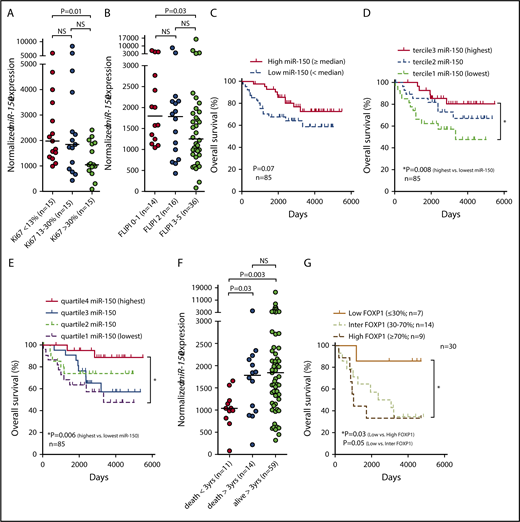
Altogether our data suggest that miR-150 might play a role not only in tFL but also in the behavior of FL B cells. Indeed, the expression level of miR-150 is associated with characteristics related to malignant B-cell biology and the outcome of patients with FL. miR-150 expression was reduced in FL samples with high Ki67 positivity (>30%; P = .01; Figure 4A) and in patients with high FLIPI score (3-5; P = .03; Figure 4B). To assess the potential prognostic impact of miR-150 levels in FL, we analyzed its expression in a cohort of FFPE samples and freshly frozen FL samples that were available (to increase the size of the cohort). The expression levels for miR-150 obtained by qRT-PCR from both sample types (ie, FFPE and freshly frozen tissues were highly concordant, which allowed us to include both sample types in the analyses; r = 0.82; P = .006; n = 10 pairs of FFPE and freshly frozen samples from the same biopsy; data not shown). The patients with FL with relatively low miR-150 levels (divided by median) had a trend toward a shorter OS than those with relatively high miR-150 levels (P = .07; n = 85; Figure 4C). The difference in OS was more pronounced and statistically significant when we compared patients with the lowest and the highest miR-150 levels based on the terciles (median survival, 9.1 years vs not reached; P = .008; hazard ratio [HR], 3.5; confidence interval [CI], 1.3-9.2; Figure 4D) or quartiles of its expression (median survival, 9.1 years vs not reached; P = .006; HR, 4.9; CI, 1.6-15.4; Figure 4E). To determine whether miR-150 is an independent predictor of OS, we performed a multivariate analysis, which included several known prognostic markers (age, sex, FLIPI, hemoglobin levels, lactate dehydrogenase levels, number of lymph node areas involved, Ann Arbor stage, presence of B symptoms, and miR-150 levels). This showed that miR-150 levels retained independent predictive power for OS (supplemental Table 4; P = .02). The prognostic value of low-level miR-150 expression was also retained (P < .05) when the patients who experienced transformation during follow-up (n = 8; 9.4%) were excluded from the OS analysis. Interestingly, miR-150 was also significantly less expressed in patients who experienced early death within 3 years from diagnosis or biopsy (transformation to tFL excluded), and similar data were obtained for occurrence of early relapse (<24 months), which suggests a potential to identify the ∼20% of patients with FL with the most aggressive disease49 (Figure 4F; supplemental Figure 17). This also supports the overall biological relevance of miR-150 for the behavior of malignant FL cells. However, this will require further analysis in a cohort of patients treated homogeneously and prospectively. It has previously been shown that high-level expression of miR-155 is associated with ABC DLBCL50,-52 and shorter survival in CLL53 ; however, we did not find any association of miR-155 levels with OS or high-grade transformation of FL (supplemental Figure 18).
The association of miR-150 expression with biological characteristics of FL and survival of patients. (A) miR-150 expression in FL patients divided according to terciles of Ki67 proliferation index (the differences were tested by Mann-Whitney test). All samples with available data on Ki67 were used in the analysis. (B) miR-150 expression in FL patients divided according to Follicular Lymphoma International Prognostic Index (FLIPI) score (the differences were tested by Mann-Whitney test). All samples with available data on FLIPI were used in the analysis. (C-E) Association of miR-150 expression with OS in FL. The OS is depicted using the Kaplan-Meier curves (with log-rank test) in the FL cohort divided by median miR-150 expression (C) or by dividing the cohort into 3 (D) or 4 (E) groups based on terciles or quartiles of miR-150 expression, respectively. For all analyses, only patients with histologically verified FL at the time of sampling (with no histopathological signs of transformation to DLBCL) were included. Patient characteristics are listed in supplemental Table 2. (F) Differences in miR-150 levels between FL patients who experienced early death within 3 years from biopsy (death <3 years) or who died later (death >3 years) or are still alive for longer than 3 years (alive >3 years) from biopsy. One patient with a follow-up time <3 years was excluded from the analysis. Similar data were obtained when only samples obtained at diagnosis were included in the analysis (54 of 84 samples; supplemental Figure 17A) or in patients experiencing early relapse (<24 months; supplemental Figure 17B). For all analyses, only patients with histologically verified FL at the time of sampling (with no histopathological signs of transformation to DLBCL) were included. The differences in miR-150 levels between individual groups were tested by Mann-Whitney test. (G) The OS in FL patients divided according to immunostaining for FOXP1 (30 FL patients with material available as tissue microarray blocks; supplemental Methods). The patients were divided into 3 groups of samples with low (≤30%), intermediate (inter; 30%-70%), or high (≥70%) percentage of FOXP1+ cells. For all analyses, only patients with histologically verified FL at the time of sampling (with no histopathological signs of transformation to DLBCL) were included. Representative images of the scoring of FOXP1 staining in FL are shown in supplemental Figure 14. NS, not significant.
The association of miR-150 expression with biological characteristics of FL and survival of patients. (A) miR-150 expression in FL patients divided according to terciles of Ki67 proliferation index (the differences were tested by Mann-Whitney test). All samples with available data on Ki67 were used in the analysis. (B) miR-150 expression in FL patients divided according to Follicular Lymphoma International Prognostic Index (FLIPI) score (the differences were tested by Mann-Whitney test). All samples with available data on FLIPI were used in the analysis. (C-E) Association of miR-150 expression with OS in FL. The OS is depicted using the Kaplan-Meier curves (with log-rank test) in the FL cohort divided by median miR-150 expression (C) or by dividing the cohort into 3 (D) or 4 (E) groups based on terciles or quartiles of miR-150 expression, respectively. For all analyses, only patients with histologically verified FL at the time of sampling (with no histopathological signs of transformation to DLBCL) were included. Patient characteristics are listed in supplemental Table 2. (F) Differences in miR-150 levels between FL patients who experienced early death within 3 years from biopsy (death <3 years) or who died later (death >3 years) or are still alive for longer than 3 years (alive >3 years) from biopsy. One patient with a follow-up time <3 years was excluded from the analysis. Similar data were obtained when only samples obtained at diagnosis were included in the analysis (54 of 84 samples; supplemental Figure 17A) or in patients experiencing early relapse (<24 months; supplemental Figure 17B). For all analyses, only patients with histologically verified FL at the time of sampling (with no histopathological signs of transformation to DLBCL) were included. The differences in miR-150 levels between individual groups were tested by Mann-Whitney test. (G) The OS in FL patients divided according to immunostaining for FOXP1 (30 FL patients with material available as tissue microarray blocks; supplemental Methods). The patients were divided into 3 groups of samples with low (≤30%), intermediate (inter; 30%-70%), or high (≥70%) percentage of FOXP1+ cells. For all analyses, only patients with histologically verified FL at the time of sampling (with no histopathological signs of transformation to DLBCL) were included. Representative images of the scoring of FOXP1 staining in FL are shown in supplemental Figure 14. NS, not significant.
In univariate analyses, high-level FOXP1 expression was associated with a significantly shorter OS in FL (median survival, 33 months vs not reached; P = .03; HR, 5.0; CI, 1.1-22.6; Figure 4G). Altogether, the adverse prognostic impact of low-level miR-150 expression and the reverse trend for FOXP1 provide support for the relevance of these genes to the biology of neoplastic FL and tFL cells.
Discussion
We have shown that the process of high-grade transformation of indolent FL to a more aggressive lymphoma is associated with a uniform downregulation of miRNA miR-150. We have demonstrated that an increase in MYC levels represses miR-150 levels in tFL. Downmodulating miR-150 leads to the upregulation of FOXP1 transcription factor, which is a direct target of miR-150 and an important regulator of B-cell activation. Additionally, some FL cases have relatively low miR-150 levels and therefore higher levels of the FOXP1, which is strongly associated with shorter OS in FL.
The histological transformation of FL to a high-grade lymphoma, usually DLBCL, is associated with clinically aggressive behavior and poor patient prognosis.1,,-4 Recently, several genetic lesions acquired during the transformation process were identified, such as MYC aberrations and CDKN2A/B or TP53 loss,6,7,15 and high AID expression was reported to increase the risk of tFL.54 However, the role of miRNAs in this process is largely unknown. Here we report the first complex profiling of miRNA expression in paired samples of FL and tFL. A recent profiling of selected miRNAs in FL-tFL (only 2 pairs of samples) suggested that 2 miRNAs (miR-17-5p and miR-31) change upon tFL.55 We have identified miRNAs miR-150, miR-31, miR-106b, miR-222, and miR-517b as differentially expressed in tFL. This partially validates and extends the previous findings, because we also observed downmodulation of miR-31 and upregulation of miR-106b, which is part of the cluster homologous to miR-17-92 on chromosome 7. It has previously been described that miRNA expression profiles in tFL (DLBCL) and primary DLBCL are not identical,51 but miR-150 had a similar expression pattern in both entities when compared with FL (Figure 1C). Notably, none of the significantly changed miRNAs are located in regions with known genomic aberrations in FL/tFL.15
We focused on studying miR-150 because it was consistently downmodulated in every tFL sample examined (fold change, ∼3.5; Figure 1B). We have previously described low miR-150 levels in aggressive CLL,22 and low levels of miR-150 have been described in aggressive non-Hodgkin lymphomas.56,-58 Here we show that MYC protein represses miR-150 expression in tFL, which is compatible with the observed gain of MYC-activating aberrations and expression in tFL.7,10,17 In our experiments, MYC silencing by siRNA increased miR-150 levels, and miR-150 levels were low in B cells from transgenic MYC-overexpressing mice. Additionally, CLL samples with activating MYC aberrations had significantly lower miR-150 levels. MYC is known to directly suppress the expression of large number of miRNAs59 and activate expression of the miR-17-92 cluster (and its homologs miR-106b-25/miR-106a-92).60 In accordance with previous findings,15,61 most of our FL samples had weak MYC expression as assessed by immunostaining. In contrast, paired tFL samples were strongly MYC+ compared with the pretransformation samples that were MYC− or showed only weak staining in a small number of cells. However, we did observe higher MYC levels (>5% positive cells) with lower miR-150 expression in a few FL samples from patients who did not have tFL.
It has recently been shown that MYC upregulates LIN28 expression in myeloid cells, which inhibits miR-150 maturation from its precursor pri-miR-150.45 We have shown that LIN28A/B is not expressed in a panel of B-cell lines and FL samples. Additionally, pri-miR-150 was effectively processed in B cells. These data suggest that the decrease in miR-150 levels in tFL and some cases of FL is mediated by MYC through a mechanism that does not involve LIN28. This conclusion is supported by the results of the MYC–chromatin immunoprecipitation experiments identifying MYC binding sites in the upstream region of miR-150 gene in B cells.
We further investigated the effect of low miR-150 levels on the expression of its direct target in B cells, namely FOXP1.22 We demonstrated that FOXP1 is upregulated with the transformation of FL to tFL, and its levels are regulated by miR-150 in this context and in FL. FOXP1 has been extensively studied in lymphoid malignancies, because its high levels are known to be associated with worse prognosis in DLBCL and MALT lymphoma,34,35,37 and FOXP1-staining is used in IHC algorithms (eg, Choi, Tally, and Visco-Young) that distinguish the non-GC vs GC DLBCL subtypes.29,38,,,,-43 FOXP1 belongs to a family of forkhead box transcription factors and has been shown to be required for early B-cell development and GC reaction.48,62 In DLBCL cells, FOXP1 acts as a key transcription factor that regulates expression of a large number of genes, including those involved in cell survival, lymphocyte activation, chemotaxis, cell cycle, and plasmacytic differentiation.29,30,33,48,63,64 We and others have shown that FOXP1 positively affects BCR signaling and NF-κB pathway and Wnt signaling in malignant B cells22,33,65 and contributes to immune escape in DLBCL by suppressing MHCII expression.63 Altogether, it has been shown that FOXP1 contributes to lymphomagenesis in vivo by affecting a number of proproliferative and prosurvival genes and in ABC-DLBCL by disrupting terminal B-cell differentiation.29 However, in vitro in DLBCL cell lines, FOXP1 silencing can have an effect on cell cycle and survival, or only on one or none of these cell characteristics.30,,-33,66 This is likely to depend on the context of other factors determining certain aspects of FOXP1 functionality (extensively discussed by Gascoyne and Banham29 ), which could also explain why FOXP1 alone was insufficient to cause lymphomagenesis in mice.48 In WSU-NHL cells, transfection with miR-150 resulted in significant reduction of S phase, but this was not observed upon silencing FOXP1, suggesting that miR-150 regulates other targets that are influencing the cell cycle, alone or together with FOXP1. However, this does not exclude the possibility that FOXP1 is involved in proliferation of primary FL cells in vivo, and its known role in the activity of NF-κB and BCR signaling suggests that it is plausible that it has some role in B-cell proliferation. Importantly, we observed a significantly higher percentage of Ki67+ cells in FL samples with high FOXP1 protein levels, which is in accordance with other studies.30,33,67
Recently, FOXP1 upregulation was associated with a process of high-grade transformation of MALT lymphoma to aggressive gastric DLBCL.66 In their report, the authors describe that downmodulation of miR-34a in gastric DLBCL leads to upregulation of FOXP1, because it is a target of miR-34a.66,68 We tested the expression of miR-34a and another FOXP1-targeting miRNA, namely miR-181a,69 in paired FL and tFL, but these miRNAs are clearly not downmodulated in most tFLs (supplemental Figure 1B-C). We also tested the expression of another known miR-150 target, namely MYB, regulation of which by miR-150 is critical in immature B cells.25 MYB expression was not influenced by miR-150 levels in our previous study in CLL,22 and we found no inverse correlation between MYB and miR-150 levels in FL/tFL (data not shown). This indicates that FOXP1 is likely to be a more relevant miR-150 target in FL/tFL B cells. We propose that MYC-induced repression of miR-150 results in upregulation of FOXP1 in malignant B cells in tFL, contributing to transformation to DLBCL, as summarized in Figure 5. Altogether, our data suggest that MYC acts to activate oncogenic miRNAs (eg, miR-17-92 family) and inhibits expression of tumor suppressor miR-150 during tFL. Previous reports have also suggested a relevance of miR-142-3p and miR-142-5p in the FL/DLBCL biology, because their mutations have been described in DLBCL70 and tFL.16 However, we detected no mutations in mature sequences of these miRNAs or miR-150 in the 24 analyzed paired FL-tFL samples (supplemental Figure 19).
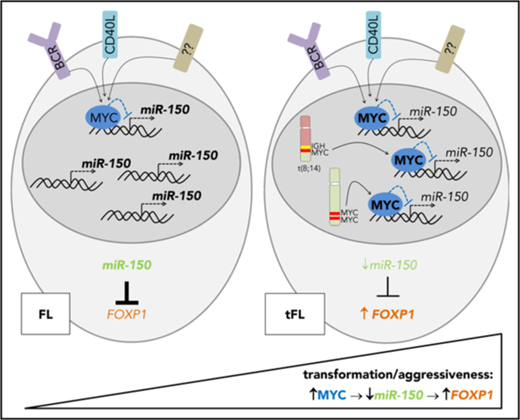
A schematic overview of the MYC/miR-150/FOXP1 role in the aggressiveness and high-grade transformation of FL. MYC overexpression caused by genomic aberrations (eg, translocations, mutations, or duplications) or B-cell activation downregulates miR-150 expression during the transformation of FL to DLBCL or in aggressive FL. Low levels of miR-150 lead to the upregulation of its target, FOXP1.
A schematic overview of the MYC/miR-150/FOXP1 role in the aggressiveness and high-grade transformation of FL. MYC overexpression caused by genomic aberrations (eg, translocations, mutations, or duplications) or B-cell activation downregulates miR-150 expression during the transformation of FL to DLBCL or in aggressive FL. Low levels of miR-150 lead to the upregulation of its target, FOXP1.
miR-150 expression could also determine the behavior of FL B cells in patients who do not undergo transformation. miR-150 expression was reduced in FL samples with high Ki67 positivity (>30%) and in patients with high FLIPI score (3-5), and furthermore, patients with FL with lower miR-150 levels had a shorter OS (median survival, 9.1 years vs not reached; P = .008; HR, 3.5; CI, 1.3-9.2]). miR-150 levels retained independent predictive power for OS (HR, 2.8; P = .02) in a multivariate analysis, which included several known prognostic markers (age, sex, FLIPI, hemoglobin levels, lactate dehydrogenase levels, number of lymph node areas involved, Ann Arbor clinical stage, presence of B symptoms, and miR-150 levels). miR-150 was also significantly lower in patients dying within 3 years from biopsy or diagnosis (FL transformation excluded) or those with early relapse after therapy. This finding suggests that measurement of miR-150 levels could be useful in identifying patients with FL with unfavorable prognosis as defined by progression within 24 months after therapy49 (supplemental Figure 17), and this should be studied prospectively in a clinical trial. The possibility to use FFPE samples to quantify miR-150 levels suggests its use as a relatively simple prognostic biomarker based on analyzing FFPE material available from routine biopsy evaluation. The circulating levels of extracellular miR-150 in serum or plasma might potentially also serve as a biomarker; however, previous findings in CLL suggest that the serum/plasma miR-150 levels reflect tumor burden and lymphocyte count rather than the biology of malignant B cells.71,72 Univariate analysis showed that high protein expression of FOXP1 as determined by IHC was associated with a significantly shorter OS in FL (median survival, 33 months vs not reached; P = .03; HR, 5.0; CI, 1.1-22.6). During preparation of this report, similar results demonstrating the association of higher FOXP1 levels with shorter failure-free survival in FL were reported.67 The adverse prognostic implications of low-level miR-150 expression and increased expression of FOXP1 support an important role in progression of tFL and FL. However, it is possible that miR-150 could also influence FL biology by regulating translation of other genes besides FOXP1 that contribute to malignant B-cell biology and their interplay with genetic aberrations in individual FL cases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
M.M. thanks Thomas J. Kipps (University of California San Diego) for inspiring discussions, Dasa Bohaciakova (Masaryk University [MU]) for protein lysates from human embryonic stem cells, and Ales Hampl (MU) for access to microtome instrumentation.
This work was supported by the Ministry of Health of the Czech Republic, grant no. NV18-03-00054. All rights reserved. Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601); MH CZ - DRO (FNBr, 65269705); the Czech Science Foundation (project no. 16-13334Y); the Ministry of Health of the Czech Republic, grant no. 16-29622A. All rights reserved; institutional support no. 00023736 from the Czech Ministry of Health.
Authorship
Contribution: K.M. performed experiments, analyzed data, and wrote the paper; J.D., K.C., V.S., G.P., and S.S. performed experiments; J.O. analyzed data; L.Z. and K.L. performed immunostaining; L. Kruzova performed cytogenetics; A.M. performed immunoglobulin rearrangement analyses; P.B. and K.M.P. performed chromatin immunoprecipitation; M. Trbusek performed the experiments with L cells; R.P., V.P., Z.P., M.J., C.K., I.S.-K., A.-I.S., O.M., H.M., L. Kren, J.M., C.S.Z., M. Trneny, A.G.E., and A.J. provided samples and clinical data and interpreted data; M.M. designed the study, interpreted data, and wrote the paper; and all authors edited and approved the paper for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.J. is University Hospital Brno, Brno, Czech Republic.
The current affiliation for Z.P. is Motol University Hospital, Prague, Czech Republic.
Correspondence: Marek Mraz, Central European Institute of Technology, Masaryk University, Kamenice 5, 625 00 Brno, Czech Republic; e-mail: marek.mraz@email.cz.

![Figure 1. MiRNAs are differentially expressed in tFL. (A) miRNAs were quantified in paired FL-tFL samples from 8 patients (analyzed by TaqMan Array Human MicroRNA Cards; supplemental Methods). Expression of each miRNA was centered on the median miRNA expression in all samples and visualized in a heatmap (MeV software). Lower expression is indicated in blue, and higher expression in yellow (linear scale). The differences in miRNA expression between FL vs tFL were tested by paired t test (P < .05; fold change ≥2). Patient characteristics are listed in supplemental Table 1 (FL patients 1-8). All tFLs were transformations (transf) to histologically verified DLBCL. All RNA samples in the analyses were isolated from FFPE tissue. (B) Validation of miR-150 levels in paired FL and tFL samples from 13 patients. The same 16 samples from panel A and additional 10 samples (5 pairs) were analyzed by individual quantitative reverse transcription polymerase chain reaction (qRT-PCR; tested by paired t test; patient characteristics listed in supplemental Table 1 [FL patients 1-13]). (C) miR-150 expression in patients who did not experience transformation during follow-up (FL non-transf) and those with FL before transformation (FL before transf; patient later transformed to DLBCL), tFL (at sampling), and primary DLBCL (tested by Mann-Whitney test). The median follow-up for patients in the nontransforming FL group was 131 months (range, 2-276 months), the median follow-up for those in the FL before transformation group was 95 months (range, 25-231 months), and the median follow-up for those in the tFL group was 98 months (range, 5-231 months). NS, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/22/10.1182_blood-2018-06-855502/5/m_blood855502f1.png?Expires=1769091573&Signature=la0CYbmpP~VzKSq3rC6qZm1zIuKdxb3oeMmXPRjS5oYMbXNs8yWvgmXcCwiOojUj~R5Ak2S38bi3FVerENubmqxCG9ykabPy6XQix5ulz9cm1yJwlM9qi3HbhF1LZ19gWZt3xL5xOZ7NHJyYrYgSKC3MSHnpVpoXd61O0h2UWwlC8m1svfXtHpzgeFG~LvqLIKPjcrsiGpvR33w7r-Im9-YQoMpjrytPRHPOKw8pIE1fdf7M78COXNCFWB9gfEbTMWE~yy0puWfu9QfOEgFTBF8fp~5RJeCTEjnIZD5aryMSYEM-zqigU4p7Rfvda5Xg3C4cendlcAwFjGYMlnOBQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. MYC represses miR-150 expression in tFL. (A-B) The differences in MYC messenger RNA (mRNA) and miR-150 levels in B cells from transgenic iMycCα mice (MYC transgene controlled by the immunoglobulin [Ig] α heavy-chain enhancer) compared with wild-type (WT) mice. The splenic B cells were purified from MYC hemizygous and control (ctrl) spleens of relatively young mice before the development of any malignancy, which was verified by flow cytometry together with the sample purity (>95% B cells postpurification; supplemental Methods). The differences between groups were tested by unpaired t test. (C) miR-150 expression in WSU-NHL B-cell line transfected with siRNA against MYC (siMYC) or siRNA negative control (siNeg. Ctrl) (n = 4). Statistical differences were tested by paired t test. (D) Intensity of MYC IHC staining in paired FL-tFL samples from all 14 patients with available material (patient characteristics listed in supplemental Table 1 [FL patients 5-10 and 12-19]). Percentages of FL-tFL samples are shown, which were scored as MYC− (0% to 1% positive cells), mostly MYC− (1% to 5% positive cells), MYC+ (10% to 40% positive cells), strongly MYC+ (40% to 70% positive cells), and very strongly MYC+ (>70% positive cells). The number in each segment of the column indicates the number of samples with the corresponding intensity of IHC staining. Each arrow indicates the change in the IHC staining category in each of the analyzed pairs (note that the angle of the line does not show the trend of increase/decrease in percentage of positive cells). Representative images of the scoring of MYC staining in FL are shown in panel E and in supplemental Figure 7. The statistical differences between 13 paired FL-tFL samples were tested by Wilcoxon matched-pairs test. For 1 patient, only the material from the tFL sample was available for IHC (included in the graph but not in the paired statistical analysis). (E) Representative examples of differences in IHC staining for MYC between paired samples of FL (upper) and tFL (lower) from 3 patients. Original magnification ×400. (F) The miR-150 expression levels in FL samples scored as MYC− (0% to 1% positive cells), mostly MYC− (1% to 5% positive cells), weakly MYC+ (5% to 10% positive cells), or MYC+ (10% to 40% positive cells) for MYC staining. P values were assessed by unpaired t test. Representative images of the scoring of MYC staining in FL are shown in supplemental Figure 7 (MYC staining performed as tissue microarray blocks; supplemental Methods). (G) Immunoblot analysis of LIN28 protein (LIN28A and LIN28B) in B-cell non-Hodgkin lymphoma (B-NHL) cell lines and primary samples. The membrane was overexposed while detecting immunocomplexes, and no band(s) appeared in any B-NHL samples. Protein lysates from human embryonic stem cells (hESCs) were used as a positive control for LIN28A/B. *Two bands identified by the anti-LIN28B antibody represent two isoforms of LIN28B protein. (H) ChIP analysis for MYC binding in the region upstream of the MIR150 transcription start site in WSU-NHL cells. DNA from immunoprecipitates was assayed by qPCR on the independent amplicons upstream of the MIR150 gene (−12.7 to −0.35 kb relative to the MIR150 transcription start site [TSS]; supplemental Figure 12). The results are represented as percentage of DNA input; the error bars indicate the standard error of the mean. Enrichment quantitation of immunoprecipitates with control IgG antibody was calculated to equal 1, and MYC IP was normalized accordingly. NS, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/22/10.1182_blood-2018-06-855502/5/m_blood855502f2.png?Expires=1769091573&Signature=xOAdFrKzzfQBTY0iMq3GXPplGLy-s62Nd5xijZVHNgGlOKPKcsfVGyvytwB8NneFGbRaREh6uD~o1IIF1KwZHgNpcppRchxdE65-68zwasAvdofiHFyCy-stMh0c0nIgyhMe2O~ZkqSjZkLj35ezMjnOg1cI~HFmjTlpgURth79V3f38io7XBJvN6wVRYxeLNAIF6AE-mIZDRGgAMAkoBmBQ6lIFfdMYOdmUuu6ttVWz61UVNtX5ds~PBc~erink92GT34wDbySkpYWzYdz5Iti8qlHuKUV13RiumnjKYqGDpqYucdyn09Ia7RCa55AHj8852hFmQnR5CTkQ4yzO0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. miR-150 regulates FOXP1 levels in FL/tFL. (Ai) Statistical analysis of the effect of synthetic miR-150 on FOXP1 levels in WSU-NHL cells (48 hours; n = 3). WSU-NHL cells were transfected with siRNA against FOXP1 (siFOXP1) or siRNA negative control (siNeg. Ctrl) or miR-150 mimic (miR-150 MIMIC) or miRNA negative control (miR Neg. Ctrl) and harvested after 48 hours. The siRNA against FOXP1 serves as a positive control. The blot images were quantified with UVItec Alliance 4.7, and the FOXP1/β-actin ratio in the first sample (siNeg. Ctrl) was arbitrarily set at 1. The differences were tested by paired t test. (Aii) Representative immunoblot of FOXP1 levels in WSU-NHL cells transfected with siRNA against FOXP1 or miR-150 mimic as described in panel Ai. *Two bands identified by the anti-FOXP1 antibody represent two isoforms of FOXP1 protein. (B) The luciferase activity in HEK293T cells cotransfected with miR-150 mimic (400 nM) and luciferase reporter construct (15 ng), containing the 3′UTR region of wild-type or mutated FOXP1 (supplemental Methods). As a negative control, we used a control miRNA mimic (miR Neg. Ctrl; 400 nM). Luciferase activity was measured 24 hours posttransfection and compared by paired t test. (C) Statistical analysis of FOXP1 staining in paired FL-tFL samples from all 14 patients with available material (patient characteristics listed in supplemental Table 1 [FL patients 5-10 and 12-19]). Percentages of samples with low (≤30%), intermediate (inter; 30%-70%), and high (≥70%) percentages of FOXP1+ cells are shown. The number in each segment of the column indicates the number of samples with the corresponding intensity of IHC staining. Each arrow indicates the change in the IHC staining category in each of the analyzed pairs (note that the angle of the line does not show the trend of increase/decrease in percentage of positive cells). The statistical differences were tested by Wilcoxon matched-pairs test. Representative images of the scoring of FOXP1 staining in FL are shown in panel D and in supplemental Figure 14. (D) Representative examples of FOXP1 IHC staining between paired samples of FL (upper) and tFL (lower). Original magnification ×400. (E) Differences in miR-150 expression levels between FL samples with low (≤30%), intermediate (30%-70%), and high (≥70%) percentages of FOXP1+ cells from 30 FL patients with available material (FOXP1 staining performed as tissue microarray blocks; supplemental Methods). P values were assessed by unpaired t test. Representative images of the scoring of FOXP1 staining in FL are shown in supplemental Figure 14. (F) Cell-cycle analysis of WSU-NHL cells transfected with synthetic miR-150 mimic or siRNA against FOXP1 or negative controls for miRNA or siRNA. Cells were harvested after 24 hours (n = 10 independent experiments) and analyzed for cell cycle (supplemental Methods; supplemental Figure 16). P values were assessed by paired t test. (G) The Ki67 proliferation index in FL samples with low (≤30%), intermediate (30%-70%), or high (≥70%) percentage of FOXP1+ cells from 27 FL patients with available material (FOXP1 staining performed as tissue microarray blocks; supplemental Methods). P values were assessed by unpaired t test. NS, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/22/10.1182_blood-2018-06-855502/5/m_blood855502f3.png?Expires=1769091573&Signature=NCcDf5nfT~HXT~Gq8O6dAf34gMm2KMYJdyq~Iho3PBuMXtvKQndhx4M6995WoGP7JGktPp0bn1Uz7tjwwi410lNov85n8ZXXs3UthAcoygCvkLzIwv2cWmbQN9QyFrfBA9WHltSQcw0-tcUFfxdwp4nclxZELwfImfH4Gxj4RrPWmtJuWMGMXVRA8szayqADJ6itrWFQxdit8XgZSNB8PgZPhk0HmXDsqRv9W8upbiyxzcqkdXQRLX8AGYoNrxJ2ytnqQ9On4lJUt0BavnJq9IilDJfaXxGkvwXD~VAbYGjKKRTP~fe2JtB8bss4TBUYIxuWNHcjLSmfZCtwMfLqDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal