
Background:
Hypomethylating agents (HMAs) can confer transfusion independence and prolong overall survival (OS) in patients with myelodysplastic syndromes (MDS), but response rates are < 50% and depend on sustained administration. In Ontario, 33% of higher risk MDS patients receive < 4 cycles AZA and have very short survival. Identifying the patients unsuitable for HMAs and the factors predictive of overall survival (OS)/leukemia free survival (LFS) would be of value. MDS-CAN, the national MDS registry prospectively evaluates patient-related factors in addition to disease factors in MDS, MDS/MPN, and oligoblastic AML patients.
Objective:
Determine the factors predictive of OS/LFS and the completion of ≥ 4 cycles of HMA, with particular focus on frailty and comorbidity.
Methods:
All patients who had received HMAs (azacytidine (AZA), decitabine, guadecitabine, ASTX727) were eligible. Frailty was assessed using the Rockwood clinical frailty scale (CFS) and the frailty index (FI) comprised of 42 deficits we previously developed. The MDS-FI was calculated using baseline measurements of comorbidities, laboratory values, Lawton Brody instrumental activities of daily living (LB-IADL), quality of life (EQ-5D), and 3 physical fitness tests. Patients who had ≤ 13 missing variables on the MDS-FI were included. Kaplan-Meier (KM) OS curves were calculated from treatment start date to death or last follow up. Univariable and multivariable analysis was done to identify significant predictors of OS, LFS and the receipt of ≥ 4 cycles of HMA.
Results:
There were 422 patients treated with an HMA (94% AZA). FI scores could be calculated in 188 patients and CFS in 169 patients. Among the 188 patients, the median age at HMA start was 73 years old (IQR 67, 79), time from diagnosis was 10 months (IQR 2, 28), 66% of patients were high/intermediate-2 risk IPSS, and 72% were high/very high risk IPSS-R. 40% were transfusion dependent, 30% had poor/very poor cytogenetics, and 10% had oligoblastic AML.
Median number of HMA cycles was 7 and 76% completed ≥ 4 cycles. The median follow up was 12 months (IQR 7, 25). 19% of patients developed AML. Actuarial median OS was 17 months (95% CI: 13-20) with 50% of deaths due to AML or progressive disease. The MDS-FI score was grouped into categories of 1, 2, and 3 (scores ≤0.2, 0.2-3, and >3, respectively), with a median score of 0.3 (IQR 0.2, 0.3). The median scores for the clinical frailty scale (CFS), Charlson comorbidity index (CCI), and MDS-specific comorbidity index (CI) were 3 (IQR 2, 4), 1 (IQR 0, 2), and 0 (IQR 0, 2) respectively. 21% of patients had cardiac comorbidity(s), 53% had ≥ 1 disability (LB-IADL), and 76% had ≥ 1 impaired symptoms or function on the EQ5D, the most common being usual activities (45%) and pain/discomfort (44%). On physical testing, 56%, 30% and 88% had partial or full deficits in grip strength, 4 meter walk and the 10x chair stand tests compared with age/sex matched reference standards.
Those who completed ≥ 4 cycles of AZA compared with those that did not were more likely to be younger (73 vs 78 years old, p=0.002), have lower risk disease (IPSS-R very low/low/intermediate: 29 versus 13%, p=0.044), have lower comorbidity (MDS-CI score: 0 vs 1, p=0.006), lower frailty scores (CFS: 2 versus 3, p=0.008) and performed better on grip strength (31 vs 26 kg, p=0.021) and the 10x chair stand test (28 vs 30s, p=0.045).
Predictive factors from univariate analysis are presented on Table 1. There was a trend towards receiving fewer HMA cycles if patients fell within higher FI categories (8 vs 7 vs 5 cycles, p=NS).
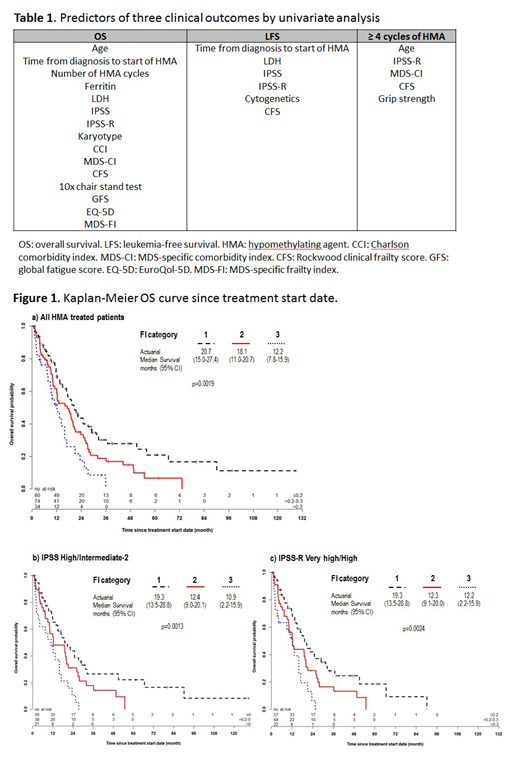
OS declined with increasing FI categories (p=0.002, Fig 1a). In subgroup analysis by IPSS or IPSS-R score, the FI further stratified the OS of patients with IPSS high/intermediate-2 (p=0.001, Fig 1b) and IPSS-R very high/high risk groups (p=0.002, Fig 1c).
The best multivariate model for OS included IPSS (p=0.001), LDH (p=0.001), and MDS-CI (p<0.001). Multivariate predictors of LFS included LDH (p=0.024) and IPSS (p=0.042). IPSS-R (p=0.011), MDS-CI (p=0.007) and grip strength (p=0.007) were independent predictors of receiving ≥4 cycles of HMA.
Conclusions:
Frailty and comorbidity provide important prognostic information for clinical outcomes in MDS patients receiving hypomethylating agents. The evaluation of patient characteristics in addition to disease parameters should be an integral part of clinical decision-making.
Wells:Alexion: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Rockwood:Alzheimer Society of Canada: Research Funding; Lundbeck: Membership on an entity's Board of Directors or advisory committees; Canadian consortium on neurodegeneration in aging and nutricia: Membership on an entity's Board of Directors or advisory committees; Foundation Family Fund: Research Funding; Pfizer: Research Funding; Capital Health research support: Research Funding; Sanofi: Research Funding; CIHR: Research Funding; Nova Scotia Health research foundation: Research Funding. Geddes:Celgene: Honoraria, Research Funding; Alexion: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Sabloff:Actinium Pharmaceuticals, Inc: Membership on an entity's Board of Directors or advisory committees; Sanofi Canada: Research Funding; Astellas Pharma Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; ASTX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Keating:Sanofi: Membership on an entity's Board of Directors or advisory committees; Hoffman La Roche: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy; Novartis: Honoraria; Celgene: Membership on an entity's Board of Directors or advisory committees. Leber:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Leitch:Celgene Corporation: Honoraria, Research Funding; Otsuka: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; Alexion: Research Funding; AbbVie: Research Funding. Yee:Takeda: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Research Funding; Hoffman La Roche: Research Funding; MedImmune: Research Funding. St-Hilaire:Teva: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Finn:Sanofi: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Ipsen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Lundbeck: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Research Funding; Alexion: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Storring:Novartis: Honoraria, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees. Nevill:Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Paladin Labs: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Shamy:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Abbie: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees. Banerji:Roche: Honoraria, Licensing fee, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; LLSC: Research Funding; Research Manitoba: Research Funding; CCMF: Research Funding; CancerCare Manitoba/University of Manitoba: Employment; CAPhO: Honoraria; BIOGEN: Other: Licensing fee; Dana-Farber Cancer Institute: Other: Licencing fee; Abbvie: Consultancy, Honoraria; CIHR: Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Astra-Zeneca: Consultancy, Honoraria. Delage:Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Buckstein:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal