Key Points
In patients with FLT3mut+ AML unfit for intensive chemotherapy, CRc rate was higher with GIL + AZA than with AZA.
OS was not significantly different between GIL + AZA and AZA in patients with FLT3mut+ AML unfit for intensive chemotherapy.
Abstract
Treatment results for patients with newly diagnosed FMS-like tyrosine kinase 3 (FLT3)-mutated (FLT3mut+) acute myeloid leukemia (AML) ineligible for intensive chemotherapy are disappointing. This multicenter, open-label, phase 3 trial randomized (2:1) untreated adults with FLT3mut+ AML ineligible for intensive induction chemotherapy to receive gilteritinib (120 mg/d orally) and azacitidine (GIL + AZA) or azacitidine (AZA) alone. The primary end point was overall survival (OS). At the interim analysis (August 26, 2020), a total of 123 patients were randomized to treatment (GIL + AZA, n = 74; AZA, n = 49). Subsequent AML therapy, including FLT3 inhibitors, was received by 20.3% (GIL + AZA) and 44.9% (AZA) of patients. Median OS was 9.82 (GIL + AZA) and 8.87 (AZA) months (hazard ratio, 0.916; 95% CI, 0.529-1.585; P = .753). The study was closed based on the protocol-specified boundary for futility. Median event-free survival was 0.03 month in both arms. Event-free survival defined by using composite complete remission (CRc) was 4.53 months for GIL + AZA and 0.03 month for AZA (hazard ratio, 0.686; 95% CI, 0.433-1.087; P = .156). CRc rates were 58.1% (GIL + AZA) and 26.5% (AZA) (difference, 31.4%; 95% CI, 13.1-49.7; P < .001). Adverse event (AE) rates were similar for GIL + AZA (100%) and AZA (95.7%); grade ≥3 AEs were 95.9% and 89.4%, respectively. Common AEs with GIL + AZA included pyrexia (47.9%) and diarrhea (38.4%). Gilteritinib steady-state trough concentrations did not differ between GIL + AZA and gilteritinib. GIL + AZA resulted in significantly higher CRc rates, although similar OS compared with AZA. Results support the safety/tolerability and clinical activity of upfront therapy with GIL + AZA in older/unfit patients with FLT3mut+ AML. This trial was registered at www.clinicaltrials.gov as #NCT02752035.
Introduction
Patients with newly diagnosed (ND) FMS-like tyrosine kinase 3 (FLT3)-mutated (FLT3mut+) acute myeloid leukemia (AML) who may be considered unfit for standard intensive induction chemotherapy (IIC) have a poor prognosis characterized by worse survival or treatment response compared with their counterparts without advanced age, multiple comorbidities, or poor performance status; presence of FLT3 mutations is also associated with worse survival.1-5 Activated FLT3 with internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutations are present in up to 35% and 14% of AML cases, respectively.6 Frequencies of FLT3-ITD and FLT3-TKD mutations in older adults occur in up to 25% and 12% in patients with AML.7-10 In a study of age-dependent frequencies of select mutations, rates of FLT3-ITD were lower in older vs younger adults (ie, 30-39 years, 39.3%; 40-49 years, 30.8%; 50-59 years, 31.4%; 60-69 years, 25.2%; and ≥70 years, 19.5%), and FLT3-TKD mutations were not age dependent (ie, 30-39 years, 6.1%; 40-49 years, 11.0%; 50-59 years, 7.9%; 60-69 years, 5.2%; and ≥70 years, 3.7%).8
Although standard induction-style chemotherapy regimens in combination with midostaurin are recommended for ND FLT3mut+ AML in patients eligible for intensive chemotherapy, there is no universal standard chemotherapy for patients with ND FLT3-mutant AML considered unfit for IIC.11-13 Compared with conventional care regimens, azacitidine (AZA) prolonged overall survival (OS) in a phase 3 trial of patients aged ≥65 years with AML.14 Recently, AZA monotherapy exhibited a significantly higher risk of death vs AZA/venetoclax in untreated patients with AML ineligible for IIC.15 For patients with FLT3mut+ AML ineligible for intensive chemotherapy, combination FLT3 inhibitors with hypomethylating agents (AZA or decitabine) has shown synergistic cytotoxicity.16-18
Gilteritinib is a FLT3 inhibitor displaying efficacy/safety in patients with FLT3mut+ relapsed/refractory (R/R) AML.19,20 In a phase 3 trial of patients with R/R FLT3mut+ AML, gilteritinib monotherapy significantly prolonged median OS vs salvage chemotherapy (9.3 vs 5.6 months; hazard ratio [HR], 0.64 [95% confidence interval (CI), 0.49-0.83; P < .001]).19 Estimated survival rates after 2 years were 20.6% for gilteritinib monotherapy and 14.2% for chemotherapy.21 In preclinical studies, gilteritinib plus AZA (GIL + AZA) impeded cell growth and induced apoptosis and differentiation of FLT3-ITD AML cell lines.22 In xenografted mouse models, combining GIL + AZA appeared synergistic and exhibited superior antitumor efficacy vs gilteritinib or AZA.22
Based on favorable preclinical data, we investigated the efficacy and safety/tolerability of GIL + AZA vs AZA in adults with ND FLT3mut+ AML ineligible for IIC in LACEWING (A Phase 3 Multicenter, Open-label, Randomized Study of ASP2215 [Gilteritinib], Combination of ASP2215 Plus Azacitidine and Azacitidine Alone in the Treatment of Newly Diagnosed Acute Myeloid Leukemia With FLT3 Mutation in Patients Not Eligible for Intensive Induction Chemotherapy; #NCT02752035). An initial gilteritinib-only arm was removed, and patients were subsequently randomized to receive GIL + AZA and AZA because of protocol changes due to updates in preferred treatment approaches for this patient population. We present efficacy, safety, and pharmacokinetic (PK) data from an interim analysis.
Methods
Study design
The randomized, open-label, phase 3 LACEWING study compared the efficacy and safety of GIL + AZA vs AZA alone in patients with ND FLT3mut+ AML ineligible for IIC from ∼185 centers in North America, Europe, and Asia/Pacific. Patients aged ≥18 years previously untreated for AML, positive for a FLT3 mutation (ITD and/or TKD), and ineligible for IIC were included. FLT3 mutation status was determined by LeukoStrat CDx FLT3 Mutation Assay (Versiti, Inc.) on blood or bone marrow aspirate samples in a central laboratory (supplemental Text, available on the Blood Web site). The assay measures the ratio of mutant FLT3 to wild-type FLT3 and uses a cutoff point of ≥0.05 for positivity. Patients aged ≥65 years were deemed ineligible for IIC per investigator, as were patients any age with selected comorbidities (supplemental Text), including heart failure (New York Heart Association functional class I-III), Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥2, and malignancies not requiring concurrent treatment. Patients with acute promyelocytic leukemia, BCR-ABL–positive leukemia, clinically active central nervous system leukemia, or major surgery/radiation therapy 4 weeks before the first study dose were excluded.
Before the randomized trial, 8 to 12 patients were planned for enrollment in a safety cohort to evaluate the safety/tolerability of combination GIL + AZA in dose-escalation cohorts of gilteritinib from 80 to 120 mg. Patients were initially treated with gilteritinib 80 mg/d (days 1-28; dose reductions/increases permitted after cycle 1) and AZA 75 mg/m2 per day (days 1-7). Safety data were analyzed during the dose-limiting toxicity (DLT) observation period (days 1-28 of cycle 1). Evaluable patients experienced a DLT or, in the absence of a DLT, received ≥23 of 28 doses of gilteritinib and at least 5 of 7 doses of AZA. Patients not evaluable for reasons other than DLT were replaced. The gilteritinib dose of 120 mg/d for the randomized trial was determined based on safety cohort data.
Randomization and treatments
Patients were initially randomized (1:1:1) to receive GIL + AZA (gilteritinib 120 mg/d orally and AZA 75 mg/m2 per day subcutaneously or IV on days 1-7), AZA 75 mg/m2 per day subcutaneously/IV on days 1 to 7, or gilteritinib 120 mg/d orally. The gilteritinib arm was removed ∼12 months (September 5, 2018) after recruitment began due to changes in preferred treatment for this patient population (ie, combination over monotherapy). AZA administration on days 8 and/or 9 was permitted according to local practice (ie, interruptions permitted ≤2 days at a time for weekends/nonworking days). Patients were subsequently randomized (2:1) to receive GIL + AZA or AZA; both regimens were administered on a 28-day cycle. Dose interruptions, reductions, and escalations were permitted per prespecified criteria (supplemental Text). Randomization was stratified based on age group (<75 years or ≥75 years) and performed via Interactive Response Technology. Patients continued treatment until they no longer received clinical benefit per investigator assessment, unacceptable toxicity, or other prespecified treatment discontinuation criteria were met (supplemental Table 1). Patients achieving response were permitted to undergo hematopoietic stem cell transplantation (HSCT) at any time per physician discretion. Continuation of gilteritinib (GIL + AZA group) after HSCT was permitted if prespecified conditions were met; continuation of AZA in either group post-HSCT was not permitted during the trial.
End points and assessments
The primary end point was OS. The key secondary efficacy end point was event-free survival (EFS), defined as time from the randomization date until date of documented relapse from complete remission (CR), treatment failure (failure to achieve CR within 6 treatment cycles), or death from any cause, whichever occurred first. Treatment failure date was randomization date if CR was not achieved after 6 cycles, if study treatment was permanently discontinued without achieving CR before completing 6 cycles, or if the patient had no postbaseline disease assessment. Secondary end points included response rates, transfusion conversion rate, remission duration, and safety/tolerability. PK analyses of gilteritinib (alone and combined with AZA) were conducted as exploratory outcomes. Additional secondary/exploratory end points were assessed but not reported herein.
Treatment response was defined per modified Cheson criteria.23 Safety was assessed by evaluating adverse events (AEs), including vital sign assessment, clinical laboratory testing, physical examination, and electrocardiogram. AE severity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. For PK assessment of gilteritinib (including calculation of trough concentrations at steady state [Ctrough]), predose plasma samples (GIL + AZA and gilteritinib groups) were collected at cycle 1 (days 1, 8, and 15) and subsequent cycles on day 1; the samples were analyzed by using a liquid chromatography with tandem mass spectrometry assay method, as previously described.24 Data on survival status, subsequent antileukemia treatments (including HSCT), and outcomes were collected for patients not withdrawing consent during long-term follow-up.
Analysis of comutations was performed by next-generation sequencing as described previously.19 Patients were categorized as having a RAS/MAPK pathway mutation if they had a mutation detected in any of the following: BRAF, CBL, KRAS, NRAS, or PTPN11.
Statistical analysis
The planned sample size of 250 patients, randomized 2:1 to receive GIL + AZA or AZA alone, would provide ≥80% power to detect a difference in OS between treatments, assuming a median survival of 16.7 months for GIL + AZA and 10 months for AZA at an overall two-sided significance level of 0.05.
This study used a group-sequential design based on OS using O’Brien-Fleming boundaries as implemented by the Lan-DeMets α/β spending method; interim and final analyses were planned. Interim analysis was planned when ∼50% (70 deaths) of the planned total number of all-cause deaths (140 deaths) occurred. Final analysis for OS was to be performed after 140 deaths occurred at a two-sided 0.049 significance level.
An independent data monitoring committee continuously assessed safety, made recommendations to the study sponsor regarding trial continuation, conducted the interim analysis based on OS, and informed the study sponsor of results. Boundary for efficacy and futility stopping was based on two-sided P values of <.003 and ≥.724, respectively.
Time-to-event end points (OS, EFS, and remission duration) were summarized by using Kaplan-Meier estimates; 95% CIs and comparisons were based on log-rank tests. Response was summarized with frequency counts and percentages, with exact 95% CIs based on binomial distribution; comparisons were based on the Cochran-Mantel-Haenszel test. Stratification factors for OS and EFS were age (<75 years vs ≥75 years), risk group (favorable/intermediate cytogenetic risk vs unfavorable cytogenetic risk/secondary AML), and FLT3 mutation status (FLT3-TKD vs FLT3-ITD low allelic ratio [<0.5] vs FLT3-ITD high allelic ratio [≥0.5]). The stratification factor for other end points was age (<75 years vs ≥75 years). Allelic ratio was determined by using the LeukoStrat CDx FLT3 Mutation Assay and calculated as ratio of peak area of FLT3-ITD mutant signal, if present, divided by peak area of FLT3 wild-type signal, if present. Prespecified sensitivity testing was performed for OS and EFS. Subgroup analyses (unstratified) were planned for OS and EFS for age group, sex, baseline ECOG PS, race, cytogenetic risk, FLT3 mutation status, and region.
All randomized patients (intention-to-treat population) were included in the efficacy analysis. All patients receiving ≥1 dose of study treatment were included in the safety analysis. All patients receiving ≥1 dose of study treatment, who had ≥1 plasma concentration data point available, and for whom time of dosing on day of sample was known were included in the PK analysis. PK parameters were estimated by using standard noncompartmental analysis.
Trial oversight
Astellas Pharma designed the trial. Data were collected by study investigators, analyzed by biostatisticians employed by Astellas Pharma US, and interpreted by authors. Institutional review board or independent ethics committee approval was obtained at all sites. The study was conducted in accordance with International Council for Harmonisation guidelines for Good Clinical Practice and Declaration of Helsinki and applicable regulations/guidelines governing clinical study conduct and ethical principles. Written informed consent was obtained from each patient before study entry. Authors had access to data used for manuscript preparation and, with writing/editorial support funded by the trial sponsor, developed and approved the manuscript.
Results
Safety cohort
Fifteen patients were enrolled and received GIL + AZA (supplemental Figure 1). One of 9 patients who received gilteritinib 80 mg/d experienced a DLT (determined related to treatment for tumor lysis syndrome); no patients receiving gilteritinib 120 mg/d (n = 6) experienced a DLT. Based on safety cohort data, the study sponsor, in consultation with investigators, decided to initiate the randomized trial at the targeted dose (gilteritinib 120 mg) alone and with AZA.
