Abstract
Background: TAK-981 is the first small-molecule inhibitor of SUMOylation to enter clinical trials. SUMOylation is a post-translational modification in which small ubiquitin-like modifier (SUMO) proteins are activated and covalently attached to substrate proteins. SUMOylation has a central role in constraining type I interferon (IFN-I)-dependent responses (Decque Nat Immunol 2016). By blocking SUMOylation, TAK-981 promotes IFN-I production and increases innate immunity. The ability of TAK-981 to promote activation of macrophages and NK cells and increase their cytotoxic/phagocytic activity provides a mechanistic rationale for its use in combination with monoclonal antibodies (mAbs) reliant on antibody-dependent cellular cytotoxicity and phagocytosis. Preclinical studies have demonstrated synergistic antitumor activity between TAK-981 and the anti-CD20 monoclonal antibody rituximab in xenograft models of human B cell lymphoma (Nakamura AACR 2019). Based on these data, and a single-agent TAK-981 study (TAK-981-1002), this phase 1b/2, open-label, dose-escalation and expansion study is investigating the safety and efficacy of TAK-981 plus rituximab in adults with CD20-positive R/R NHL (NCT04074330); here, we report data from the phase 1b dose-escalation part of the study.
Methods: Eligible pts were aged ≥18 years with CD20-positive, R/R aggressive B-cell NHL (aNHL) or indolent NHL (iNHL). aNHL included diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and grade 3b follicular lymphoma (FL); iNHL included grade 1-3a FL and marginal zone lymphoma. aNHL pts had to have received prior R-CHOP or equivalent plus 1 additional line of therapy in the R/R setting; iNHL pts had to be refractory to rituximab or another anti-CD20 mAb, and to have received at least 1 prior therapy for R/R disease. TAK-981 IV was given at increasing doses (starting: 10 mg) on days 1 and 8 (QW) or days 1, 4, 8, and 11 (BIW) of 21-day cycles. Rituximab 375 mg/m 2 IV was given on days 1, 8, and 15 of cycle 1 and on day 1 thereafter. Dose escalation was based on adaptive Bayesian logistic regression modelling with overdose control based on the posterior probability of having a dose-limiting toxicity (DLT). Primary phase 1b objectives were safety, tolerability, and recommended phase 2 dose (RP2D) of TAK-981 in combination with rituximab; data cutoff was 28 June 2021.
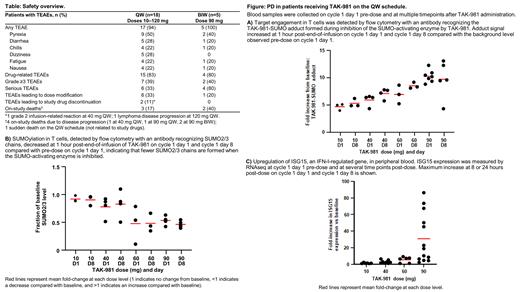
Results: 24 pts have been enrolled and treated: 19 QW (10-120 mg) and 5 BIW (90 mg); 4 are currently on treatment. Enrollment continues in the 90 mg BIW and 120 mg QW cohorts. Median age was 65 years (range 29-80); 67% were male. No DLTs have been reported to date. RP2D and schedule will be determined based on safety, pharmacokinetic (PK), and pharmacodynamic (PD) data. Treatment-emergent adverse events (TEAEs) are shown in the Table. Most common TEAEs (≥15%) were similar between the QW/BIW schedules with the exception of dizziness (2 pts at 10 mg, 2 at 40 mg, and 1 at 90 mg) and hypokalemia (1 pt at 40 mg, 2 at 90 mg, and 1 at 120 mg); all QW. TEAEs were consistent with induction of IFN signalling (transient flu-like symptoms: fever, chills, fatigue) and with those observed in the single-agent study (data on file); no further TAK-981- or immune-related TEAEs were observed. Grade ≥3 TEAEs related to TAK-981 were reported in 2 pts: grade 3 atrial fibrillation (ongoing cardiac history) and grade 4 neutropenia; both in the 40 mg QW cohort and transient. In this population of rituximab-refractory pts, there were 5 objective responses in 17 response-evaluable pts: 4 partial responses (2 at 10 mg, FL and DLBCL; 1 at 60 mg, DLBCL; 1 at 90 mg, primary mediastinal thymic large B-cell lymphoma) and 1 complete response (40 mg, MCL), all in the QW cohort. TAK-981 PK was linear and declined in a tri-phasic manner. TAK-981 exhibited PD activity in peripheral blood including target engagement, decreased SUMOylation, and increased IFN-regulated gene expression (Figure).
Conclusion: The combination of TAK-981 and rituximab was well tolerated in pts across each dose level and schedule. TAK-981 PK showed minimal accumulation after repeat dosing and PD assays confirmed inhibition of SUMOylation and activation of IFN-I signalling. More importantly, the combination of TAK-981 and rituximab resulted in promising clinical activity (ORR 29%) in the R/R setting, supporting the continued development of this combination in pts with NHL.
Assouline: Johnson&Johnson: Current equity holder in publicly-traded company; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; Takeda: Research Funding; Roche/Genentech: Research Funding; Eli Lilly: Research Funding; Novartis: Honoraria, Research Funding; Amgen: Current equity holder in publicly-traded company, Research Funding; Gilead: Speakers Bureau; Jewish General Hospital, Montreal, Quebec: Current Employment. Mehta: Affirmed; Kite/Gilead; Roche-Genetech; Celgene/BMS; Oncotartis; Innate Pharmaceuticals; Seattle Genetics; Incyte; Takeda; Fortyseven Inc/Gilead; TG Therapeutics; Merck; Juno Pharmaceuticals/Bristol Myers Squibb: Research Funding; Seattle Genetics; Incyte; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics; Incyte; TG Therapeutics: Consultancy. Phillips: Bayer: Consultancy, Research Funding; ADCT, BeiGene, Bristol Myers Squibb, Cardinal Health, Incyte, Karyopharm, Morphosys, Pharmacyclics, Seattle Genetics: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Other: received travel expenses from Incyte, Research Funding. Danilov: Rigel Pharm: Honoraria; Bristol-Meyers-Squibb: Honoraria, Research Funding; Gilead Sciences: Research Funding; Pharmacyclics: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; TG Therapeutics: Consultancy, Research Funding; Takeda Oncology: Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding; SecuraBio: Research Funding; Bayer Oncology: Consultancy, Honoraria, Research Funding. Doucet: Amgen: Consultancy; Novartis: Consultancy; Astra-Zeneca: Consultancy; BMS: Consultancy; Jannsen: Consultancy; Servier: Consultancy; Abbvie: Consultancy; Seattle Genetics: Consultancy; Roche: Consultancy; Gilead: Consultancy. Park: Morphosys: Membership on an entity's Board of Directors or advisory committees; G1 Therapeutics: Consultancy; Gilead: Speakers Bureau; Takeda: Research Funding; Rafael Pharma: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Seattle Genetics: Research Funding, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Teva: Consultancy, Membership on an entity's Board of Directors or advisory committees. Berg: Takeda: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Gomez-Pinillos: Takeda: Current Employment. Martinez: Takeda: Current Employment. Chao: Takeda: Current Employment. Berger: Takeda Development Center Americas, Inc.: Current Employment. Gibbs: Takeda: Current Employment. Friedlander: Takeda: Current Employment. Ward: Takeda: Current Employment. Proscurshim: Takeda Pharmaceuticals: Current Employment, Current holder of individual stocks in a privately-held company. Caimi: TG Therapeutics: Consultancy; Kite: Consultancy; ADC Therapeutics: Consultancy; Verastem: Consultancy; Amgen: Consultancy; XaTek Inc.: Patents & Royalties; Celgene: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal