Key Points
Ravulizumab every 8 weeks is noninferior to eculizumab every 2 weeks across all efficacy end points in eculizumab-experienced PNH patients.
Patients with PNH may be safely and effectively switched from labeled-dose eculizumab every 2 weeks to ravulizumab every 8 weeks.
Abstract
Ravulizumab, a new complement component C5 inhibitor administered every 8 weeks, was noninferior to eculizumab administered every 2 weeks in complement-inhibitor–naive patients with paroxysmal nocturnal hemoglobinuria (PNH). This study assessed noninferiority of ravulizumab to eculizumab in clinically stable PNH patients during previous eculizumab therapy. In this phase 3, open-label, multicenter study, 195 PNH patients on labeled-dose (900 mg every 2 weeks) eculizumab for >6 months were randomly assigned 1:1 to switch to ravulizumab (n = 97) or continue eculizumab (n = 98). Primary efficacy end point was percentage change in lactate dehydrogenase (LDH) from baseline to day 183. Key secondary end points included proportion of patients with breakthrough hemolysis, change in Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue score, transfusion avoidance, and stabilized hemoglobin. In 191 patients completing 183 days of treatment, ravulizumab was noninferior to eculizumab (Pinf < .0006 for all end points), including percentage change in LDH (difference, 9.21% [95% confidence interval (CI), −0.42 to 18.84], P = .058 for superiority), breakthrough hemolysis (difference, 5.1 [95% CI, −8.89 to 18.99]), change in FACIT-Fatigue score (difference, 1.47 [95% CI, −0.21 to 3.15]), transfusion avoidance (difference, 5.5 [95% CI, −4.27 to 15.68]), and stabilized hemoglobin (difference, 1.4 [95% CI, −10.41 to 13.31]). The most frequently reported adverse event was headache (26.8%, ravulizumab; 17.3%, eculizumab). No meningococcal infections or discontinuations due to adverse events occurred. Patients with PNH may be safely and effectively switched from labeled-dose eculizumab administered every 2 weeks to ravulizumab administered every 8 weeks. This trial was funded by Alexion Pharmaceuticals, Inc., and is registered at www.clinicaltrials.gov as #NCT03056040.
Introduction
The discovery that uncontrolled complement system activation plays a key role in the pathogenesis of paroxysmal nocturnal hemoglobinuria (PNH),1 atypical hemolytic uremic syndrome,2 and myasthenia gravis3,4 was established upon results of several trials demonstrating the efficacy and safety of complement-inhibitor therapy to treat these serious and potentially life-threatening diseases.5-12 Eculizumab (Soliris; Alexion Pharmaceuticals, Inc., Boston, MA), the only approved complement inhibitor for PNH,13,14 is associated with sustained improvements in intravascular hemolysis, anemia, thrombotic events, transfusion independence, survival, and quality of life.5-7,15,16 Although eculizumab therapy is highly effective, up to 27% of eculizumab-treated patients may experience breakthrough hemolysis,17-19 resulting in a return of PNH symptoms and increased risk of serious complications. In addition, the treatment burden associated with an every-2-week dosing regimen of an IV infusion may negatively impact quality of life.20
Ravulizumab (ALXN1210) is a new complement component 5 (C5) inhibitor that produces immediate, complete, and sustained inhibition of C5 with an extended, 8-week dosing interval.21,22 Ravulizumab binds to C5 with high affinity and prevents hemolysis by inhibiting formation of C5a and C5b.23 In ravulizumab, 4 amino acid substitutions in the complementarity-determining and Fc regions of eculizumab result in enhanced endosomal dissociation of C5 and recycling to the vascular compartment through the neonatal Fc receptor pathway.22 These modifications result in a terminal half-life that is approximately fourfold longer than that of eculizumab.21,22,24
Results of phase 1b/2 studies in complement-inhibitor–naive patients with PNH demonstrate that ravulizumab provides rapid and sustained reduction in complement-mediated hemolysis at dosing intervals up to 12 weeks and overall improvement of PNH-related symptomatology and quality of life.25 In the largest phase 3 study in complement-inhibitor–naive PNH patients conducted to date, ravulizumab was shown to be noninferior to eculizumab for all end points, including transfusion avoidance, lactate dehydrogenase (LDH) normalization, percentage change in LDH levels, change in Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue score, breakthrough hemolysis, and hemoglobin stabilization.26 In this phase 3 study, we assessed the noninferiority of ravulizumab vs eculizumab in patients with PNH on stable eculizumab therapy.
Methods
Trial oversight and study design
ALXN1210-PNH-302 was a multicenter, randomized, open-label, active-controlled study conducted in 49 centers in 11 countries (registered at www.clinicaltrials.gov as #NCT03056040 and EudraCT as #2016-002026-36, CHAMPION 302). This study was performed in accordance with the principles of the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines.
The study consisted of a 4-week screening period followed by a 26-week randomized treatment period and an extension period during which all patients received ravulizumab for up to 2 years (supplemental Appendix, section 2; supplemental Figure 1, available on the Blood Web site). Patients were stratified according to transfusion history and were randomly assigned (1:1) to 26 weeks of open-label treatment with IV ravulizumab or eculizumab. History of major adverse vascular events was not a component of the randomization stratification criteria. Patients randomly assigned to the ravulizumab treatment group received weight-based dosing: a loading dose on day 1 followed by maintenance doses of ravulizumab (on day 15 and every 8 weeks thereafter) (supplemental Appendix, section 2; supplemental Figure 1). Patients randomly assigned to the eculizumab treatment group received 900 mg every 2 weeks. At the end of the 26-week treatment period, ravulizumab-treated patients continued weight-based maintenance dosing of ravulizumab, whereas eculizumab-treated patients were switched to open-label ravulizumab for the extension period. Eculizumab-treated patients received a weight-based loading dose of ravulizumab followed 2 weeks later by weight-based maintenance doses every 8 weeks.
Patients
The study enrolled adult patients (≥18 years of age) who had documented diagnoses of PNH, confirmed by high-sensitivity flow cytometry evaluation of red blood cells and white blood cells with granulocyte or monocyte clone size of ≥5% and who were clinically stable on eculizumab treatment. Eligible patients must have received eculizumab treatment of ≥6 months at labeled dose before study entry, had an LDH level ≤1.5× the upper limit of normal (ULN; 246 U/L) at screening, and been vaccinated against Neisseria meningitidis <3 years before dosing or at the time of study drug initiation to reduce the risk of meningococcal infections. Key exclusion criteria included LDH value >2× the ULN in the 6 months before day 1, major adverse vascular event (supplemental Appendix, section 2) within 6 months before day 1, platelet count 30 × 109/L, absolute neutrophil count <0.5 × 109/L, body weight <40 kg at screening, history of bone marrow transplantation, and history of N meningitidis infection (supplemental Appendix, section 2). All participants gave written informed consent prior to study participation.
Outcomes
Efficacy end points
The primary efficacy end point was hemolysis, as directly measured by percentage change in LDH levels from baseline to day 183. Key secondary efficacy end points were proportion of patients with breakthrough hemolysis, defined as at least 1 new or worsening symptom or sign of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, shortness of breath [dyspnea], anemia [hemoglobin <10 g/dL], major adverse vascular event [including thrombosis], dysphagia, or erectile dysfunction) in the presence of elevated LDH ≥2× the ULN after prior reduction of LDH to <1.5× the ULN on treatment; change from baseline in quality of life, assessed with the FACIT-Fatigue Scale Version 4.0; transfusion avoidance, defined as the proportion of patients who remained transfusion free and did not require a transfusion per protocol-specified guidelines; and proportion of patients with stabilized hemoglobin, defined as avoidance of a ≥2-g/dL decrease in hemoglobin level from baseline in the absence of transfusion.
Additional secondary efficacy end points included total number of units of packed red blood cells transfused, proportion of patients with LDH in the normal range, change in European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 scale (Version 3.0), change in clinical manifestations of PNH (fatigue, hemoglobinuria, abdominal pain, shortness of breath, chest pain, dysphagia, and erectile dysfunction), and proportion of patients experiencing major adverse vascular events. Change in free C5 concentration over time was also assessed.
Safety
Adverse events were recorded by type, incidence, and severity. Antidrug antibodies were also assessed. A safety review committee monitored safety, and an independent data monitoring committee monitored data for cases of meningococcal infection.
Statistical analysis
The planned sample size of ∼192 enrolled patients provided 90% power to demonstrate noninferiority of ravulizumab to eculizumab at a 1-sided α level of 0.025, a 10% dropout rate, and a noninferiority margin of 15% for percentage change in LDH from baseline to day 183. The primary efficacy end point of percentage change in LDH from baseline to day 183 was analyzed by mixed model for repeated measures with the fixed, categorical effects of treatment, study visit, and study visit by treatment group interaction as well as the fixed covariate of baseline LDH and the stratification randomization indicator of packed red blood cell transfusion history. The Kenward-Roger approximation was used to estimate denominator degrees of freedom. A difference in percentage change in LDH from baseline to day 183 between ravulizumab and eculizumab treatment groups along with a 2-sided 95% confidence interval (CI) were calculated. Ravulizumab treatment was concluded to be noninferior to eculizumab if the lower bound of the 95% CI for the difference (ravulizumab − eculizumab) was greater than the noninferiority margin of −15%.
The key secondary end points were tested for noninferiority in a hierarchical manner provided that noninferiority was declared for the primary end point. If noninferiority was established for all key secondary end points, then superiority was assessed via a closed-testing procedure, using a 2-sided 0.05 test for each parameter, in the following order: percentage change in LDH, FACIT-Fatigue, breakthrough hemolysis, stabilized hemoglobin, and transfusion avoidance (supplemental Appendix, section 2). If at any point noninferiority or superiority was not reached, then all subsequent tests were stopped. Post hoc P values were calculated for testing of noninferiority (Pinf) relative to the prespecified noninferiority margins to assess the strength of evidence of the study results.
For the primary end point of percentage change in LDH and the secondary end point of breakthrough hemolysis, estimates of the treatment difference were based upon (eculizumab − ravulizumab), while the remainder of the end points were based upon (ravulizumab − eculizumab). This calculation was made so that all positive estimates of treatment effects indicated a greater effect of ravulizumab, and all negative treatment estimates indicated a greater effect of eculizumab.
Efficacy analyses were performed on the full analysis set (all patients who received at least 1 dose of ravulizumab or eculizumab). Safety analyses were performed on the safety set, defined as all patients who received at least 1 dose of ravulizumab or eculizumab. The pharmacodynamic analysis was performed on all patients who received at least 1 dose of study drug and who had evaluable data. All analyses were performed using SAS release (SAS Institute Inc., Cary, NC), version 9.4 or higher, or other validated statistical software.
Results
Patient characteristics
Of 208 patients screened for eligibility, 197 met all entry criteria and were randomly assigned (1:1) to ravulizumab or eculizumab. Two patients withdrew before receiving study drug, and 195 received treatment (ravulizumab, n = 97; eculizumab, n = 98) (supplemental Appendix, section 3; supplemental Figure 2). All received meningococcal vaccination if they had not received it in the past 3 years. Of the 195 patients who received treatment, 191 completed the 26-week treatment period (ravulizumab, n = 96; eculizumab, n = 95). Four patients discontinued the study, 1 in the ravulizumab group (patient decision) and 3 in the eculizumab group (patient decision, lack of efficacy, and pregnancy; n = 1 for each). Patient demographics and baseline clinical characteristics were well balanced between treatment groups (Table 1). On average, patients had received eculizumab therapy for 5.8 years before study entry. All patients who received treatment (100%) received all planned infusions of study medication.
Demographic and baseline clinical characteristics
| Characteristic . | Ravulizumab (n = 97) . | Eculizumab (n = 98) . | Total (N = 195) . |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 50 (51.5) | 48 (49.0) | 98 (50.3) |
| Female | 47 (48.5) | 50 (51.0) | 97 (49.7) |
| Age at first infusion of study drug, mean (SD), y | 46.6 (14.4) | 48.8 (14.0) | 47.7 (14.2) |
| Race, no. (%) | |||
| White | 50 (51.5) | 61 (62.2) | 111 (56.9) |
| Asian | 23 (23.7) | 19 (19.4) | 42 (21.5) |
| Japanese | 5 (5.2) | 7 (7.1) | 12 (6.2) |
| African American | 5 (5.2) | 3 (3.1) | 8 (4.1) |
| Other/multiple | 3 (3.1) | 1 (1.0) | 4 (2.1) |
| Not reported/unknown | 16 (16.5) | 14 (14.3) | 30 (15.4) |
| Weight, mean (SD), kg | 72.4 (16.8) | 73.4 (14.6) | 72.9 (15.7) |
| Height, mean (SD), cm | 168.3 (10.1) | 168.8 (9.9) | 168.5 (10.0) |
| Years on eculizumab before first study infusion | 6.0 (3.5) | 5.6 (3.5) | 5.8 (3.5) |
| Patients with packed red blood cells/whole blood transfusions received within 1 y before first dose, no. (%) | 13 (13.4) | 12 (12.2) | 25 (12.8) |
| Age at PNH diagnosis, mean (SD), y | 34.1 (14.4) | 36.8 (14.1) | 35.5 (14.3) |
| Time from PNH diagnosis to consent, mean (SD), y | 12.4 (8.4) | 11.9 (9.4) | 12.2 (8.9) |
| LDH, mean (SD),* U/L | 228.0 (48.7) | 235.2 (49.7) | 231.6 (49.2) |
| PNH clone size, mean (SD), % | |||
| Type II red blood cells | 14.9 (19.6) | 16.3 (23.6) | 15.6 (21.6) |
| Type III red blood cells† | 44.6 (30.5) | 43.5 (29.7) | 44.0 (30.0) |
| Total red blood cells | 60.6 (32.5) | 59.5 (31.4) | 60.1 (31.9) |
| Granulocyte | 82.6 (23.6) | 84.0 (21.4) | 83.3 (22.5) |
| Monocyte | 85.6 (20.5) | 86.1 (19.7) | 85.9 (20.0) |
| Hemoglobin, g/L, mean (SD)‡ | 110.8 (18.4) | 109.1 (18.4) | Not available |
| Haptoglobin, g/L, mean (SD)§ | 0.283 (0.235) | 0.255 (0.174) | Not available |
| History of major adverse vascular events, no. (%) | 28 (28.9) | 22 (22.4) | 50 (25.6) |
| History of aplastic anemia, no. (%) | 34 (35.1) | 39 (39.8) | 73 (37.4) |
| Characteristic . | Ravulizumab (n = 97) . | Eculizumab (n = 98) . | Total (N = 195) . |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 50 (51.5) | 48 (49.0) | 98 (50.3) |
| Female | 47 (48.5) | 50 (51.0) | 97 (49.7) |
| Age at first infusion of study drug, mean (SD), y | 46.6 (14.4) | 48.8 (14.0) | 47.7 (14.2) |
| Race, no. (%) | |||
| White | 50 (51.5) | 61 (62.2) | 111 (56.9) |
| Asian | 23 (23.7) | 19 (19.4) | 42 (21.5) |
| Japanese | 5 (5.2) | 7 (7.1) | 12 (6.2) |
| African American | 5 (5.2) | 3 (3.1) | 8 (4.1) |
| Other/multiple | 3 (3.1) | 1 (1.0) | 4 (2.1) |
| Not reported/unknown | 16 (16.5) | 14 (14.3) | 30 (15.4) |
| Weight, mean (SD), kg | 72.4 (16.8) | 73.4 (14.6) | 72.9 (15.7) |
| Height, mean (SD), cm | 168.3 (10.1) | 168.8 (9.9) | 168.5 (10.0) |
| Years on eculizumab before first study infusion | 6.0 (3.5) | 5.6 (3.5) | 5.8 (3.5) |
| Patients with packed red blood cells/whole blood transfusions received within 1 y before first dose, no. (%) | 13 (13.4) | 12 (12.2) | 25 (12.8) |
| Age at PNH diagnosis, mean (SD), y | 34.1 (14.4) | 36.8 (14.1) | 35.5 (14.3) |
| Time from PNH diagnosis to consent, mean (SD), y | 12.4 (8.4) | 11.9 (9.4) | 12.2 (8.9) |
| LDH, mean (SD),* U/L | 228.0 (48.7) | 235.2 (49.7) | 231.6 (49.2) |
| PNH clone size, mean (SD), % | |||
| Type II red blood cells | 14.9 (19.6) | 16.3 (23.6) | 15.6 (21.6) |
| Type III red blood cells† | 44.6 (30.5) | 43.5 (29.7) | 44.0 (30.0) |
| Total red blood cells | 60.6 (32.5) | 59.5 (31.4) | 60.1 (31.9) |
| Granulocyte | 82.6 (23.6) | 84.0 (21.4) | 83.3 (22.5) |
| Monocyte | 85.6 (20.5) | 86.1 (19.7) | 85.9 (20.0) |
| Hemoglobin, g/L, mean (SD)‡ | 110.8 (18.4) | 109.1 (18.4) | Not available |
| Haptoglobin, g/L, mean (SD)§ | 0.283 (0.235) | 0.255 (0.174) | Not available |
| History of major adverse vascular events, no. (%) | 28 (28.9) | 22 (22.4) | 50 (25.6) |
| History of aplastic anemia, no. (%) | 34 (35.1) | 39 (39.8) | 73 (37.4) |
Normal range, 120 to 246 U/L.
Erythrocytes with complete deficiency in glycosylphosphatidylinositol-anchored proteins, including complement regulatory proteins CD59 and CD55.30
Normal range, 11.5 to 16.0 g/dL (women) and 13.0 to 17.5 g/dL (men).
Normal range, 0.4 to 2.4 g/dL.
Primary end point
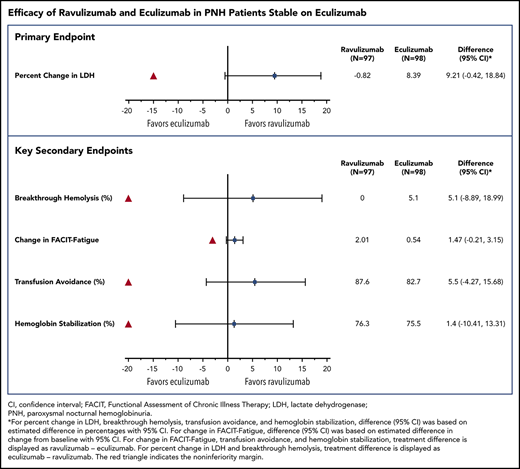
Ravulizumab achieved noninferiority compared with eculizumab (Figure 1A; Table 2) for the primary end point of percentage change in LDH, with the point estimate for treatment difference favoring ravulizumab. The least-squares estimate of the mean (standard error) in percentage change in LDH from baseline to day 183 showed a decrease of 0.82% (3.033%) for the ravulizumab group and an increase of 8.39% (3.041%) for the eculizumab group, with a treatment difference (ravulizumab − eculizumab) of 9.21% (95% CI, −0.42% to 18.84%). The lower bound of the 95% CI for the difference was −0.42%, which exceeded the protocol-specified noninferiority margin of −15%, indicating that ravulizumab is noninferior to eculizumab with a Pinf < .0006.
Treatment effect. (A) Primary end point. The red triangle indicates the noninferiority margin. [1] Difference (Diff) (95% CI) was based on estimated difference in percentage with 95% CI. [2] Treatment difference was estimated for eculizumab − ravulizumab. (B) Secondary end point. The red triangle indicates the noninferiority margin. [1] For the end points transfusion avoidance (TA), breakthrough hemolysis (BTH), and stabilized hemoglobin (HGB-S), Diff (95% CI) was based on estimated differences in percentage with 95% CI. For FACIT-Fatigue, Diff (95% CI) was based on estimated difference in change from baseline with 95% CI. [2] Treatment difference was estimated for ravulizumab − eculizumab, except for breakthrough hemolysis, where treatment difference was based on eculizumab − ravulizumab. PCHG, percentage change.
Treatment effect. (A) Primary end point. The red triangle indicates the noninferiority margin. [1] Difference (Diff) (95% CI) was based on estimated difference in percentage with 95% CI. [2] Treatment difference was estimated for eculizumab − ravulizumab. (B) Secondary end point. The red triangle indicates the noninferiority margin. [1] For the end points transfusion avoidance (TA), breakthrough hemolysis (BTH), and stabilized hemoglobin (HGB-S), Diff (95% CI) was based on estimated differences in percentage with 95% CI. For FACIT-Fatigue, Diff (95% CI) was based on estimated difference in change from baseline with 95% CI. [2] Treatment difference was estimated for ravulizumab − eculizumab, except for breakthrough hemolysis, where treatment difference was based on eculizumab − ravulizumab. PCHG, percentage change.
Primary and key secondary efficacy outcomes at day 183
| . | Ravulizumab (n = 97) . | Eculizumab (n = 98) . | Statistic for comparison . | Treatment effect . | Noninferiority margin . | Conclusion* . |
|---|---|---|---|---|---|---|
| Primary end point | ||||||
| LDH, least squares mean % change (95% CI) | −0.82 (−7.8, 6.1) | 8.4 (1.5, 15.3) | Difference in percentage change from baseline | 9.2 (−0.42 to 18.8) | −15% | Noninferior |
| Key secondary efficacy end points | ||||||
| Breakthrough hemolysis rate, % (95% CI) | 0 (0 to 3.7) | 5.1 (1.7 to 11.5) | Difference in rate | 5.1 (−8.9 to 19.0) | −20% | Noninferior |
| FACIT-Fatigue score, least squares mean change (95% CI) | 2.0 (0.6 to 3.4) | 0.54 (−0.8 to 1.9) | Difference in change from baseline | 1.5 (−0.2 to 3.2) | −3.0 | Noninferior |
| Transfusion avoidance rate, % (95% CI) | 87.6 (81.1 to 94.2) | 82.7 (75.2 to 90.2) | Difference in rate | 5.5 (−4.3 to 15.7) | −20% | Noninferior |
| Stabilized hemoglobin rate, % (95% CI) | 76.3 (67.8 to 84.8) | 75.5 (67.0 to 84.0) | Difference in rate | 1.4 (−10.4 to 13.3) | −20% | Noninferior |
| . | Ravulizumab (n = 97) . | Eculizumab (n = 98) . | Statistic for comparison . | Treatment effect . | Noninferiority margin . | Conclusion* . |
|---|---|---|---|---|---|---|
| Primary end point | ||||||
| LDH, least squares mean % change (95% CI) | −0.82 (−7.8, 6.1) | 8.4 (1.5, 15.3) | Difference in percentage change from baseline | 9.2 (−0.42 to 18.8) | −15% | Noninferior |
| Key secondary efficacy end points | ||||||
| Breakthrough hemolysis rate, % (95% CI) | 0 (0 to 3.7) | 5.1 (1.7 to 11.5) | Difference in rate | 5.1 (−8.9 to 19.0) | −20% | Noninferior |
| FACIT-Fatigue score, least squares mean change (95% CI) | 2.0 (0.6 to 3.4) | 0.54 (−0.8 to 1.9) | Difference in change from baseline | 1.5 (−0.2 to 3.2) | −3.0 | Noninferior |
| Transfusion avoidance rate, % (95% CI) | 87.6 (81.1 to 94.2) | 82.7 (75.2 to 90.2) | Difference in rate | 5.5 (−4.3 to 15.7) | −20% | Noninferior |
| Stabilized hemoglobin rate, % (95% CI) | 76.3 (67.8 to 84.8) | 75.5 (67.0 to 84.0) | Difference in rate | 1.4 (−10.4 to 13.3) | −20% | Noninferior |
Testing of the noninferiority hypothesis is assessed by comparing the bolded limit of the 95% CI to the noninferiority margin.
A conclusion of noninferiority indicates that the noninferiority margin is larger or smaller than the lower or upper bound of the 95% CI indicated in boldface.
Key secondary end points
Treatment with ravulizumab also achieved noninferiority compared with eculizumab for all 4 key secondary end points, with all point estimates for treatment difference favoring ravulizumab (Figure 1B; Table 2). No patients in the ravulizumab group experienced breakthrough hemolysis compared with 5 (5.1%) patients in the eculizumab group (difference, 5.1% [95% CI, −8.89% to 18.99%, Pinf < .0004). Of these 5 patients with breakthrough hemolysis, 4 had 1 event each and 1 had 3 events, the third of which resulted in hospitalization and subsequent study discontinuation because of lack of efficacy (this patient subsequently received eculizumab 1200 mg every 2 weeks). Four of the 7 breakthrough hemolysis events were associated with time-matched free C5 ≥ 0.5 μg/mL, a level associated with incomplete inhibition of hemolysis.13,14 One of these was also associated with infection. Additionally, 2 events with complete C5 inhibition were associated with infection. One breakthrough hemolysis event was of unknown etiology. Least-squares mean (standard error) change in FACIT-Fatigue total score was 2.01 (0.697) in the ravulizumab group and 0.54 (0.704) in the eculizumab group (difference, 1.47 [95% CI, −0.21 to 3.15], Pinf < .0001). The percentage of patients with a ≥3-point improvement in FACIT-Fatigue score was similar between the ravulizumab and eculizumab groups (37.1% vs 33.7%). Eighty-five of 97 patients (87.6%) receiving ravulizumab and 81 of 98 patients (82.7%) receiving eculizumab avoided transfusion, with a between-group difference of 5.5% (95% CI, −4.27% to 15.68%, Pinf < .0001), whereas 74 of 97 patients (76.3%) receiving ravulizumab and 74 of 98 patients (75.5%) receiving eculizumab achieved stabilized hemoglobin levels (difference, 1.4% [95% CI, −10.41% to 13.31%], Pinf < .0005).
Because noninferiority was achieved for the primary end point and all 4 key secondary end points, superiority testing of percentage change in LDH was performed. Although LDH levels increased by 8.4% in the eculizumab group and decreased 0.82% in the ravulizumab group at day 183, the difference did not reach statistical significance for superiority (P = .058). Accordingly, no further hierarchical testing was performed.
Subgroup analyses were performed for the randomization stratification variable of transfusion history and for sex, race, region, and age for the primary end point and key secondary end points. No sensitive subgroups were identified.
Additional secondary end points
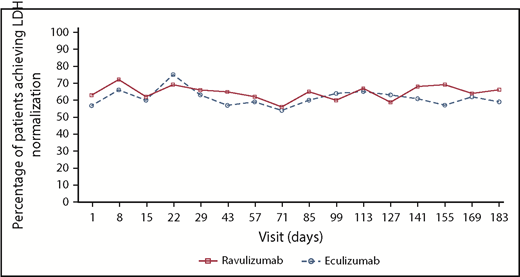
The mean (standard deviation [SD]) total number of packed red blood cell units transfused during the treatment period was comparable in the ravulizumab (4.3 [4.76]) and eculizumab (3.4 [3.01]) treatment groups (Table 3). As expected in a patient population that was clinically stable on eculizumab therapy, the proportion of patients who achieved normalization in LDH was relatively stable over time. At day 183, LDH normalization was achieved by 64 of 97 patients (66.0%) treated with ravulizumab and 58 of 98 patients (59.2%) treated with eculizumab (Figure 2). Similarly, mean LDH values were within the normal range at baseline and generally sustained over time (supplemental Appendix, section 3; supplemental Figure 3). Baseline European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 subscale scores reflected a patient population with stable disease, and changes in scores during the study were similar in both treatment groups (supplemental Appendix, section 4; supplemental Table 1). Similarly, shifts in clinical manifestations of PNH were infrequent in both treatment groups, and no patient experienced a major adverse vascular event (Table 3).
Other secondary efficacy outcomes at day 183
| . | Ravulizumab (n = 96) . | Eculizumab (n = 95) . | ||
|---|---|---|---|---|
| Total number of packed red blood cell units transfused, mean (SD) | — | 4.3 (4.76)† | — | 3.4 (3.01)‡ |
| Patients with major adverse vascular events, n (%) | — | 0 (0) | — | 0 (0) |
| . | Ravulizumab (n = 96) . | Eculizumab (n = 95) . | ||
|---|---|---|---|---|
| Total number of packed red blood cell units transfused, mean (SD) | — | 4.3 (4.76)† | — | 3.4 (3.01)‡ |
| Patients with major adverse vascular events, n (%) | — | 0 (0) | — | 0 (0) |
| . | Baseline* . | Day 183 . | Baseline* . | Day 183 . |
|---|---|---|---|---|
| Clinical manifestations of PNH, n (%) | ||||
| Fatigue | 29 (30.2) | 42 (43.8) | 38 (40) | 36 (37.9) |
| Abdominal pain | 5 (5.2) | 5 (5.2) | 6 (6.3) | 12 (12.6) |
| Dyspnea | 6 (6.3) | 6 (6.3) | 10 (10.5) | 17 (17.9) |
| Dysphagia | 2 (2.1) | 5 (5.2) | 2 (2.1) | 5 (5.2) |
| Chest pain | 0 (0) | 2 (2.1) | 1 (1.1) | 5 (5.2) |
| Hemoglobinuria | 4 (4.2) | 8 (8.3) | 7 (7.4) | 9 (9.5) |
| Erectile dysfunction§ | 5 (10.0) | 6 (12.0) | 7 (14.6) | 6 (12.5) |
| . | Baseline* . | Day 183 . | Baseline* . | Day 183 . |
|---|---|---|---|---|
| Clinical manifestations of PNH, n (%) | ||||
| Fatigue | 29 (30.2) | 42 (43.8) | 38 (40) | 36 (37.9) |
| Abdominal pain | 5 (5.2) | 5 (5.2) | 6 (6.3) | 12 (12.6) |
| Dyspnea | 6 (6.3) | 6 (6.3) | 10 (10.5) | 17 (17.9) |
| Dysphagia | 2 (2.1) | 5 (5.2) | 2 (2.1) | 5 (5.2) |
| Chest pain | 0 (0) | 2 (2.1) | 1 (1.1) | 5 (5.2) |
| Hemoglobinuria | 4 (4.2) | 8 (8.3) | 7 (7.4) | 9 (9.5) |
| Erectile dysfunction§ | 5 (10.0) | 6 (12.0) | 7 (14.6) | 6 (12.5) |
Baseline was defined as the last nonmissing value before the first dose of study drug.
n = 97.
n = 98.
n = 50 male patients in the ravulizumab group and n = 48 male patients in the eculizumab group.
Percentage of patients achieving LDH normalization over time in the ravulizumab and eculizumab treatment groups. LDH normalization is defined as proportion of patients who achieved LDH level ≤1× the ULN (246 U/L).
Percentage of patients achieving LDH normalization over time in the ravulizumab and eculizumab treatment groups. LDH normalization is defined as proportion of patients who achieved LDH level ≤1× the ULN (246 U/L).
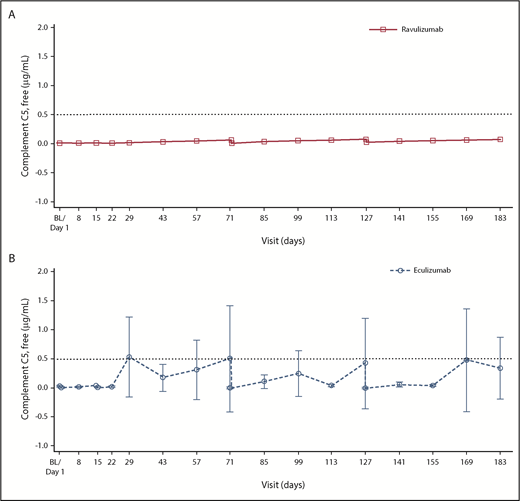
Mean serum free C5 concentrations were suppressed to <0.5 µg/mL by the end of the first infusion and at all subsequent visits for all patients receiving ravulizumab; however, this threshold was not consistently met in the eculizumab group (Figure 3).
Mean (95% CI) free C5 levels in the ravulizumab and eculizumab groups over time. (A-B) A gyros-based fluorescence assay was used for patients who received ravulizumab (A), and an electrochemiluminescence immunoassay was used for patients who received eculizumab (B). Baseline (BL) is defined as the last nonmissing value before first dose of study drug. Day 29, 43, 57, 85, 99, 113, 141, 155, and 169 data are from anytime for the ravulizumab group and predose for the eculizumab group. Horizontal line indicates free C5 level of 0.5 µg/mL. Free C5 levels <0.5 µg/mL are associated with complete inhibition of C5 activity.
Mean (95% CI) free C5 levels in the ravulizumab and eculizumab groups over time. (A-B) A gyros-based fluorescence assay was used for patients who received ravulizumab (A), and an electrochemiluminescence immunoassay was used for patients who received eculizumab (B). Baseline (BL) is defined as the last nonmissing value before first dose of study drug. Day 29, 43, 57, 85, 99, 113, 141, 155, and 169 data are from anytime for the ravulizumab group and predose for the eculizumab group. Horizontal line indicates free C5 level of 0.5 µg/mL. Free C5 levels <0.5 µg/mL are associated with complete inhibition of C5 activity.
Safety and tolerability
Ravulizumab and eculizumab were well tolerated in this study. An overview of adverse events is shown in Table 4. The most frequently reported adverse event occurring in 3% or more of patients in either treatment group was headache, which occurred in 26.8% of patients treated with ravulizumab and in 17.3% of patients treated with eculizumab. Twelve patients experienced serious adverse events (4 ravulizumab patients and 8 eculizumab patients). Pyrexia and hemolysis were the only serious adverse events reported by >1 patient (3 and 2 patients, respectively, all in the eculizumab group). There were no deaths and no cases of meningococcal infection. Serious infections occurred in 2 patients (2.1%) in the ravulizumab group (influenza and lower respiratory tract infection [without positive culture]) and in 1 eculizumab-treated patient (1.0%) (acute pyelonephritis [causative agent unknown]). None of these serious adverse events led to discontinuation from the study. Four patients discontinued the study, 1 in the ravulizumab group (withdrawal by subject) and 3 in the eculizumab group (withdrawal by subject, lack of efficacy [3 breakthrough hemolysis events], and pregnancy). No adverse events led to withdrawal of study drug during the randomized treatment period. There were no treatment-emergent antidrug antibodies in patients treated with ravulizumab.
Adverse events
| Variable . | Ravulizumab (n = 97) . | Eculizumab (n = 98) . |
|---|---|---|
| Patients with adverse events | 85 (87.6) | 86 (87.8) |
| Most common adverse events (≥5% of patients in either treatment group) | ||
| Headache | 26 (26.8) | 17 (17.3) |
| Nasopharyngitis | 21 (21.6) | 20 (20.4) |
| Upper respiratory tract infection | 18 (18.6) | 10 (10.2) |
| Diarrhea | 9 (9.3) | 7 (7.1) |
| Pyrexia | 9 (9.3) | 5 (5.1) |
| Nausea | 8 (8.2) | 9 (9.2) |
| Constipation | 7 (7.2) | 5 (5.1) |
| Influenza-like illness | 7 (7.2) | 8 (8.2) |
| Abdominal pain | 6 (6.2) | 9 (9.2) |
| Anemia | 6 (6.2) | 3 (3.1) |
| Fatigue | 6 (6.2) | 6 (6.1) |
| Vomiting | 6 (6.2) | 4 (4.1) |
| Cough | 5 (5.2) | 10 (10.2) |
| Pain in extremity | 5 (5.2) | 4 (4.1) |
| Rhinitis | 5 (5.2) | 4 (4.1) |
| Oropharyngeal pain | 4 (4.1) | 9 (9.2) |
| Chest pain | 3 (3.1) | 9 (9.2) |
| Dizziness | 3 (3.1) | 7 (7.1) |
| Musculoskeletal pain | 2 (2.1) | 5 (5.1) |
| Dyspnea | 0 (0.0) | 6 (6.1) |
| Patients with serious adverse events | 4 (4.1) | 8 (8.2) |
| Meningococcal infections | 0 | 0 |
| Death | 0 | 0 |
| Patients with adverse events leading to withdrawal of study drug | 0 | 0 |
| Patients with serious adverse events leading to withdrawal of study drug | 0 | 0 |
| Variable . | Ravulizumab (n = 97) . | Eculizumab (n = 98) . |
|---|---|---|
| Patients with adverse events | 85 (87.6) | 86 (87.8) |
| Most common adverse events (≥5% of patients in either treatment group) | ||
| Headache | 26 (26.8) | 17 (17.3) |
| Nasopharyngitis | 21 (21.6) | 20 (20.4) |
| Upper respiratory tract infection | 18 (18.6) | 10 (10.2) |
| Diarrhea | 9 (9.3) | 7 (7.1) |
| Pyrexia | 9 (9.3) | 5 (5.1) |
| Nausea | 8 (8.2) | 9 (9.2) |
| Constipation | 7 (7.2) | 5 (5.1) |
| Influenza-like illness | 7 (7.2) | 8 (8.2) |
| Abdominal pain | 6 (6.2) | 9 (9.2) |
| Anemia | 6 (6.2) | 3 (3.1) |
| Fatigue | 6 (6.2) | 6 (6.1) |
| Vomiting | 6 (6.2) | 4 (4.1) |
| Cough | 5 (5.2) | 10 (10.2) |
| Pain in extremity | 5 (5.2) | 4 (4.1) |
| Rhinitis | 5 (5.2) | 4 (4.1) |
| Oropharyngeal pain | 4 (4.1) | 9 (9.2) |
| Chest pain | 3 (3.1) | 9 (9.2) |
| Dizziness | 3 (3.1) | 7 (7.1) |
| Musculoskeletal pain | 2 (2.1) | 5 (5.1) |
| Dyspnea | 0 (0.0) | 6 (6.1) |
| Patients with serious adverse events | 4 (4.1) | 8 (8.2) |
| Meningococcal infections | 0 | 0 |
| Death | 0 | 0 |
| Patients with adverse events leading to withdrawal of study drug | 0 | 0 |
| Patients with serious adverse events leading to withdrawal of study drug | 0 | 0 |
Values are reported as n (%) of patients.
Discussion
This 26-week, active-controlled study of 195 patients with PNH who were clinically stable on labeled-dose eculizumab treatment for a mean for 5.8 years demonstrated that ravulizumab administered every 8 weeks effectively inhibited complement-mediated hemolysis and had a safety profile similar to that of eculizumab.5-7 Ravulizumab met the primary end point (percentage change in LDH from baseline to day 183) and all key secondary end points, showing noninferiority to biweekly treatment with 900 mg eculizumab, the current standard of care for PNH.13,14 Point estimates consistently favored ravulizumab treatment over eculizumab treatment for the primary end point and all 4 key secondary efficacy end points, although none of the results from this noninferiority trial demonstrated superiority.
Given that the patients enrolled in this study were stable on eculizumab therapy and LDH levels were within the normal range at baseline, and given the highly efficacious nature of eculizumab, differences between treatments were expected to be minimal.15-17 However, 5 patients receiving eculizumab experienced breakthrough hemolysis, whereas no events of breakthrough hemolysis were observed in patients switched to ravulizumab. Among 7 episodes of breakthrough hemolysis, 4 were associated with inadequate C5 inhibition, 2 were primarily associated with infection, and 1 was of unclear etiology. Breakthrough hemolysis occurring as a result of complement-amplifying conditions such as infection may occur irrespective of free C5 and remains a possibility with the use of eculizumab and ravulizumab. Moreover, no major adverse vascular events were observed in either treatment group.
These results are qualitatively similar to those observed in patients naive to C5 inhibitor therapy,26 providing further evidence that ravulizumab provides complete inhibition of C5 to levels <0.5 μg/mL compared with eculizumab, which did not provide complete inhibition in all patients, and may mitigate the observed incidence and clinical sequelae of breakthrough hemolysis that has been observed with labeled-dose eculizumab.17-19
The safety profile of ravulizumab was consistent with that of eculizumab in the clinical trial program in PNH.5,7,27 Overall, the types and incidences of adverse events were comparable to those of other ravulizumab25,26 and eculizumab trials,5,7,27 with headache being the most commonly reported adverse event. The serious infections noted in this study resolved without sequelae. Although an increased risk of meningococcal infections has been reported in recipients of complement inhibitor therapy,13,14,28,29 no such infections were observed in the present trial.
This study demonstrates that patients with PNH may be safely and effectively switched from eculizumab (900 mg administered every 2 weeks) to ravulizumab administered every 8 weeks while maintaining the high level of efficacy, safety, and quality of life previously achieved with eculizumab. For all efficacy end points, ravulizumab achieved noninferiority compared with eculizumab. The complete and sustained C5 inhibition associated with ravulizumab may account for the consistent results across end points. For stable patients receiving label-dose eculizumab therapy, providing an effective treatment duration that is 4 times longer between infusions by switching to ravulizumab given every 8 weeks is likely to result in a substantially reduced burden of treatment, fewer occurrences of breakthrough hemolysis and their clinical consequences, better quality of life, and greater likelihood of retention on long-term therapy.
Presented at 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
Qualified academic investigators may request participant-level, de-identified clinical data and supporting documents (statistical analysis plan and protocol) pertaining to this study. Further details regarding data availability, instructions for requesting information, and our data disclosure policy are available at the Alexion Web site (http://alexion.com/research-development).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Medical writing and editorial support were provided by Lynn Brown and Traci Stuve of ApotheCom. The authors thank Lori Volles, Rodrigo Pavani, and Masayo Ogawa of Alexion Pharmaceuticals for their contribution to the implementation of the study. Statistical analysis, pharmacokinetic and pharmacodyamic assessments, and editorial review were provided by Rasha Aguzzi, Rajendra Pradhan, and Stephan Ortiz of Alexion Pharmaceuticals. Editorial review was provided by Kenneth Pomerantz of Alexion Pharmaceuticals, Inc. The authors also thank the investigators of ALXN1210-PNH-302, who are listed in the supplemental Appendix, section 1. The sponsor and investigators thank the patients and their families for their participation in, and support for, this clinical study.
This study was supported by Alexion Pharmaceuticals, Inc.
Authorship
Contribution: A.G.K., A.H., J.S., and S.N. recruited patients, collected data, analyzed and interpreted the data, contributed to the manuscript, and approved the final version; S.T.R., A.I.D., S.O., and L.S., developed the protocol, analyzed and interpreted the data, contributed to the manuscript, and approved the final version; S.L., R.W., A.G., J.W.L., E.O.G., C.I.P., and A.R. recruited patients, contributed to the manuscript, and approved the final version; F.A.G.-F. recruited patients, collected data, contributed to the manuscript, and approved the final version; E.B. contributed to the study protocol, contributed to the manuscript, and approved the final version; and A.R. and R.P.d.L. developed the protocol, recruited patients, collected data, analyzed and interpreted the data, contributed to the manuscript, and approved the final version.
Conflict-of-interest disclosure: A.G.K., A.H., R.W., F.A.G.-F., A.G., E.O.G., A.R., and S.N. have received honoraria and consulting fees from Alexion Pharmaceuticals, Inc. S.T.R., L.S., A.I.D., and S.O. are employees and stockholders of Alexion Pharmaceuticals, Inc. J.W.L. has received honoraria, consulting fees, and research support (to Seoul St. Mary’s Hospital) from Alexion Pharmaceuticals, Inc. E.B. is a former employee and stockholder of Alexion Pharmaceuticals, Inc. J.S. has received consultancies, honoraria, and advisory board membership for Alexion Pharmaceuticals, Inc. A.R. has received consultancies and honoraria from Alexion, Roche, and Novartis and research funding from Alexion and Roche. R.P.d.L. has received consultancies, honoraria, and research funding from Alexion, Novartis, and Pfizer and research funding from Amgen. The remaining authors declare no competing financial interests.
A complete list of study investigators can be found in the supplemental Appendix, section 1.
Correspondence: Austin G. Kulasekararaj, Department of Haematological Medicine, King’s College Hospital, NHS Foundation Trust, Denmark Hill, London SE5 9RS, United Kingdom; e-mail: austin.kulasekararaj@nhs.net.

![Figure 1. Treatment effect. (A) Primary end point. The red triangle indicates the noninferiority margin. [1] Difference (Diff) (95% CI) was based on estimated difference in percentage with 95% CI. [2] Treatment difference was estimated for eculizumab − ravulizumab. (B) Secondary end point. The red triangle indicates the noninferiority margin. [1] For the end points transfusion avoidance (TA), breakthrough hemolysis (BTH), and stabilized hemoglobin (HGB-S), Diff (95% CI) was based on estimated differences in percentage with 95% CI. For FACIT-Fatigue, Diff (95% CI) was based on estimated difference in change from baseline with 95% CI. [2] Treatment difference was estimated for ravulizumab − eculizumab, except for breakthrough hemolysis, where treatment difference was based on eculizumab − ravulizumab. PCHG, percentage change.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/6/10.1182_blood-2018-09-876805/4/m_blood876805f1.png?Expires=1770152523&Signature=wZWZSjpeBYVX7zYOI1MA0Kw8K3LJEOabk4O9bfpe1hNRoHLOUWAL0saH0YWIjP8QNMfPO~3KaGc8h9YQY7lyoTOcjW5lktFKuCcoGWyJ5Wu9WEZN09EdloASgQSVW~qWntsVUJDTfPPTQUT7zRhI7TcjqsltSzJd1zCcULDoMFO3SpOVxksUB9iG8YG4-A6P6h8WDWYbD~bFnpJw2E7LCXcQ0d1ZkPlsu-QQDpvfMvYmgS4G6-23qtC96JBKCr5DbzQoIx01VhLi1d0aXyvVAAZFlwYjyQzWFkxs9IUdjuZO8o3bd3xF6R7djmUwiY2aoPoQcXH4nZ71nddSffl5Zg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)