Patient demographic characteristics and disposition
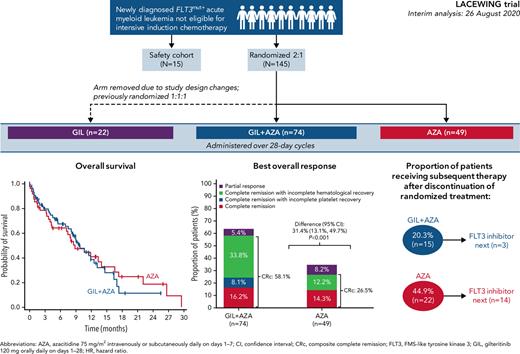
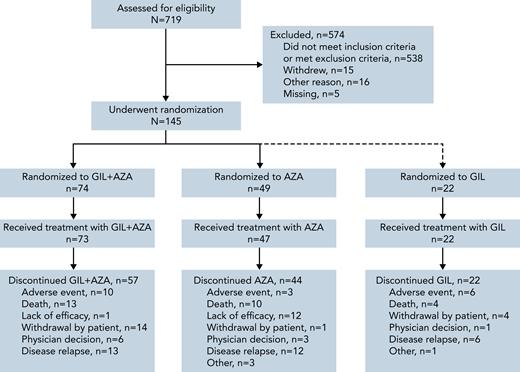
As of August 26, 2020, a total of 123 patients were randomized to receive GIL + AZA (n = 74) and AZA (n = 49) (Figure 1); 39 (52.7%) and 31 (63.3%) deaths occurred in each arm, respectively. Patients randomized to gilteritinib alone under earlier versions of the protocol continued to receive treatment and assessments per protocol; data from these patients are included, where relevant. The accrual period for randomized patients (N = 145, including 22 patients randomized to receive gilteritinib alone) was December 4, 2017, to August 6, 2020. Median age was 78 years with GIL + AZA and 76 years with AZA; ECOG PS ≥2 was observed in 47.3% and 32.7% of patients. FLT3-ITD was the only FLT3 mutation present in 78.4% and 81.6% of patients receiving GIL + AZA and AZA (Table 1); FLT3-TKD alone was present in 18.9% and 14.3%, and both ITD and TKD mutations were present in 2.7% and 4.1% of patients treated with GIL + AZA and AZA. A total of 4.1% (n = 3) and 12.2% (n = 6) of patients had secondary AML in the 2 groups. Data for gilteritinib alone are shown in supplemental Table 2. Median exposure duration was 112 days for gilteritinib in the GIL + AZA arm (n = 73); AZA exposure was 98 and 99 days for the GIL + AZA and AZA (n = 47) arms. Subsequent AML therapy (including clinical trial participation) was received by 20.3% (n = 15) of patients on GIL + AZA and 44.9% (n = 22) on AZA (supplemental Table 3); median time to first subsequent therapy was 8.2 and 4.5 months for the 2 arms. Hypomethylating agents (monotherapy or combined therapy) were subsequently used in 8 regimens for patients who received GIL + AZA (n = 7) and 9 regimens for patients who received AZA (n = 9). In the AZA arm, 14 patients initiated subsequent treatment with a FLT3 inhibitor; 10 initiated off-study gilteritinib. Most common reasons for discontinuation of GIL + AZA and AZA were relapse (17.6% and 24.5%), death (17.6% and 20.4%), lack of efficacy (1.4% and 24.5%), withdrawal by patient (18.9% and 2.0%), and AEs (13.5% and 6.1%). Thirty-day and 60-day mortality, from time of randomization, was similar between arms (6 patients per arm at 30 days and 10 patients on GIL + AZA vs 9 patients on AZA at 60 days).
Patient demographic and disease characteristics
| Parameter . | GIL + AZA (n = 74) . | AZA (n = 49) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 42 (56.8) | 28 (57.1) |
| Female | 32 (43.2) | 21 (42.9) |
| Race, n (%) | ||
| White | 46 (62.2) | 29 (59.2) |
| Asian | 20 (27.0) | 13 (26.5) |
| Age, y | ||
| Mean ± standard deviation | 77.4 ± 5.6 | 76.7 ± 5.3 |
| Median (minimum, maximum) | 78.0 (59, 90) | 76.0 (61, 88) |
| Age group, n (%)∗ | ||
| <75 y | 21 (28.4) | 13 (26.5) |
| ≥75 y | 53 (71.6) | 36 (73.5) |
| Body surface area, m2 | ||
| n | 73 | 47 |
| Mean ± standard deviation | 1.75 ± 0.22 | 1.75 ± 0.25 |
| Median (minimum, maximum) | 1.72 (1.4, 2.7) | 1.72 (1.2, 2.5) |
| Baseline ECOG PS, n (%) | ||
| 0-1 | 38 (51.4) | 32 (65.3) |
| ≥2 | 35 (47.3) | 16 (32.7) |
| Missing | 1 (1.4) | 1 (2.0) |
| Baseline FLT3 mutation type, n (%) | ||
| ITD alone | 58 (78.4) | 40 (81.6) |
| TKD (D835/I836) alone | 14 (18.9) | 7 (14.3) |
| ITD with TKD (D835/I836) | 2 (2.7) | 2 (4.1) |
| Baseline FLT3 mutation status, n (%)∗ | ||
| ITD allelic ratio < 0.5 | 25 (33.8) | 18 (36.7) |
| ITD allelic ratio ≥ 0.5 | 35 (47.3) | 24 (49.0) |
| TKD | 14 (18.9) | 7 (14.3) |
| Cytogenetic risk status, n (%)∗,† | ||
| Favorable | 2 (2.7) | 0 |
| Intermediate | 51 (68.9) | 36 (73.5) |
| Unfavorable | 8 (10.8) | 5 (10.2) |
| Others (unknown, missing) | 13 (17.6) | 8 (16.3) |
| Parameter . | GIL + AZA (n = 74) . | AZA (n = 49) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 42 (56.8) | 28 (57.1) |
| Female | 32 (43.2) | 21 (42.9) |
| Race, n (%) | ||
| White | 46 (62.2) | 29 (59.2) |
| Asian | 20 (27.0) | 13 (26.5) |
| Age, y | ||
| Mean ± standard deviation | 77.4 ± 5.6 | 76.7 ± 5.3 |
| Median (minimum, maximum) | 78.0 (59, 90) | 76.0 (61, 88) |
| Age group, n (%)∗ | ||
| <75 y | 21 (28.4) | 13 (26.5) |
| ≥75 y | 53 (71.6) | 36 (73.5) |
| Body surface area, m2 | ||
| n | 73 | 47 |
| Mean ± standard deviation | 1.75 ± 0.22 | 1.75 ± 0.25 |
| Median (minimum, maximum) | 1.72 (1.4, 2.7) | 1.72 (1.2, 2.5) |
| Baseline ECOG PS, n (%) | ||
| 0-1 | 38 (51.4) | 32 (65.3) |
| ≥2 | 35 (47.3) | 16 (32.7) |
| Missing | 1 (1.4) | 1 (2.0) |
| Baseline FLT3 mutation type, n (%) | ||
| ITD alone | 58 (78.4) | 40 (81.6) |
| TKD (D835/I836) alone | 14 (18.9) | 7 (14.3) |
| ITD with TKD (D835/I836) | 2 (2.7) | 2 (4.1) |
| Baseline FLT3 mutation status, n (%)∗ | ||
| ITD allelic ratio < 0.5 | 25 (33.8) | 18 (36.7) |
| ITD allelic ratio ≥ 0.5 | 35 (47.3) | 24 (49.0) |
| TKD | 14 (18.9) | 7 (14.3) |
| Cytogenetic risk status, n (%)∗,† | ||
| Favorable | 2 (2.7) | 0 |
| Intermediate | 51 (68.9) | 36 (73.5) |
| Unfavorable | 8 (10.8) | 5 (10.2) |
| Others (unknown, missing) | 13 (17.6) | 8 (16.3) |
Unknown category includes missing and data not allowed to be collected.
Stratification factors for analysis.
Patients may have reported more than one cytogenetic risk status.
Overall survival
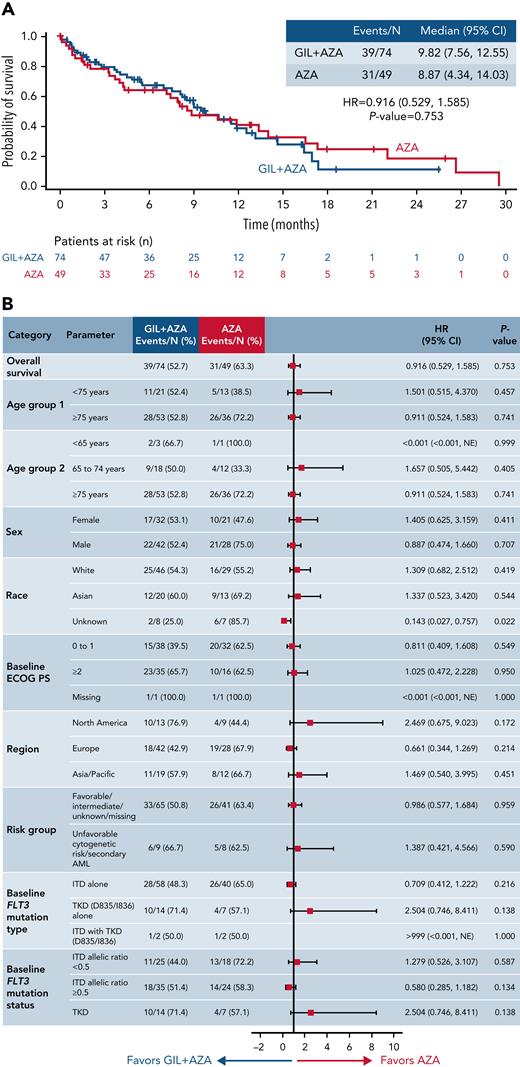
After a median follow up of 9.76 months for GIL + AZA and 17.97 months for AZA, median OS was 9.82 and 8.87 months, respectively (HR, 0.916; 95% CI, 0.529-1.585; P = .753) (Figure 2A). Based on recommendations from the independent data monitoring committee meeting in December 2020 to stop the study for futility based on protocol-specified boundary for futility, the study sponsor terminated enrollment. Subgroups favoring GIL + AZA vs AZA for median OS were ECOG PS 0 to 1 (13.17 months vs 11.89 months; HR, 0.811 [95% CI, 0.409-1.608]) and FLT3-ITD allelic ratio ≥0.5 (10.68 months vs 4.34 months; HR, 0.580 [95% CI, 0.285-1.182]) (Figure 2B). AZA was favored over GIL + AZA for median OS in patients with FLT3-TKD (11.89 months vs 4.86 months; HR, 2.504 [95% CI, 0.746-8.411]), although caution with interpretation is warranted due to small sample sizes and wide CIs for risk of death. Kaplan-Meier survival estimates according to baseline FLT3 mutation status are shown in supplemental Figure 2. Factors contributing to OS findings were analyzed; they included confounding effects from subsequent AML therapy (Figure 3), higher proportions of patients with baseline ECOG PS ≥2 in the GIL + AZA arm, differences in follow-up duration due to randomization ratio change from 1:1 to 2:1 to receive GIL + AZA or AZA, and the timing of OS analysis after early termination of study enrollment. Data for gilteritinib alone are provided in the supplemental Text.
OS. (A) Kaplan-Meier estimates show the primary end point of OS for the intention-to-treat population. Tick marks indicate censored data. (B) Forest plot for OS in prespecified subgroups. Data represent unstratified analyses. NE, not estimable.
OS. (A) Kaplan-Meier estimates show the primary end point of OS for the intention-to-treat population. Tick marks indicate censored data. (B) Forest plot for OS in prespecified subgroups. Data represent unstratified analyses. NE, not estimable.
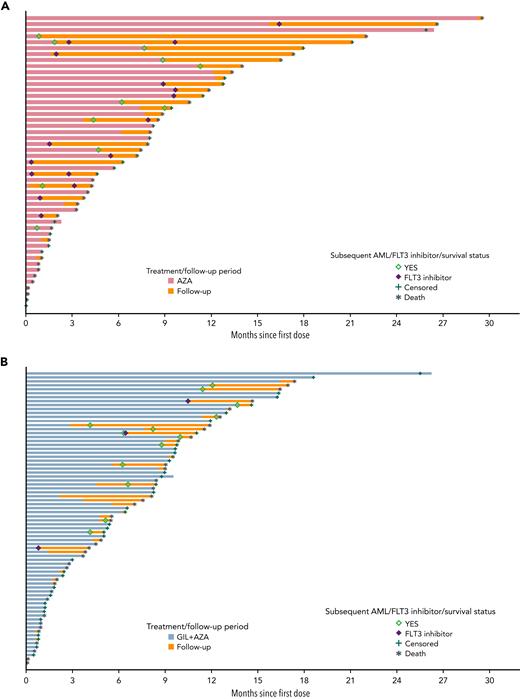
Survival status, follow-up, and use of FLT3 inhibitors by individual patients among treatments. Follow-up, including survival status and treatment and follow-up periods, is shown for individual patients in the (A) AZA alone and (B) GIL + AZA groups who received subsequent AML therapy with FLT3 inhibitors.
Survival status, follow-up, and use of FLT3 inhibitors by individual patients among treatments. Follow-up, including survival status and treatment and follow-up periods, is shown for individual patients in the (A) AZA alone and (B) GIL + AZA groups who received subsequent AML therapy with FLT3 inhibitors.
To allow for adequate numbers, OS was also analyzed in subsets of patients with selected comutations at a data cutoff date of November 24, 2021. Of 125 patients receiving any gilteritinib (≥1 dose of gilteritinib in gilteritinib-alone or GIL + AZA) throughout the trial, 60 had comutated NPM1, 17 had NPM1 + DNMT3a comutations, and 27 had RAS/MAPK comutation at baseline. NPM1, NPM1 + DNMT3a, and RAS/MAPK comutations were present in 21, 5, and 3 patients in the AZA arm (n = 54), respectively. Numerically longer survival was observed with any gilteritinib (gilteritinib alone or GIL + AZA) vs AZA, particularly in patients with NPM1+DNMT3a comutations, similar to the ADMIRAL (A Phase 3 Open-Label, Multicenter, Randomized Study of ASP2215 Versus Salvage Chemotherapy in Patients With Relapsed or Refractory Acute Myeloid Leukemia [AML] With FLT3 Mutation) study in patients with R/R AML19; observations in patients with NPM1 + DNMT3a and RAS/MAPK comutations were limited by small sample sizes in the AZA arm (supplemental Table 4; supplemental Figure 3). Findings were not statistically significant.
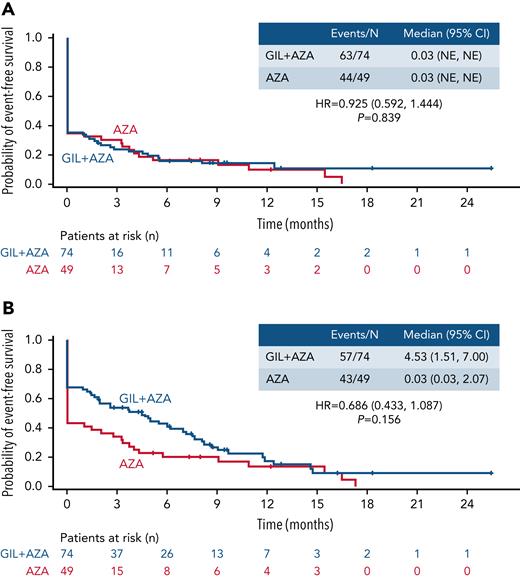
Event-free survival
A total of 107 events (GIL + AZA, n = 63; AZA, n = 44) occurred across the GIL + AZA and AZA arms. Median EFS (including relapse events from CR, treatment failure, and death) was 0.03 month in both arms (HR, 0.925; 95% CI, 0.592-1.444; P = .839) (Figure 4A). Sensitivity analysis of EFS, defined based on composite CR (CRc) (100 events across both arms), revealed a median EFS of 4.53 months for GIL + AZA and 0.03 month for AZA (HR, 0.686; 95% CI, 0.433-1.087; P =.156) (Figure 4B). Considering the potential impact on EFS of assigning randomization date as treatment failure date, a post hoc analysis of EFS without and with treatment failure defined at date of permanent discontinuation or end of 6 cycles of treatment was conducted (supplemental Figure 4).
EFS. (A) EFS based on CR, treatment failure, or all-cause death. (B) EFS based on CRc, treatment failure, or all-cause death. Tick marks indicate censored data. EFS is defined as the time from the date of randomization until the date of documented relapse from CR (panel A) or CRc (panel B), treatment failure (failure to achieve CR within 6 cycles of treatment), or all-cause death, whichever occurred first. NE, not estimable.
EFS. (A) EFS based on CR, treatment failure, or all-cause death. (B) EFS based on CRc, treatment failure, or all-cause death. Tick marks indicate censored data. EFS is defined as the time from the date of randomization until the date of documented relapse from CR (panel A) or CRc (panel B), treatment failure (failure to achieve CR within 6 cycles of treatment), or all-cause death, whichever occurred first. NE, not estimable.
Response rates
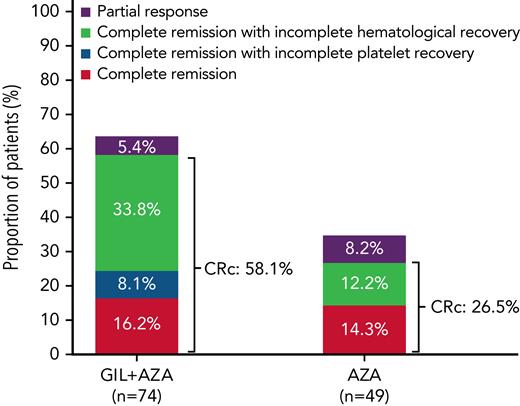
CR rates for GIL + AZA vs AZA were similar (16.2% vs 14.3%). Median time to CR was 116.5 days for GIL + AZA and 95.0 days for AZA; median time to response in patients who achieved CRc or partial response was 31.0 and 50.0 days, respectively. CRc rates were 58.1% for GIL + AZA and 26.5% for AZA (difference, 31.4%; 95% CI, 13.1-49.7; P < .001) (Figure 5). Subgroup analyses for CRc rates show that GIL + AZA was favored vs AZA in patients with an FLT3-ITD allelic ratio ≥0.5 (71.4% vs 20.8%; risk difference, –23.6% [95% CI, −37.3 to −9.9; P = .003]) (supplemental Figure 5). Median time to CRc was 57.0 days for both treatments. Additional response details are provided in Table 2. Transfusion conversion rates for patients with evaluable transfusion status postbaseline were 20.3% (n = 15) for GIL + AZA and 26.5% (n = 13) for AZA (difference, −6%; 95% CI, −23.0 to 10.4; P = .420). Median duration of CR had not been reached for the GIL + AZA group, with 10 of 12 patients remaining in CR. Median CR duration was 8.57 months for 7 patients in the AZA group who achieved CR. Median duration of CRc was 9.92 months (95% CI, 6.28-not estimable) and 9.23 months (95% CI, 0.95-14.09) in the GIL + AZA and AZA groups. Higher rates of CRc were observed in patients receiving any gilteritinib (gilteritinib alone or GIL + AZA) vs AZA for the following comutations: NPM1, NPM1 + DNMT3a, and RAS/MAPK (supplemental Table 5).
Comparison of CRc between treatment groups. A graphical depiction of the incidence of CRc in patients with ND FLT3mut+ AML ineligible for IIC who received GIL + AZA and AZA is shown.
Comparison of CRc between treatment groups. A graphical depiction of the incidence of CRc in patients with ND FLT3mut+ AML ineligible for IIC who received GIL + AZA and AZA is shown.
Summary of best overall response and response rates
| Parameter/variable rate . | GIL + AZA (n = 74) . | AZA (n = 49) . | Treatment difference (95% CI); P . |
|---|---|---|---|
| Response and composite response rate comparisons | |||
| CR | 12 (16.2) | 7 (14.3) | 2.0 (–12.5 to 16.5); .762 |
| CRh | 7 (9.5) | 1 (2.0) | 7.5 (–1.9 to 17.0); .100 |
| CR/CRh | 19 (25.7) | 8 (16.3) | 9.5 (–6.4 to 25.4); .212 |
| CRc∗ | 43 (58.1) | 13 (26.5) | 31.4 (13.1 to 49.7); <.001 |
| Response (CRc + PR) | 47 (63.5) | 17 (34.7) | 28.7 (9.7 to 47.6); .002 |
| Best overall response rate | |||
| CR | 12 (16.2) | 7 (14.3) | NA |
| CRp | 6 (8.1) | 0 | NA |
| CRi | 25 (33.8) | 6 (12.2) | NA |
| PR | 4 (5.4) | 4 (8.2) | NA |
| Not evaluable | 2 (2.7) | 1 (2.0) | NA |
| No response | 10 (13.5) | 17 (34.7) | NA |
| Parameter/variable rate . | GIL + AZA (n = 74) . | AZA (n = 49) . | Treatment difference (95% CI); P . |
|---|---|---|---|
| Response and composite response rate comparisons | |||
| CR | 12 (16.2) | 7 (14.3) | 2.0 (–12.5 to 16.5); .762 |
| CRh | 7 (9.5) | 1 (2.0) | 7.5 (–1.9 to 17.0); .100 |
| CR/CRh | 19 (25.7) | 8 (16.3) | 9.5 (–6.4 to 25.4); .212 |
| CRc∗ | 43 (58.1) | 13 (26.5) | 31.4 (13.1 to 49.7); <.001 |
| Response (CRc + PR) | 47 (63.5) | 17 (34.7) | 28.7 (9.7 to 47.6); .002 |
| Best overall response rate | |||
| CR | 12 (16.2) | 7 (14.3) | NA |
| CRp | 6 (8.1) | 0 | NA |
| CRi | 25 (33.8) | 6 (12.2) | NA |
| PR | 4 (5.4) | 4 (8.2) | NA |
| Not evaluable | 2 (2.7) | 1 (2.0) | NA |
| No response | 10 (13.5) | 17 (34.7) | NA |
Data are expressed as n (%).
CRh, complete remission with partial hematologic recovery; CRp, complete remission with incomplete platelet recovery; NA, not applicable; PR, partial response.
CRc is equal to CR + CRp + CRi.
Safety/tolerability
AE rates were 100% with GIL + AZA and 95.7% with AZA; grade ≥3 AEs occurred in 95.9% and 89.4% of patients, respectively (Table 3). Most common AEs occurring with GIL + AZA were pyrexia (47.9%), diarrhea (38.4%), febrile neutropenia (35.6%), constipation (34.2%), and nausea (32.9%); common AEs with AZA were pyrexia (34.0%), anemia (34.0%), neutropenia (27.7%), and thrombocytopenia (23.4%). The most common grade ≥3 AE was febrile neutropenia (35.6%) for GIL + AZA and anemia (27.7%) for AZA.
Events occurring in ≥20% of patients in either treatment group
| AE . | GIL + AZA (n = 73) . | AZA (n = 47) . | ||
|---|---|---|---|---|
| All grades . | Grade ≥ 3∗ . | All grades . | Grade ≥ 3∗ . | |
| Overall | 73 (100.0) | 70 (95.9) | 43 (91.5) | 42 (89.4) |
| Pyrexia | 35 (47.9) | 7 (9.6) | 16 (34.0) | 0 |
| Diarrhea | 28 (38.4) | 5 (6.8) | 8 (17.0) | 0 |
| Febrile neutropenia | 26 (35.6) | 26 (35.6) | 10 (21.3) | 9 (19.1) |
| Constipation | 25 (34.2) | 0 | 10 (21.3) | 0 |
| Nausea | 24 (32.9) | 1 (1.4) | 10 (21.3) | 1 (2.1) |
| Anemia | 23 (31.5) | 18 (24.7) | 16 (34.0) | 13 (27.7) |
| Thrombocytopenia | 22 (30.1) | 20 (27.4) | 11 (23.4) | 9 (19.1) |
| Pneumonia | 19 (26.0) | 15 (20.5) | 8 (17.0) | 8 (17.0) |
| Neutropenia | 18 (24.7) | 16 (21.9) | 13 (27.7) | 10 (21.3) |
| Aspartate aminotransferase increased | 18 (24.7) | 4 (5.5) | 3 (6.4) | 0 |
| Vomiting | 18 (24.7) | 2 (2.7) | 8 (17.0) | 0 |
| Asthenia | 17 (23.3) | 5 (6.8) | 7 (14.9) | 0 |
| Edema peripheral | 16 (21.9) | 0 | 5 (10.6) | 0 |
| Hypokalemia | 16 (21.9) | 6 (8.2) | 10 (21.3) | 4 (8.5) |
| Decreased appetite | 15 (20.5) | 3 (4.1) | 7 (14.9) | 1 (2.1) |
| Neutrophil count decreased | 14 (19.2) | 14 (19.2) | 4 (8.5) | 4 (8.5) |
| Platelet count decreased | 14 (19.2) | 13 (17.8) | 9 (19.1) | 9 (19.1) |
| Hyponatremia | 14 (19.2) | 9 (12.3) | 3 (6.4) | 1 (2.1) |
| Sepsis | 4 (5.5) | 4 (5.5) | 5 (10.6) | 5 (10.6) |
| AE . | GIL + AZA (n = 73) . | AZA (n = 47) . | ||
|---|---|---|---|---|
| All grades . | Grade ≥ 3∗ . | All grades . | Grade ≥ 3∗ . | |
| Overall | 73 (100.0) | 70 (95.9) | 43 (91.5) | 42 (89.4) |
| Pyrexia | 35 (47.9) | 7 (9.6) | 16 (34.0) | 0 |
| Diarrhea | 28 (38.4) | 5 (6.8) | 8 (17.0) | 0 |
| Febrile neutropenia | 26 (35.6) | 26 (35.6) | 10 (21.3) | 9 (19.1) |
| Constipation | 25 (34.2) | 0 | 10 (21.3) | 0 |
| Nausea | 24 (32.9) | 1 (1.4) | 10 (21.3) | 1 (2.1) |
| Anemia | 23 (31.5) | 18 (24.7) | 16 (34.0) | 13 (27.7) |
| Thrombocytopenia | 22 (30.1) | 20 (27.4) | 11 (23.4) | 9 (19.1) |
| Pneumonia | 19 (26.0) | 15 (20.5) | 8 (17.0) | 8 (17.0) |
| Neutropenia | 18 (24.7) | 16 (21.9) | 13 (27.7) | 10 (21.3) |
| Aspartate aminotransferase increased | 18 (24.7) | 4 (5.5) | 3 (6.4) | 0 |
| Vomiting | 18 (24.7) | 2 (2.7) | 8 (17.0) | 0 |
| Asthenia | 17 (23.3) | 5 (6.8) | 7 (14.9) | 0 |
| Edema peripheral | 16 (21.9) | 0 | 5 (10.6) | 0 |
| Hypokalemia | 16 (21.9) | 6 (8.2) | 10 (21.3) | 4 (8.5) |
| Decreased appetite | 15 (20.5) | 3 (4.1) | 7 (14.9) | 1 (2.1) |
| Neutrophil count decreased | 14 (19.2) | 14 (19.2) | 4 (8.5) | 4 (8.5) |
| Platelet count decreased | 14 (19.2) | 13 (17.8) | 9 (19.1) | 9 (19.1) |
| Hyponatremia | 14 (19.2) | 9 (12.3) | 3 (6.4) | 1 (2.1) |
| Sepsis | 4 (5.5) | 4 (5.5) | 5 (10.6) | 5 (10.6) |
Including AEs of grade ≥3 severity occurring in at least 10% of patients in either treatment group.
Serious AEs (SAEs) were reported by 87.7% of patients receiving GIL + AZA and 63.8% receiving AZA (supplemental Table 6). Treatment-related SAEs were reported in 61.6% and 25.5% of patients, respectively. When analyzing treatment-related SAEs according to individual therapy, AZA-related AEs occurred in 47.9% (n = 35) of GIL + AZA–treated patients and 25.5% (n = 12) of AZA-treated patients; gilteritinib-related AEs occurred in 53.4% (n = 39) on combination therapy (supplemental Table 7). Treatment-related AEs adjusted for treatment exposure were also higher with GIL + AZA vs AZA. SAEs occurring in ≥20% of patients included febrile neutropenia (GIL + AZA, 35.6%; AZA, 21.3%) and pneumonia (GIL + AZA, 20.5%; AZA, 17.0%). AEs led to death in 19 (26.0%) patients receiving GIL + AZA and 11 (23.4%) receiving AZA. AEs occurring in >1 patient included pneumonia (n = 5), sepsis (n = 4), cardiac arrest (n = 3), and Clostridium difficile, AML, and pulmonary hemorrhage (n = 2, each). AEs leading to death were considered treatment related in 4 patients in each group.
Gastrointestinal hemorrhage, an AE of special interest identified during animal studies, occurred in 12.3% (n = 9) and 6.4% (n = 3) of patients in the GIL + AZA and AZA groups, respectively. In the GIL + AZA arm, 9 patients had 15 gastrointestinal hemorrhage treatment-emergent AEs; 10 events resolved, 11 were not considered related to gilteritinib, and 13 were not considered related to AZA. Several patients had gastrointestinal hemorrhage treatment-emergent AEs that occurred in proximity to their subsequent death (n = 3) and treatment discontinuation (n = 2). Six patients continued receiving gilteritinib and 8 patients continued receiving AZA without dose interruption or modification. Grade ≥3 treatment-related gastrointestinal hemorrhage occurred in 5.5% (n = 4) of patients receiving GIL + AZA and in none receiving AZA. None of the gastrointestinal hemorrhage AEs was fatal. Another AE of special interest, QT prolongation, occurred in 13.7% (grade ≥3, 5.5% [n = 4]) of patients receiving GIL + AZA and did not occur in patients receiving AZA.
PK parameters
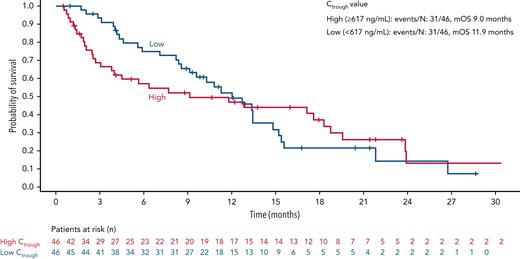
No substantial differences in gilteritinib Ctrough values were observed (supplemental Figure 6) between GIL + AZA and gilteritinib (before removal). However, on cycle 1, day 15, median gilteritinib Ctrough was 585 ng/mL (GIL + AZA and gilteritinib) in contrast to a Ctrough of 279 ng/mL observed with gilteritinib monotherapy in ADMIRAL involving patients with R/R AML (Table 4). Reasons for this difference are being evaluated. High (≥617 ng/mL) vs low (<617 ng/mL) Ctrough values may be associated with early treatment discontinuation (18 vs 29 patients), dose interruption (18 patients vs 13 patients), or dose reduction (10 patients vs 4 patients). Comparison of composite study day discontinuation (“DPR” defined as discontinuation, pause ≥7 days, or dose reduction) stratified according to median Ctrough values showed early DPR in patients with high vs low Ctrough values and median discontinuation at 1.3 months vs 3.7 months, respectively (supplemental Figure 7). Median OS was 2.9 months longer for low vs high Ctrough (11.9 months vs 9.0 months) (Figure 6). No apparent relationship was observed between Ctrough and response rates or grade of neutropenia or thrombocytopenia (supplemental Figure 8).
Comparison of gilteritinib Ctrough values at steady state across studies
| Parameter . | LACEWING trial . | ADMIRAL trial∗ . | ||
|---|---|---|---|---|
| Population | ND FLT3mut+ AML ineligible for IIC | R/R FLT3mut+ AML | ||
| Treatment | GIL + AZA and gilteritinib alone | Gilteritinib monotherapy | ||
| Parameter . | LACEWING trial . | ADMIRAL trial∗ . | ||
|---|---|---|---|---|
| Population | ND FLT3mut+ AML ineligible for IIC | R/R FLT3mut+ AML | ||
| Treatment | GIL + AZA and gilteritinib alone | Gilteritinib monotherapy | ||
| Cycle/day . | Cycle 1, day 15 . | Cycle 2, day 1 . | Cycle 1, day 15 . | Cycle 2, day 1 . |
|---|---|---|---|---|
| No. of values | 82 | 57 | 231 | 219 |
| Median | 584.5 | 583 | 279 | 311 |
| -fold diff pop PK | 2.1 | 2.1 | 1.0 | 1.1 |
| Mean | 698 | 721 | 330 | 372 |
| -fold diff pop PK | 2.1 | 2.2 | 1.0 | 1.1 |
| %CV | 62% | 71% | 68% | 80% |
| Cycle/day . | Cycle 1, day 15 . | Cycle 2, day 1 . | Cycle 1, day 15 . | Cycle 2, day 1 . |
|---|---|---|---|---|
| No. of values | 82 | 57 | 231 | 219 |
| Median | 584.5 | 583 | 279 | 311 |
| -fold diff pop PK | 2.1 | 2.1 | 1.0 | 1.1 |
| Mean | 698 | 721 | 330 | 372 |
| -fold diff pop PK | 2.1 | 2.2 | 1.0 | 1.1 |
| %CV | 62% | 71% | 68% | 80% |
CV, coefficient of variation; diff, difference; pop, population.
Data not previously presented for ADMIRAL trial.
OS according to gilteritinib Ctrough at steady state. OS is shown in patients stratified according to high (≥617 ng/mL) or low (<617 ng/mL) gilteritinib serum Ctrough at steady state. Tick marks indicate censored data. mOS, median OS.
OS according to gilteritinib Ctrough at steady state. OS is shown in patients stratified according to high (≥617 ng/mL) or low (<617 ng/mL) gilteritinib serum Ctrough at steady state. Tick marks indicate censored data. mOS, median OS.
Discussion
In previously untreated patients with FLT3mut+ AML who were elderly or considered ineligible for IIC, OS after treatment with GIL + AZA was not statistically significantly different than AZA, with a median OS ∼1 month longer in the GIL + AZA group. However, OS in patients with AML with high FLT3-ITD allelic burden (ratio ≥0.5) was substantially longer after GIL + AZA treatment, with a median OS improvement over AZA of 6.3 months. This finding suggests that a higher allelic ratio may reflect disease which is more FLT3 dependent and/or driven by FLT3 mutations and potentially indicates that patients with higher allelic ratios may receive more benefit from combination therapy with gilteritinib. Possible reasons for nonsignificant OS findings include effect of subsequent AML therapies (known confounder in survival studies25), a greater proportion of patients with worse functional status in the GIL + AZA group vs AZA group, differences in follow-up time due to study design change during study course, and OS analysis timing after early study termination. Compared with LACEWING, other phase 3 trials of treatments for patients with ND AML with advanced age or ineligible for intensive chemotherapy had lower proportions of patients with baseline ECOG PS ≥214,26-28 or a minority with FLT3 mutations.15,29 Notably, time to next therapy for patients receiving AZA was shorter, and proportions of patients with a subsequent therapy were double that of patients receiving GIL + AZA; 10 of 49 patients originally randomized to receive AZA subsequently initiated gilteritinib. A placebo-controlled, double-blind study design may have mitigated the effect of subsequent AML therapies, including commercially available FLT3 inhibitors, and specifically gilteritinib, on the primary end point of OS.
Notably, CRc rates were significantly higher in patients treated with GIL + AZA (58.1%) vs AZA (26.5%; P < .001), with comparable CR rates. Higher CRc rates in patients with high FLT3-ITD allelic burden, shown in CRc subgroup analyses, may indicate that a larger proportion of leukemic cells are carrying the mutation and a more critical dependence on FLT3-ITD for leukemic cell growth and survival than in cases with a lower allelic ratio in which other signaling mechanisms may be involved. In addition, CRc findings with GIL + AZA vs AZA could reflect a more myelosuppressive regimen that might not, or not yet, display effects on other outcomes. Interestingly, the proportion of patients receiving GIL + AZA with CR with incomplete hematologic recovery (CRi) was more than double that observed with AZA. Patients achieving CR with incomplete platelet recovery or CRi with GIL + AZA may not have had sufficient follow-up time to develop CR. This difference was not due to HSCT, as no patients received posttreatment HSCT before enrollment termination. In the US Food and Drug Administration’s analysis of randomized controlled trials of patients with ND AML who were eligible for intensive chemotherapy, regardless of intervention, patients achieving CR had better OS than those with CRi or CR with incomplete platelet recovery as the best response, who in turn, had better OS than patients with no response.30 Improvements in efficacy measures may be delayed in patients receiving less intensive chemotherapy.31 Underlying biological mechanisms may explain these differences, as gilteritinib treatment in patients with R/R FLT3mut+ AML might eliminate/differentiate leukemic blasts but may not eradicate the inherent leukemia clone.32
The GIL + AZA safety profile was consistent with known AE profiles of each individual therapy; no new, concerning safety signals were identified with combination therapy. AE incidence, including serious and grade ≥3 events, was higher with combination treatment. Although AE rates were higher when adjusted for exposure and by treatment relatedness, events leading to death were comparable. The higher AE rate with GIL + AZA could be related to the higher proportion of patients with ECOG PS ≥2; additional analyses are needed for confirmation. No major differences in treatment-emergent SAEs specific to anemia, neutropenia, and thrombocytopenia were observed between groups; however, the proportion of patients with gastrointestinal hemorrhage was higher with GIL + AZA. Several patients had gastrointestinal hemorrhage events occurring in proximity to their death or treatment discontinuation, suggesting that events may have occurred in the context of disease progression. Although patients receiving GIL + AZA may have been sicker based on poorer PS vs those receiving AZA, these data align with target organ toxicities identified in nonclinical studies.
Given moderate effects of hypomethylating monotherapy in older/unfit patients with ND AML ineligible for IIC, combining therapies with a FLT3 inhibitor plus hypomethylating agent has been evaluated here and elsewhere15,33 to potentially enhance clinical efficacy without excessive toxicity. In a phase 2 study, sorafenib + AZA in patients with relapsed FLT3-ITD–mutant AML resulted in a response rate of 46% (27% with complete response with incomplete count recovery, 16% with complete response, and 3% with partial response).33 Recent data from a phase 3 trial of venetoclax and AZA in older, unfit patients with ND AML reported no statistical improvement in OS after combination therapy vs AZA in patients with FLT3mut+ AML.15 This trial was not powered to detect such a difference, and notably, FLT3-ITD or FLT3-TKD mutations were only present in ∼20% of patients. In patients with high FLT3-ITD allelic burden (allelic ratio ≥0.5), combination venetoclax + AZA yielded a CR rate of 11% (n/N = 1/9),34 whereas in LACEWING, GIL + AZA exhibited a CR rate of 22.9% (n/N = 8/35), suggesting beneficial effects of gilteritinib (including 80 and 120 mg/d dose) and supporting exploration of triple therapy. Effective treatments for patients with FLT3mut+ AML ineligible for intensive chemotherapy remain a critical unmet need.
Gilteritinib Ctrough was consistent between single and combination therapy. Curiously, Ctrough values in patients with ND FLT3mut+ AML ineligible for IIC were twofold greater than in patients with R/R FLT3mut+ AML (some eligible for high-intensity chemotherapy regimens). A similar increase in exposure was observed for erlotinib, also predominately cleared by cytochrome P450 3A metabolism, in older patients with non–small cell lung cancer.35 Lower Ctrough values may be associated with higher median OS, although not with other efficacy or selected safety outcomes.
GIL + AZA exhibited favorable clinical activity over AZA in patients with ND FLT3mut+ AML considered unfit for IIC based on higher CRc rates, particularly for patients with FLT3-ITD allelic ratio ≥0.5, with no new safety signals. Although no difference in OS was observed, confounding factors included use of subsequent therapies (specifically FLT3 inhibitors, including gilteritinib monotherapy) in patients receiving AZA alone and differences in PS between groups. Overall, these results support safety, tolerability, and activity of GIL + AZA in this patient population. Analysis in patients with FLT3-ITD–positive AML with high allelic burden or better PS indicates improved clinical activity in these subgroups, which may warrant further exploration.
Acknowledgments
This study was funded by Astellas Pharma, Inc. Medical writing/editorial support was provided by Stephanie Phan, Cheryl Casterline, and Elizabeth Hermans, from Peloton Advantage, LLC, an OPEN Health company, and funded by the study sponsor.
Authorship
Contribution: E.S.W., P.M., M.D.M., J.-H.L., M.H., T.N., W.-C.C., K.L., J.E., J.K.A., V.H., A.-M.W., E.P., S.L., R.W., N.P., J.E.H., S.C.G., E.S.R., and R.V.T. were involved in the conception of the study design; E.S.W., S.L., R.W., N.P., J.E.H., S.C.G., E.S.R., and R.V.T. analyzed the study data; C.G.-P contributed to the acquisition and interpretation of study data; all authors contributed to the acquisition and interpretation of study data; and all authors participated in the drafting of the manuscript, provided final approval of the submitted version, and agree to be held accountable for all aspects of the work.
Conflict-of-interest disclosure: E.S.W. has received honoraria from Stemline, Kura, Pfizer, and DAVA Oncology; participated in advisory boards with AbbVie, Astellas, BMS/Celgene, Genentech, Gilead, GlaxoSmithKline, Jazz, Kite Pharmaceuticals, Kura Oncology, Novartis, Pfizer, Stemline, and Takeda; and participates in data monitoring committees for AbbVie and Rafael Pharmaceuticals. P.M. has participated in advisory boards and received honoraria from Astellas. M.D.M. has participated in advisory boards for Astellas, AbbVie, Trillium Therapeutics, Celgene, and Bristol Myers Squibb; and has received research funding from AbbVie. J.-H.L. has received honoraria from AbbVie Korea and Astellas Korea; and has participated in advisory boards for Astellas and AbbVie. M.H. has received honoraria from Jazz Pharmaceuticals, Janssen, and Novartis; participated in advisory boards for AbbVie, BMS/Celgene, Daiichi Sankyo, Jazz Pharmaceuticals, Novartis, Pfizer, Roche, and Tolremo; and has received research funding from Astellas, Bayer Pharma AG, BerGenBio, Daiichi Sankyo, Jazz Pharmaceuticals, Karyopharm, Novartis, Pfizer, and Roche. T.N. has received honoraria from Nippon Shinyaku, Bristol Myers Squibb, and Otsuka Pharma; and reports study funding from Astellas, Daiichi Sankyo, and Fujifilm. K.L. has received grants from Novartis, Takeda, Janssen, and AbbVie; and consulting fees from Novartis, Takeda, AbbVie, iQone, Astellas, Astra, and BeiGene. J.E. has received a grant from Novartis; honoraria from Astellas; and participated in advisory boards for Novartis, AbbVie, Jazz Pharmaceuticals, Pfizer, Celgene, and Daiichi Sankyo. J.K.A. has received advisory or consulting fees from AbbVie, Amgen, Astellas, bluebird bio, Curio Science, Daiichi Sankyo, Kura Oncology, Stemline, Syros, and Theradex; research funding at an institutional level for the conduct of trials from ALX Oncology, Amgen, Aptos, Astellas, Aprea, BioSight, BMS, Boehringer Ingelheim, Celgene, Fujifilm, ImmunoGen, Kartos, Kura Oncology, Loxo, and Takeda; reimbursement for travel from BioSight; and serves on a data monitoring committee for GlycoMimetics. V.H. has received honoraria fees from Novartis; travel support from Novartis and BMS; and participated in advisory boards for Incyte, Novartis, BMS, and AbbVie. E.P. has received consulting fees from KCR US, Inc.; honoraria from Amgen, Novartis, Servier, and Angelini Pharma; and travel support from Novartis, Servier, Angelini Pharma, Bristol Myers Squibb, Jazz Pharmaceuticals, Pfizer, and Astellas Pharma. J.E.H. reports holding stock for Ligacept, LLC. S.L., N.P., J.E.H., S.C.G., E.S.R., and R.V.T. are employees of Astellas. The remaining authors declare no competing financial interests.
The current affiliation for R.V.T. is Takeda Pharmaceutical Company, Boston, MA.
Correspondence: Eunice S. Wang, Roswell Park Comprehensive Cancer Center, 665 Elm St, Buffalo, NY 14203; e-mail: eunice.wang@roswellpark.org.
References
Author notes
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. The Astellas criteria on data sharing are provided at: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal